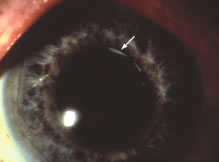
A milder form of phacoanaphylactic uveitis is known to occur following extracapsular cataract extraction, with or without posterior chamber intraocular lens implantation. In these cases the granulomatous inflammation develops at between 1 week and 6 months after the surgical procedure. This form of phacoanaphylaxis is believed to reflect limited immune response to a small amount of lens protein remaining in the ocular cavity.8,9 In some patients a sympathetic response may occur in the opposite eye.
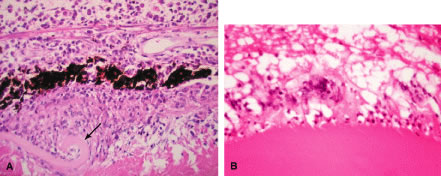
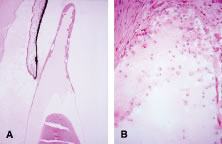
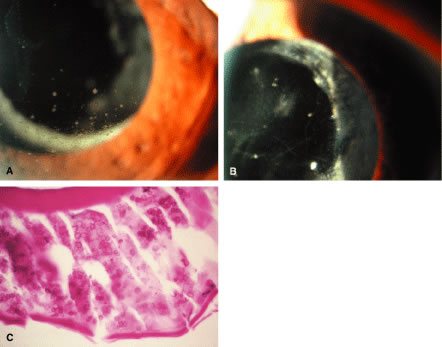
Histopathologically, enucleated eyes with severe phacoanaphylactic endophthalmitis show a zonal type of granulomatous inflammation concentrated around the disrupted lens capsule and exposed lens cortex or nucleus5 (Fig. 2). Typically, a zone of polymorphonuclear leukocytes is present around the lens cortex; the cortical fibers show degenerative changes in the form of fragmentation and disintegration. Surrounding the polymorphonuclear leukocytes is a zone of mononuclear cells, which consists primarily of macrophages and epithelioid cells but is admixed with a few scattered multinucleated giant cells. Some of these giant cells show features of foreign body type cells, whereas others exhibit features typical of Langhans' cells (see Fig. 2B). A third zone, made up of lymphocytes, surrounds these epithelioid cells. This lymphocytic zone may also contain fibroblasts or granulation tissue. The surrounding iris, ciliary body, and anterior choroid are usually infiltrated by lymphocytes and plasma cells. This lymphoplasmacytic infiltration is marked in the anterior uvea and tends to be of mild to moderate degree in the posterior uvea. The retina may show some perivascular lymphocytic infiltration. In many cases, extensive iridolenticular adhesions are present (see Fig. 2A), often accompanied by chronic inflammatory cells in the anterior chamber and in angle structures. The endothelial surface of the cornea may contain collections of mononuclear cells.
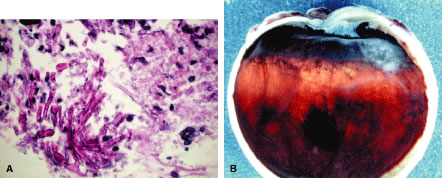
Eyes enucleated during more chronic stages of phacoanaphylactic endophthalmitis may show only a few polymorphonuclear leukocytes around the disrupted lens. However, a zone of granulomatous inflammation can be seen, made up of epithelioid cells admixed with large number of multinucleated giant cells, as can a surrounding zone of lymphocytes and fibroblasts. These eyes may also exhibit fibrosis that encloses the granulomatous inflammatory mass. Such eyes usually show a cyclitic membrane, tractional retinal detachment, osseous metaplasia of the retinal pigment epithelium, and phthisis.
ETIOLOGY
Even though the precise cause of the initial events that evoke the inflammation in phacoanaphylactic endophthalmitis is unknown, the inflammation is believed to be initiated by an autoimmune process directed at the lens protein. Reports in the past have suggested that lens protein is organ specific and, under normal conditions, is sequestered from the immune system by the lens capsule.10,11 However, in recent years it has been documented that most healthy people, with no demonstrable ocular disease, have anti-lens antibodies,12 and when they do undergo extracapsular cataract surgery, they rarely develop phacoanaphylaxis. Based on these observations it is less likely that phacoanaphylaxis is an immune reaction to the so-called sequestered lens antigen that is released by traumatic or surgical disruption of the lens capsule. Moreover, experimental animal studies have shown that lens proteins are not sequestered solely in the lens capsule and, in addition, immunologically these proteins are not organ specific. Based on experimental animal studies, Marak13 proposed a hypothesis for the pathogenesis of phacoanaphylaxis. According to this theory, development of phacoanaphylactic endophthalmitis is dependent on abrogation of normal tolerance to lens protein.
Experimentally, granulomatous inflammation can be induced around surgically disrupted lens in various strains of rats that have been sensitized to lens protein.13 Histopathologically, the enucleated eyes of these animals show features very similar to those observed in human phacoanaphylaxis. Immunologic investigations of such animals demonstrated that the lens-induced uveitis is an antibody-mediated autoimmune disease that develops when normal immune tolerance (T-cell tolerance) is terminated by stimulation of B cells with gram-negative bacterial lipopolysaccharide.13 Based on such experimental studies, it appears that phacoanaphylactic endophthalmitis in humans could be an anti-lens protein antibody-mediated localized immune complex disease that is triggered by traumatic release of lens protein and stimulation of B cells.
DIFFERENTIAL DIAGNOSIS
The intraocular inflammatory disorders that should be differentiated from phacoanaphylactic endophthalmitis include infectious (bacterial or fungal) endophthalmitis, phacogenic nongranulomatous uveitis, phacolytic glaucoma, localized endophthalmitis, and sympathetic ophthalmia. Exogenous bacterial endophthalmitis following a penetrating wound to the globe or following cataract extraction may present with signs of severe intraocular inflammation and hypopyon. However, unlike phacoanaphylactic endophthalmitis, it is unusual to observe large keratic precipitates in bacterial endophthalmitis. Some cases of bacterial or fungal endophthalmitis may be difficult to differentiate from phacoanaphylaxis. In such cases a definitive diagnosis can be established by histologic examination and by culture studies of the intraocular inflammatory exudate.
Exogenous fungal endophthalmitis may present with several clinical features that are typical of low-grade phacoanaphylactic endophthalmitis. These include delayed onset of ocular inflammation, hypopyon and inflammatory exudate in the anterior chamber and vitreous cavity, and puffball vitreous opacities. Furthermore, some of these patients may exhibit keratic precipitates similar to those noted in phacoanaphylactic endophthalmitis, but cultures and histologic studies of the intraocular exudate should point to the proper diagnosis. Phacoanaphylactic endophthalmitis may prove difficult to differentiate from phacogenic nongranulomatous uveitis, phacolytic glaucoma, and localized endophthalmitis, entities that are discussed in detail subsequently in this text.
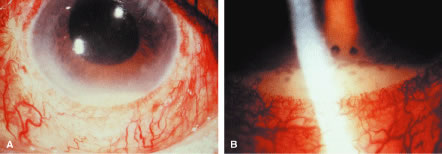
Phacoanaphylactic endophthalmitis occurs typically as a uniocular inflammatory process involving the traumatized eye but can present occasionally as bilateral intraocular inflammation.11 Such bilateral lens-induced intraocular inflammation must be differentiated from sympathetic ophthalmia, which is always a bilateral disease. Patients with sympathetic ophthalmia rarely develop hypopyon. Moreover, sympathetic ophthalmia typically results in angiographic findings of hyperfluorescence and focal leakage at the level of the retinal pigment epithelium during the early arteriovenous phase, with pooling of dye in the subretinal space during late stages of angiography. Echographic evaluation should also be useful because phacoanaphylactic endophthalmitis causes inflammatory swelling of the anterior uvea, whereas sympathetic ophthalmia induces marked swelling of the posterior uvea. It should be noted that in some cases of penetrating injury to the globe, both phacoanaphylactic endophthalmitis and sympathetic ophthalmia may coexist in one eye, and the opposite eye may show just sympathetic ophthalmia.
LABORATORY INVESTIGATIONS
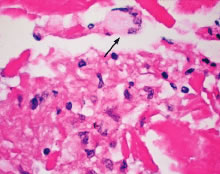
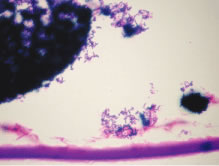
Even though phacoanaphylactic uveitis could be mediated by anti-lens antibody, serologic detection of such antibody is not diagnostic. As noted earlier, many people without any ocular disease show circulating anti-lens antibody in their serum. Similarly, patients who have undergone uncomplicated extracapsular cataract extraction, with no subsequent intraocular inflammation, have demonstrated serologic evidence of anti-lens antibody. Echography in typical cases of phacoanaphylaxis reveals a swollen iris, ciliary body, and anterior choroid and evidence of marked inflammation around the lens. Histopathologic examination of an anterior chamber tap or vitrectomy specimens can be helpful also in establishing the correct diagnosis, because these specimens will typically contain epithelioid histiocytes mixed with polymorphonuclear leukocytes and giant cells (Fig. 3). Cultures of these specimens are negative for bacteria and fungi.
|
TREATMENT
The management of phacoanaphylactic endophthalmitis includes surgical removal of the offending antigen, namely the lens material. Such a surgical procedure should be carried out early in the course of intraocular inflammation, and, in severe cases, the patient should also be treated with topical and systemic corticosteroids. Even though the corticosteroids can reduce the severity of ocular inflammation, these agents do not eliminate the source of the ocular inflammation. Meticulous surgical removal of the lens material, supplemented with cycloplegic and corticosteroid administration, may result in preservation of useful vision with minimal ocular morbidity.
In patients with a history of phacoanaphylactic endophthalmitis following trauma or extracapsular cataract extraction, it is essential to recognize the risk for similar inflammation to develop in the fellow eye following extracapsular cataract extraction. Accordingly, removal of a cataractous lens by intracapsular cataract extraction is recommended in such cases.