In the early stages, trachoma presents as a follicular conjunctivitis, with papillary hypertrophy and diffuse inflammatory infiltration involving the whole conjunctiva, including the upper tarsal conjunctiva.1 As trachoma progresses, cicatrization of the conjunctiva appears as fine linear and small stellate scars in mild cases and as broader confluent deep scars in severe cases.
In communities with hyperendemic trachoma, children acquire chlamydial eye infection in the first year of life. These young children usually develop a mucopurulent or papillary conjunctivitis without apparent follicle formation. In communities with endemic trachoma, concomitant eye infections with other bacterial and viral agents are common and appear to contribute to the inflammatory intensity of trachoma and to the keratitis.3,32 With active infectious trachoma, there is usually only a minimal to moderate amount of mucopurulent discharge, except during episodes of bacterial conjunctivitis, when the discharge is more abundant.
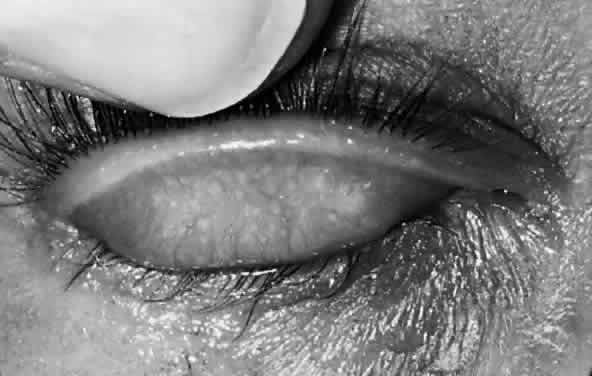
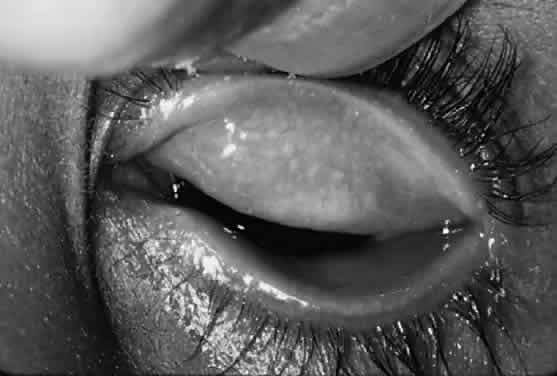
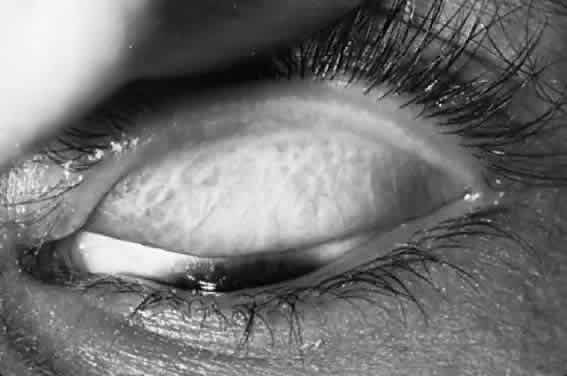
After the first year of life, lymphoid follicles are a regular feature of trachoma (Figs. 1 and 2). These conjunctival lesions are flat or elevated, opaque or clear, have a yellow or gray color, and appear avascular. They vary in diameter from 0.2 to 2.0 mm. The entire conjunctiva is involved by the inflammation but follicle formation in the conjunctiva of the upper tarsus is one of the cardinal signs of trachoma. Follicles may become necrotic, so that they rupture on slight pressure, extruding their contents onto the conjunctival surface. In such cases, the conjunctiva is extremely friable, so small blood vessels rupture on everting the tarsal plate, resulting in hemorrhage into follicles or into the adjacent subepithelial conjunctiva. Not all cases—even those that are severe—develop these large necrotic follicles.
|
|
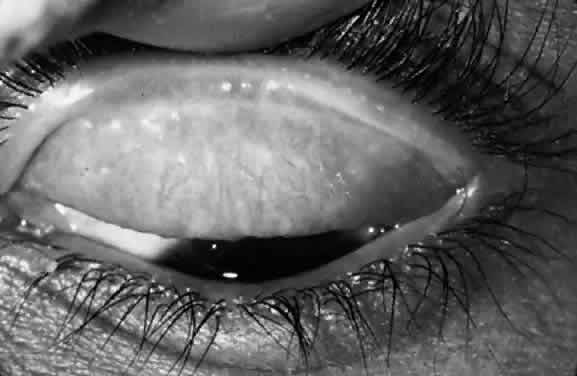
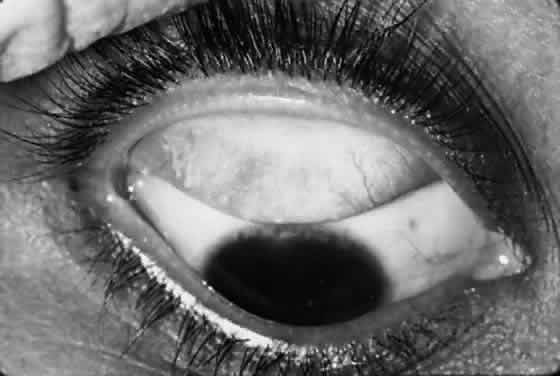
Scars of the conjunctiva may form in a stellate pattern at the site of necrotic follicles. Other scarring appears as fine short lines or broader confluent scars (Figs. 3 and 4). Larger deep scars appear to form in cases with particularly severe trachoma and may not be due simply to necrosis of follicles (Fig. 5). In some cases, a distinctive horizontal scar (line of von Arlt) forms in the upper tarsus, where the ascending and descending subconjunctival vessels meet about a third of the distance from the lid margin to the upper tarsal border.
|
|
|
This scar formation also affects the canaliculi and lacrimal duct with obstruction and cutaneous fistula.33 Occlusion of the tear outflow can cause tearing and often leads to the development of dacryocystitis in adult life.
Linear scars that form just adjacent to the lid margin are particularly damaging because of the lid distortion that they cause. Cicatricial changes occur throughout the conjunctiva but are most apparent and damaging on the tarsal conjunctiva. One result of extensive scarring is that destruction of goblet cells occurs in severely scarred eyes, with a loss of mucus secretion. The loss of mucus and distortion of the upper lid lead to inadequate surface wetting of the cornea.
The drooping or half-closed lids in active trachoma have been attributed to the weight of the lid, to inflammatory involvement of Müller's muscle in the upper lid, and to reflex blepharospasm caused by keratitis-induced photophobia. In late cicatricial trachoma, there may also be destruction of Müller's muscle.
The major potentially blinding sequela of trachoma are distortion of the lids, particularly the upper lid; trichiasis (misdirection of individual lashes); and entropion (inward deformation of the lid margin; Fig. 6).1 Severe conjunctival scarring from trachoma can lead to defects in lid closure and loss of the mucus in tears, with inadequate surface wetting of the cornea. The abrasion of the cornea by wiry lashes—especially when aggravated by even minor foreign-body injury or by inadequate wetting of the cornea—can further predispose the cornea to traumatic and infectious damage.
|
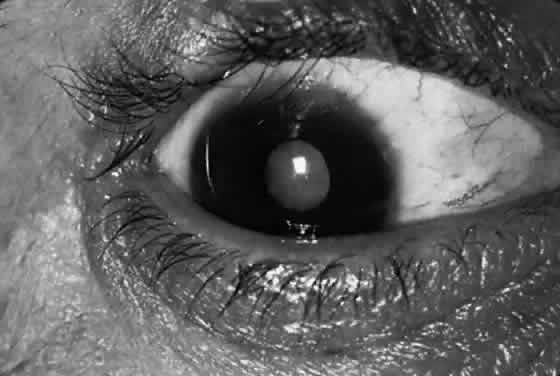
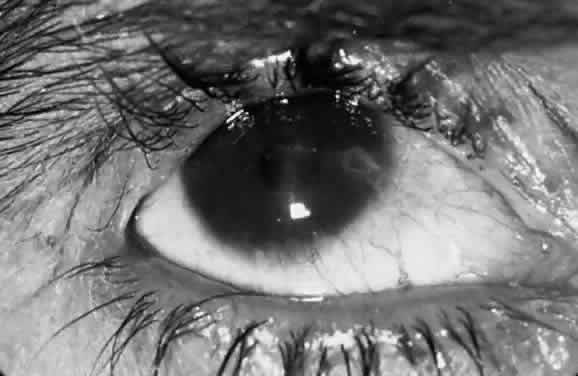
Corneal lesions in trachoma include focal inflammatory infiltrates of the epithelium and anterior stroma, with the formation of a superficial fibrovascular membrane (vascular pannus; Fig. 7). There may be lymphoid follicles at the superficial limbus, which resolve leaving characteristic depressions—Herbert's pits.12
|
The early keratitis in trachoma presents as diffuse epithelial keratitis, and small infiltrates can form in the epithelium and anterior stroma, usually at the limbus but occasionally in the central cornea. This is soon followed by extension of the superficial vessels from the limbus. These vessels form an even advancing border as they extend onto the cornea (vascular pannus). During active inflammation, there is often a hazy infiltrate around the vessels and discrete inflammatory infiltrates in advance of the pannus. Permanent superficial scarring occurs between the vessels. Particularly severe cases may have marked infiltration and swelling of the pannus, with abundant vascularization (pannus crassus). Pannus ulcers are horizontal, oval, epithelial defects that appear in the upper conjunctiva in front of the vascular pannus and resemble the epithelial ulcers in vernal catarrh.
Pannus formation is always more extensive at the upper limbus but may involve the entire limbus. Deep corneal vessels or irregular extensions of the pannus are usually caused by other lesions, such as bacterial corneal ulcers after trauma. The inturned lashes and exposure also may cause diffuse anterior stromal scarring of the cornea. The bluish-grey elevated lesions of Salzmann's nodular dystrophy are a common finding in adults with trachoma pannus and appear to be related to corneal vascularization, not to lid distortion. In older adults, there may be marked lipid deposition in the pannus, forming a lipoidal degeneration of the upper limbus.
In older patients with extensive scarring of the upper limbus, the corneal epithelium appear to be roughened with diffuse fluorescein staining. This may be caused by replacement of limbal stem cells by conjunctival epithelium.
The extent of pannus formation in children does not appear to be directly related to the disease intensity or extent of conjunctival scarring. In younger patients, marked pannus formation can occur with relatively mild lid scarring, but lesser degrees of pannus may accompany even severe intensity conjunctival inflammation.
CLINICAL CLASSIFICATION
Clinical signs of the upper tarsal conjunctiva and cornea have been used to describe clinical trachoma in the whole eye. To provide a more precise description of inflammatory and cicatricial disease in individual cases, a detailed system was developed, based on the scoring of lymphoid follicles (F), diffuse inflammation and papillary hypertrophy (P), conjunctival scarring (C), lid distortion and inturned lashes (T/E), and corneal opacities (CC) (Table 1).1 The intensity of inflammatory trachoma includes four categories—severe, moderate, mild, and insignificant—which are of prognostic value in the individual case (Table 2). As noted, the prevalence of chlamydial infection parallels this clinical intensity scale.
TABLE 1. Clinical Scoring of Inflammatory Signs for Detailed Epidemiologic
and Therapeutic Studies of Trachoma1
| Scores for Upper Tarsal Follicles (F) | |
| F0 | No follicles |
| F1 | Follicles present but no more than five in zones 2 and 3 together |
| F2 | More than five follicles in zones 2 and 3 together but fewer than five in zone 3 |
| F3 | Five or more follicles in each of the three zones |
| Scores for Upper Tarsal Papillary Hypertrophy and Diffuse | |
| Infiltration (P) | |
| P0 | Absent: normal appearance |
| P1 | Minimal: individual vascular tufts (papillae) prominent, but deep subconjunctival vessels on the tarsus not obscured |
| P2 | Moderate: more prominent papillae, and normal vessels appear hazy, even when seen by the naked eye |
| P3 | Pronounced: conjunctiva thickened and opaque, normal vessels on the tarsus are hidden over more than half of the surface |
| Conjunctival Scarring (C) | |
| C0 | No scarring on the conjunctiva |
| C1 | Mild: fine scattered scars on the upper tarsal conjunctiva, or scars on other parts of the conjunctiva |
| C2 | Moderate: more severe scarring but without shortening or distortion of the upper tarsus |
| C3 | Severe: scarring with distortion of the upper tarsus |
| Trichiasis or Entropion (T/E) | |
| T/E0 | No trichiasis or entropion |
| T/E1 | Lashes deviated towards the eye but not touching the globe |
| T/E2 | Lashes touching the globe but not rubbing on the cornea |
| T/E3 | Lashes constantly rubbing on the cornea |
| Corneal Scarring (CC) | |
| CC0 | Absent |
| CC1 | Minimal scarring or opacity but not involving the visual axis and with clear central cornea |
| CC2 | Moderate scarring or opacity involving the visual axis, with the pupillary margin visible through the opacity |
| CC3 | Severe central scarring or opacity with the pupillary margin not visible through the opacity |
TABLE 2. Intensity of Inflammatory Trachoma
| Intensity | Key Sign | Follicles | Papillae |
| Severe | P3 | F3 (or F2 or F1)* | P3† |
| Moderate | F3 | F3† | P2 |
| Mild | F2 | F2† | P0, P1, or P2 |
| Trivial (insignificant or absent) | F2 or F1 | F0 or F1 | P0, P1, or P2 |
*The follicles may be obscured by severe papillary hypertrophy and diffuse infiltration.
†Key sign.
Subsequently, a simplified grading was developed for use in trachoma control efforts based on primary health care workers (Table 3).34 This simplified system records the presence or absence of the same signs as the detailed grading system in Table 1. The grading schemes are compared in Table 4.34
TABLE 3. Simplified Grading of Trachoma Currently Recommended for Trachoma
Control Programs
TT—trichiasis/entropion
TF—5 or more follicles on the central tarsal conjunctiva
TI—intense papillary reaction of the tarsal conjunctiva
TS—trachomatous scarring
CO—central cornea opacities
TABLE 4. Comparison of Detailed and Simplified Trachoma Grading Systems
| Simplified Signs | Detailed Scoring | |
| Trichiasis | TT | T/E 1, 2, 3 |
| Tarsal follicles | TF | F 2, 3 |
| Infiltration and papillary hypertrophy | TI | P 3 |
| Conjunctival scarring | TS | C 1, 2, 3 |
| Corneal scarring | CO | CC 2, 3 |
This simplified system is in widespread use but can result in overdiagnosis of trachoma. Two examples occurred in Nepal: in Eastern Nepal, TF (five or more follicles on the central tarsal conjunctiva) was diagnosed in 22% of children and TI (intense papillary reaction of the tarsal conjunctiva) in 6.7% but no trachomatous scarring could be detected. The investigators correctly judged that without scarring, there was no need to intervene for trachoma.35 Our group found a similar situation in Western Nepal, with 7% of children with TF and 1% with TI; despite the occurrence of severe scarring and trichiasis in older adults, no chlamydial agent was found by a sensitive DNA amplification test, indicating that the follicular conjunctivitis was not active trachoma (T. Lietman, personal communication). In such situations, other signs (conjunctival scarring or Herbert's pits) are needed to definitively diagnose trachoma.
In the older MacCallan classification, trachoma cases are classified in stages by the findings in the conjunctiva alone.1 This classification describes the evolution of the disease but does not have prognostic value. Trachoma stage I and stage II are the early and established phases of inflammatory trachoma, with lymphoid follicles and papilla but without conjunctival scarring. Stage IIA is characterized by mature (i.e., large necrotic follicles) and stage IIB by papillary hypertrophy that has obscured the follicles and underlying tarsal vessels (P3 or severe intensity). In stage III, conjunctival scarring is present with the conjunctival signs of stages I or II. In stage IV, the acute inflammatory signs of the conjunctiva found in stages I and II have resolved, leaving scars in the conjunctiva. If sufficiently severe, these scars can contract further to produce lid distortion and trichiasis. In stage IV, the disease was thought to be noninfectious but the newer diagnostic tests frequently identify chlamydial infection in these putatively healed cases. The MacCallan classification does not describe the degree of inflammation or identify cases with visually disabling lesions and hence is not as useful for prognosis as the newer classification.
Communities with blinding trachoma can be recognized by the presence of those with severe visual loss due to corneal opacity and a substantial prevalence of potentially disabling trachomatous lesions, particularly trichiasis or entropion. These irreversible changes appear as the long-term outcome of prolonged or recurrent inflammatory disease of moderate or severe intensity. Communities with nonblinding trachoma may have a low prevalence of potentially blinding lesions but do not have a substantial prevalence of trachomatous visual loss. In some areas where living standards have improved, trachomatous trichiasis and corneal scarring affect many adults but active inflammatory trachoma in children is relatively mild. In this setting, trachoma control programs should emphasize surgical correction of inturned lids but the need for community-wide antibiotic treatment should be based on the prevalence and severity of conjunctival scarring in children younger than 10 years, in addition to the prevalence of inflammatory trachoma.
In communities with trachoma, infection by C. trachomatis is always present but other ocular microbial pathogens appear to contribute significantly to the intensity of trachoma and probably to the lesions that impair vision.3,7,8
CLINICAL DIAGNOSIS
If there is uncertainty concerning the presence or absence of trachoma in a given community or area, it is essential to use strict diagnostic criteria to identify those with trachoma.1 For this purpose, individual cases must have at least two of the following signs:
- Follicles on the upper tarsal conjunctiva
- Limbal follicles or their sequela, Herbert's pits
- Typical conjunctival scarring
- Vascular pannus most marked at the superior limbus.
If there is still a question about the presence of active, infectious trachoma in a community, the presence of infection with C. trachomatis should be required in a substantial proportion of tested children to confirm the diagnosis.
Once the presence of endemic trachoma has been established in a community, the occurrence of one of these signs in an individual case is sufficient to establish the diagnosis in surveys to measure endemicity. In many communities where blinding trachoma was once prevalent, improved conditions have resulted in a reduction in the severity or disappearance of infectious trachoma in children, although trichiasis and corneal opacity are still common in adults.8 Because many trachoma programs limit examinations of children to the diagnosis of tarsal follicles (TF) and severe tarsal inflammation (TI) but not trachomatous scarring (TS), the rate of active trachoma may be exaggerated because of a high prevalence of follicular and papillary conjunctivitis attributable to other causes.
MICROBIOLOGIC DIAGNOSIS
Laboratory tests in trachoma control programs may be used for several reasons: to support the clinical diagnosis of the disease in individual cases, to measure the presence and prevalence of agent in communities where trachoma is endemic or thought to be endemic, to monitor individuals or communities for the effect of therapy, to estimate the total exposure of a population to chlamydial infection, to monitor shifts in serotypes in a given population that may indicate introduction of an agent from outside the community or possible transmission of nonocular serotypes.
C. trachomatis infections are diagnosed by identifying the agent in clinical specimens or by a rising antibody titer. The microscopic examination of Giemsa-stained smears was the mainstay of diagnosis from the first decade of this century and is still a useful tool for field studies but has a low sensitivity.11,36 The first isolation of C. trachomatis strains was reported by T'ang and associates37 in 1957 from Peking, who recovered trachoma strains by inoculating the yolk sacs of fertile hen's eggs. Isolation techniques use specially treated cell cultures for isolation. With the availability of cultivated chlamydial strains, standardized (commercial) procedures have become available.38
To identify infection of individuals with C. trachomatis, the available procedures include:
- Morphologic identification of chlamydial inclusions by Giemsa-stained smears; rarely
by iodine staining
- Isolation of agent in cell cultures
- Direct fluorescein-conjugated monoclonal antibodies (DFA) to species-specific (MOMP) or
group-specific antigen (lipopolysaccharide— LPS)
- Identification of chlamydial antigen by enzyme-labeled immunoassay
- Probe for the nucleic acid of the chlamydia or the associated plasmid of C. trachomatis
- DNA amplification techniques
- PCR
- LCR
- PCR
- Detection of antibody to C. trachomatis in serum, tears, or other secretions.
Cytology
For C. trachomatis infections of the eye, several cytologic techniques are available: staining of conjunctival smears with Giemsa stain, iodine, and fluorescein or peroxidase-conjugated monoclonal antibodies.
In Giemsa-stained smears, chlamydial organisms are seen as clusters of distinct particles (inclusions) in the cytoplasm of conjunctival epithelial cells. With an oil-immersion lens (40× to 100× ), the elementary bodies stain reddish-purple and the larger initial bodies a deep blue, like most bacteria.11 The common sources of misdiagnosis of cytoplasmic chlamydial inclusion in Giemsa-stained smears include pigment granules, keratin, nuclear extrusions, goblet cells, eosinophilic granules, and bacteria. In trachoma, the accompanying conjunctival cytology can be used as a guide to screening smears: inclusions are usually found only when polymorphonuclear leukocytes and separated epithelial cells are present; the prevalence of chlamydial inclusions increases progressively with the presence of other inflammatory cells (lymphocytes, plasma cells, macrophages, and multinucleated giant cells).
Iodine staining with Lugol's iodine identifies the glycogen matrix of inclusions. Direct iodine staining of conjunctival smears is insensitive but the technique was once used to identify inclusions in cell culture.
Staining of conjunctival smears with fluorescein-conjugated polyclonal antibody produced in rabbits was done in several research laboratories in the 1960s and 1970s.16 DFA to C. trachomatis MOMP or to chlamydial LPS are widely available to detect chlamydial agent in smears.39 With the DFA, individual elementary bodies are identified as brightly fluorescing extracellular particles but intracellular inclusions are not well identified. For trachoma, DFA is 80% as sensitive as culture and highly specific.36,40 The technique requires a fluorescent microscope and an experienced microscopist who can recognize the typical morphology of free elementary bodies. Commercial DFA reagents are widely available for the diagnosis of genital infections, however.
Isolation
Isolation of C. trachomatis in cell culture has been the definitive way to identify the agent. Although this procedure was the gold standard used to judge other tests for detecting C. trachomatis, there are still only a few laboratories prepared to perform chlamydial isolation.38,40
Identification of Antigen
Many immunoassay procedures to detect chlamydial antigen in clinical specimens are available.38,40 Test kits for single specimens that use this technology are available for office and small hospital laboratories but are less sensitive.
DNA Amplification Techniques
Polymerase chain reaction identifies DNA of the plasmid carried by all C. trachomatis strains (Chlamydia Amplicor, Roche). The LCR (LCX, Abbott) uses a different technique of DNA amplification for the same plasmid DNA. Both tests are 90% to 95% sensitive and have more than 99% specificity.38,40
Serologic Diagnosis
Detection of antibody in serum or tears usually is not useful for the diagnosis of ocular chlamydial infections in patients because the infections are chronic and endemic in populations with trachoma.40 Because patients are rarely tested during the early phases of infection, serologic testing is usually done with a single serum or tear specimen rather than paired specimens. Testing for antibodies has been applied in epidemiologic studies, however.
Serologic Tests
The two major serologic tests are the complement fixation test, which detects antibody to LPS chlamydial group antigen (including C. psittaci and C. pneumoniae strains) and the indirect immunofluorescent technique (micro-IF), which identifies antibody to C. trachomatis antigens. In most cases of trachoma, the microIF test detects IgG antibody, indicating infection at some time in the past.
Comparison of Procedures
In endemic trachoma, the PCR and LCR DNA amplification tests are more sensitive (more than 90%) than DFA and enzyme-labeled immunoassay (60% to 80%).40 Giemsa-staining of smears is clearly less sensitive in detecting chlamydial agent; isolation in cell culture is less available but has good sensitivity and specificity; micro-IF testing for antibodies may be useful for epidemiologic studies but is rarely useful for individual cases.38,40