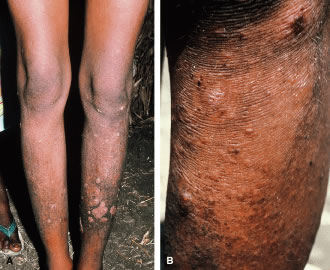
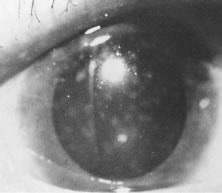
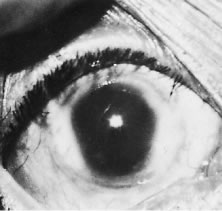
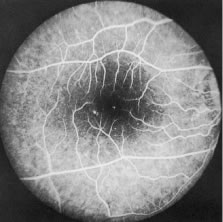
1. Report of WHO Expert Committee: Onchocerciasis and its control. Technical
Report Series No. 852. Geneva, World Health Organization, 1995 2. Prost A: The burden of blindness in adult males in the savanna villages of West
Africa exposed to onchocerciasis. Trans R Soc Trop Med Hyg 80:525, 1986 3. Report of WHO Expert Committee: Epidemiology of Onchocerciasis. Technical
Report Series No. 752. Geneva, World Health Organization, 1987 4. Duke BOL: Onchocerciasis. In Johnson GJ, Minassian DC, Weale R (eds): The
Epidemiology of Eye Disease. London, Chapman and Hall 1998, pp 227–247 5. Anderson J, Fuglsang H, Marshall TF deC: Studies on onchocerciasis in the United Cameroon Republic. III. A
four-year follow-up of 6 rain forest and 6 Sudan-savanna villages. Trans R Soc Trop Med Hyg 70:362, 1976 6. Mackenzie CD, Williams JF, Sisley BE et al: Variations in host responses and the pathogenesis of human onchocerciasis. Rev Infect Dis 7:802, 1985 7. Budden FH: The natural history of ocular onchocerciasis over a period of 14–15 years
and the effect on this of a single course of suramin therapy. Trans R Soc Trop Med Hyg 70:484, 1976 8. Duke BOL: The population dynamics of Onchocerca volvulus in the human host. Trop Med Parasitol 44:61, 1993 9. Vincent JA, Lustigman S, Zhang S et al: A comparison for the newer tests for the diagnosis of onchocerciasis. Ann Trop Med Parasitol 94:253, 2000 10. Budden FH: Route of entry of Onchocerca volvulus microfilariae into the eye [Letter]. Trans R Soc Trop Med Hyg 70:265, 1976 11. Gallin M, Edmonds K, Ellner JJ et al: Cell-mediated responses in human infection with Onchocerca volvulus. J Immunol 140:1999, 1988 12. Ottesen EA: Immune responsiveness and the pathogenesis of human onchocerciasis. J Infect Dis 171:659, 1995 13. Francis H, Awadzi K, Ottesen EA: The Mazzotti reaction following treatment of onchocerciasis with diethylcarbamazine: Clinical
severity as a function of infection intensity. Am J Trop Med Hyg 34:529, 1985 14. Buck AA: Onchocerciasis: Symptomatology, Pathology, Diagnosis. Geneva, World
Health Organization, 1974 15. Neumann E, Gunders AE: Pathogenesis of the posterior segment lesion of ocular onchocerciasis. Am J Ophthalmol 75:82, 1973 16. Chan CC, Nussenblatt RB, Kim MK et al: Immunopathology of ocular onchocerciasis. 11. Anti-retinal autoantibodies
in serum and ocular fluids. Ophthalmology 94:439, 1987 17. Van der Lelij A, Rothova A, Stilma JS et al: Cell-mediated immunity against human retinal extract, S-antigen and interphotoreceptor
retinoid binding protein in onchocercal chorioretinopathy. Invest Ophthalmol Vis Sci 31:2031, 1990 18. Garner A: Pathology of ocular onchocerciasis: Human and experimental. Trans R Soc Trop Med Hyg 70:374, 1976 19. Rodger FC: The pathogenesis and pathology of ocular onchocerciasis. IV. The pathology. Am J Ophthalmol 49:327, 1960 20. Murdoch ME, Hay RJ, Mackenzie CD et al: A clinical classification and grading system of the cutaneous changes in
onchocerciasis. Br J Dermatol 129:260, 1993 21. Murphy RP, Taylor HR, Greene BM: Chorioretinal damage in onchocerciasis. Am J Ophthalmol 98:519, 1984 22. Newland HS, White AT, Greene BM et al: Ocular manifestations of onchocerciasis in a rain forest area of West Africa. Br J Ophthalmol 75:163, 1991 23. Bird AC, Anderson J, Fuglsang H: Morphology of posterior segment lesions of the eye in patients with onchocerciasis. Br J Ophthalmol 60:2, 1976 24. Bird AC, El-Sheikh H, Anderson J et al: Changes in visual function and in the posterior segment of the eye during
treatment of onchocerciasis with diethylcarbamazine citrate. Br J Ophthalmol 64:191, 1980 25. Semba RD, Murphy RP, Newland HS et al: Longitudinal study of lesions of the posterior segments in onchocerciasis. Ophthalmology 97:1334, 1990 26. Murdoch IE, Jones BR, Cousens S et al: Visual field constriction as a cause of blindness or visual impairment. Bull World Health Organ 75:141, 1997 27. Cousens S, Cassels-Brown A, Murdoch I et al: Impact of annual ivermectin treatment on progression of onchocercal visual
field loss. Bull World Health Organ 75:229, 1997 28. Abiose A, Jones BR, Cousens S et al: Reduction in incidence of optic nerve disease with annual ivermectin to
control onchocerciasis. Lancet 341(8838):130, 1993 29. Abiose A: Onchocercal eye disease and the impact of Mectizan treatment. Ann Trop Med Parasitol 92:S11, 1998 30. Schulz-Key H: A simple technique to assess the total number of Onchocerca volvulus microfilariae in skin snips. Tropenmed Parasitol 29:51, 1978 31. Taylor HR, Munoz B, Keyvan-Larijani E et al: Reliability of skin snips in the diagnosis of onchocerciasis. Am J Trop Med Hyg 41:467, 1989 32. Boatin BA, Toé L, Alley ES et al: Diagnostics in onchocerciasis: Future challenges. Ann Trop Med Parasitol, 92:S41, 1998 33. Taylor HR, Duke BOL, Munoz B: The selection of communities for treatment of onchocerciasis with ivermectin. Trop Med Parasitol 43:267, 1992 34. World Health Organization: Methods for community diagnosis of onchocerciasis
to guide ivermectin-based Control in Africa. Report of an informal
consultation held in Ouagadougou from 19–21 November 1991. Document
TDR/TDE/ONCHCERCIASIS/92.2. Geneva, WHO, 1992 35. Bradley JE, Trenholme KR, Gillepsie AJ et al: A sensitive serodiagnostic test for onchocerciasis using a cocktail of
recombinant antigens. Am J Trop Med Hyg 48:198, 1993 36. Toe L, Boatin BA, Adjami A et al: Detection of Onchocerca volvulus infection by 0–150 polymerase chain
reaction analysis of skin scratches. J Infect Dis 178:282, 1998 37. Etya'alé DE: Mectizan as a stimulus for the development of novel partnerships: The international
organization's perspective. Ann Trop Med Parasitol 92:S73, 1998 38. Dadzie KY: Control of onchocerciasis: Challenges for the future. Ann Trop Med Parasitol 92:S65, 1998 39. Campbell WC, Fisher MH, Stapley EO et al: Ivermectin: A potent new antiparasitic agent. Science 221:823, 1983 40. Aziz MA, Diallo S, Diop IM et al: Efficacy and tolerance of ivermectin in human onchocerciasis. Lancet 2(8291):171, 1982 41. Greene BM, Taylor HR, Cupp EW et al: Comparison of ivermectin and diethylcarbamazine in the treatment of onchocerciasis. N Engl J Med 313:133, 1985 42. Taylor HR Murphy RP, Newland HS et al: Comparison of the treatment of ocular onchocerciasis with ivermectin and
diethylcarbamazine. Arch Ophthalmol 104:863, 1986 43. Pacque MC, Dukuly Z, Greene BM et al: Community-based treatment of onchocerciasis with ivermectin: Acceptability
and early adverse reactions. Bull World Health Organ 67:721, 1989 44. Greene BM, Dukuly ZK, Munoz B et al: Comparison of 6-, 12-, and 24-monthly dosing with ivermectin for treatment
of onchocerciasis. J Infect Dis 163:376, 1991 45. Murdoch IE, Abiose A, Babalola OE et al: Ivermectin and onchocercal optic neuritis: Short-term effects. Eye 8:456, 1994 46. Whitworth JA, Gilbert CE, Mabey DM et al: The effect of repeated doses of ivermectin on ocular onchocerciasis: Community-based
trial in Sierra Leone. Lancet 338(8775):1100, 1991 47. World Health Organization: The effect of repeated ivermectin treatment
on ocular onchocerciasis. Report of an informal consultation. WHO/Document/TDE/ONCHO/93.3. Geneva, World Health Organization, 1993 48. Whitworth JA, Gilbert CE, Mabey DM et al: Effects of repeated doses of ivermectin on ocular onchocerciasis: Community-based
trial in Sierra Leone. Ophthalmology 103:1001, 1996 49. Chippaux JP, Boussinesq M, Fobi G: Effect of repeated ivermectin treatments on ocular onchocerciasis: Evaluation
after six to eight doses. Ophthalmic Epidemiol 64:299, 1999 50. Duke BOL, Pacque MC, Munoz B et al: Viability of adult Onchocerca volvulus after 6 2-weekly doses of ivermectin. Bull World Health Organ 69:163, 1991 51. Taylor HR, Pacque MC, Munoz B et al: Impact of mass treatment of onchocerciasis with ivermectin on the transmission
of infection. Science 250:116, 1990 52. Cupp EW, Bernardo MJ, Kiszewski AE et al: The effects of ivermectin on transmission of Onchocerca volvulus. Science 231:740, 1986 53. Remme J, Baker RHA, de Sole G et al: A community trial of ivermectin in the onchocerciasis focus of Asubende, Ghana. I. Effect
on the microfilarial reservoir and the transmission
of Onchocerca volvulus. Trop Med Parasitol 40:367, 1989 54. Foege WH: Ten years of Mectizan. Ann Trop Med Parasitol 92:S7, 1998 55. Pacque MC, Munoz B, Poetschke G et al: Pregnancy outcome after inadvertent ivermectin treatment during community-based
distribution. Lancet 336:1486, 1990 56. Pacque MC, Munoz B, Greene BM et al: Community-based treatment of onchocerciasis with ivermectin: Safety, efficacy
and acceptability of yearly treatment. J Infect Dis 163:381, 1991 57. World Health Organization: Community-directed treatment with ivermectin: A
practical guide for trainers of community-directed distributors, the
African Program for Onchocerciasis Control (APOC). Geneva, World Health
Organization, 1998 58. Gardon J, Gardon-Wendel N, Damanga-Ngangue et al: Serious reactions after mass treatment of onchocerciasis with ivermectin
in an area endemic for Loa loa infection. Lancet 350:18, 1997 59. Boussinesq M, Gardon J: Prevalences of Loa loa microfilaraemia throughout the area endemic for
the infection. Ann Trop Med Parasitol 91:573, 1997 60. Taylor MJ, Bandi C, Hoerauf A et al: Wolbachia bacteria of filarial nematodes: A target for control? Parasitol Today 16:179, 2000 61. Ladzins J, Kron M: New molecular targets for filariasis drug discovery. Parasitol Today 15:305, 1999 |