TABLE 1. Physical Properties of Viscoelastic Substances
| Healon | Healon GV | Amvise | Amvise Plus | Chondroitin Sulfate | Viscoat | |
| Resting viscosity (cps)* | >200,000 | 2,000,000 | 100,000 | 102,000 | 17,000 at 50% | 41,000 |
| Dynamic viscosity (cps)= | 40,000–64,000 | 80,000 | 40,000 | 55,000 | 30 at 20% | 40,000 |
| Color | Clear | Clear | Clear | Clear | Yellow | Clear |
| Pseudoplasticity | +++ | ++++ | +++ | +++ | No | ++ |
| Contact angle | 60° | = | 60° | = | = | 52° |
| Occucoat | Vitrax | ProVisc | Healon 5 | Cellugel | ||
| Resting viscosity (cps)* | = | 40,000 | = | 7 million | ||
| Dynamic viscosity (cps)= | 4000 | 30,000 | 39,000 | 60,000–80,000 | 30,000 | |
| Color | Clear | Clear | Clear | Clear | Clear | |
| Pseudoplasticity | + | +++ | +++ | ++++ | ++ | |
| Contact angle | 52° | = | = | = |
*At shear rate of zero, Data from Arschinoff S, Personal communication.
+|At shear rate of 2/s, 25°C.
= Not available.
Pseudoplasticity key: + = slight; +++ = good; ++ = fair; ++++ = excellent.
Modified from Liesegang TJ: Viscoelastic substances in ophthalmology, Surv Opththalmol 34 p. 268–293, 1990
TABLE 2. Physical Properties of Various Viscoelastic Materials
| Healon 5 | Healon GV | Healon | Vitrax | Amvisc | Amvisc Plus | |
| Source | Rooster combs | Rooster combs | Rooster combs | Rooster combs | Rooster combs | Rooster combs |
| Manufacturer Molecular mass (daltons) | Pfizer/Pharmacia | Pfizer/Pharmacia 5 × 106 | Pfizer/Pharmacia 2.5-3.8 × 106 | Advanced Medical Optics 5 × 105 | Bausch & Lomb 2 × 106 | Bausch & Lomb 2 × 106 |
| Content | 2.3% sodium Hyaluronate | 1.4% sodium hyaluronate | 1% sodium hyaluronate | 3% sodium hyaluronate | 1% sodium hyaluronate | 1.6% sodium hyaluronate |
| pH Buffer solvent | 7.0–7.5 phosphate-buffered saline | 7.0–7.5 phosphate-buffered saline | 7.0–7.5 phosphate-buffered saline | 7.0–7.5 physiological BSS | 6.5–7.2 physiological saline | 7.2 physiological saline |
| Osmalality (mOsm/kg H2O) | 320 | 302 | 309 | 310 | 318 | 340 |
| Concentration (mg/mL) | 23 | 14 | 10 | 30 | 10 | 16 |
| Viscoat | Occucoat | ProVisc | Cellugel | |||
| Source | Bacterial fermentation (sodium hyaluronate): shark fin cartilage (sodium chondroitin sulfate) | Wood pulp | Bacterial fermentation | Wood pulp | ||
| Manufacturer | Alcon | Bausch & Lomb | Alcon | Alcon | ||
| Molecular mass (daltons) | 500 × 103; 25 × 103 | 86 × 103 | 1.9 × 106 | 3 × 105 | ||
| Content | 3% sodium hyaluronate; 4% sodium chondroitin sulfate | 2% HPMC | 1% sodium hyaluranate | 2% HPMC | ||
| pH Buffer solvent | 7.0–7.5 physiological phosphate buffer | 7.2 BSS and variable buffers | 7.25 physiological sodium chloride phosphate buffer | N/A | ||
| Osmalality (mOsm/kg H2O) | 360 | 319 | 310 | 315 | ||
| Concentration (mg/mL) | Sodium hyaluronate 30; Chondroitin sulfate 40 | 20 | 10 | N/A | ||
Viscoelasticity
Elasticity refers to the ability of a solution to return to its original shape after being stressed. The rheologic property of viscoelasticity is the essence of the usefulness of these materials as surgical tools in ophthalmology. Elasticity allows the anterior chamber to reform after deformation by depression on the cornea when external forces are released. A nonelastic solution, such as balanced salt solution (BSS), will show no such reformation after release of forces.
The terms viscosity and viscoelasticity are not synonymous. Viscosity, viscoelasticity, and pseudoplasticity are, however, interrelated. The amount of elasticity of an elastic compound increases with increasing molecular weight and greater chain length of the molecules. Unfortunately, comparison of the different OVDs with regard to elasticity is not easily made because of the different ways and nonuniform expression of values by the various manufacturers.
Viscosity
Viscosity (Table 1) reflects a solution's resistance to flow, which is, in part, a function of the molecular weight of the substance. Viscosity of OVDs is measured in centipoise (cPs) or centistokes (cSt), which are measures of the resistance to flow relative to a given shear force. Liquid solutions are generally considered to have viscosities of less than 10,000 cSt at rest, whereas solutions with resting viscosities greater than 100,000 cSt are gel-like. The higher the solution's molecular weight, the more it resists flow. Molecular weight, on the other hand, reflects the size of the solution's molecules. Viscosity is dependent on the degree of movement of a solution, which is also known as the shear rate, and varies inversely with temperature. The viscosity of a solution can be increased by increasing either the concentration or the molecular weight of the solution.
To facilitate optimal intraocular manipulation, an OVD should maintain space and protect tissues (possess a high viscosity at low shear rates), allow movement of instruments, aid in IOL (Intraocular Lens) implantation (possess a moderate viscosity at medium shear rates), and allow easy introduction into the eye through a small cannula (possess a low viscosity at high shear rates).2 At the present time, no single OVD fulfills all of these requirements optimally.
Pseudoplasticity
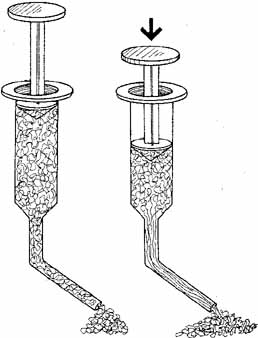
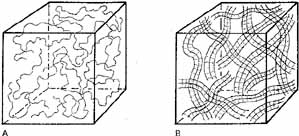
Pseudoplasticity refers to a solution's ability to transform when under pressure, from a gel-like substance to a more liquid substance. The more pseudoplastic a material is, the more rapidly it changes from being highly viscous at rest to a thin, watery solution at high shear rates. A change in molecular structure accompanies this pseudoplastic behavior. In clinical terms, a high molecular weight, high viscosity OVD at rest (zero shear force) acts as an excellent lubricant and coats tissues and maintains space very well. When under the influence of stress (i.e., a high shear rate), however, the OVD will become an elastic molecular system behaving as an excellent shock absorbing gel. The highest shear rates occur when a solution is passed through a cannula, and viscosity becomes independent of molecular weight. When the molecules align themselves in the direction of flow, the viscosity is determined solely by the concentration. Pseudoplastic solutions, therefore, have a low viscosity at high shear rates and can be extruded easily through a small diameter cannular (27 or 30 gauge) (Fig. 1) It is important to emphasize that the viscosity of a viscoelastic substance at rest (0 shear rate) is a function of concentration, molecular weight, and the size of the flexible molecular coils of the material (Figs.2A and 2B). At high shear rates, the viscosity is independent of molecular weight and is determined mainly by the concentration.3
Surface Tension
The coating ability of an OVD is not only determined by the surface tension of the material itself, but also by the surface tension of the contact tissue, surgical instrument, or IOL. By measuring the angle formed by a drop of the OVD on a flat surface (the contact angle), the coating ability of a substance can be estimated. Lower surface tension and lower contact angle indicate a better ability to coat. In this respect, a solution of sodium hyaluronate has a significantly higher surface tension and contact angle than does a solution of chondroitin sulfate, sodium hyaluronate/chondroitin sulfate in combination, or HPMC, thus indicating these latter solutions provide superior coating.3 A comparison of the various physical properties of OVDs is summarized in Tables 1 and 2.