SIR HAROLD RIDLEY
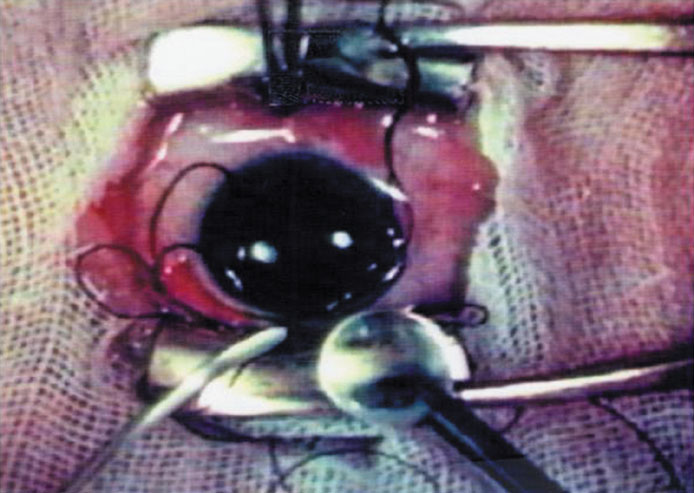
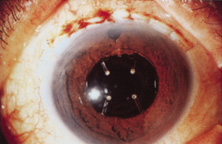
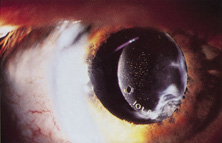
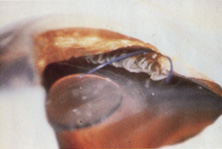
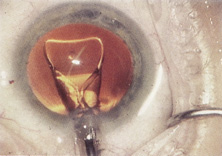
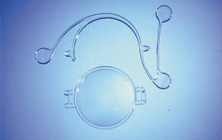
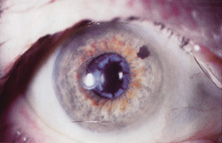
Credit for the invention and first implantation of the IOL is given to Sir Harold Ridley of London (Fig. 1)1–8 Details regarding Ridley and his invention are provided in a 1996 biography (Apple DJ, Sims JD: Harold Ridley and the Invention of the Intraocular Lens). Ridley's first IOL surgery was accomplished as a two-step procedure. The ECCE was performed on November 29, 1949. He waited for the eye to become quiet and stable and implanted the IOL secondarily a few months later on February 8, 1950. During the next 12 years, approximately 1000 Ridley IOLs were implanted. These operations were described as successes in 70%, failures caused by dislocation in 20%, and secondary glaucoma in 10%, which sometimes required explantation.9
|
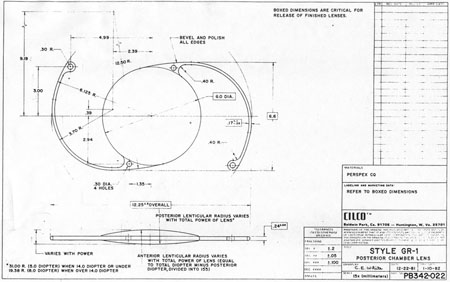
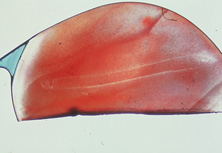
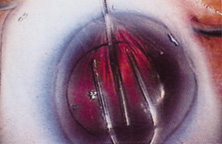
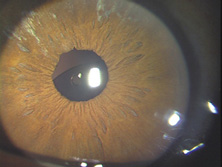
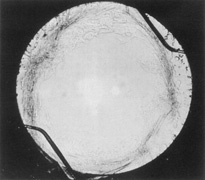
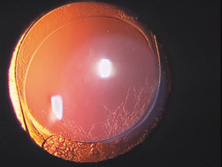
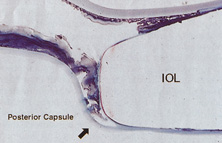
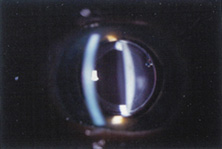
Ridley had been inspired by the tolerance of British fighter pilots' eyes to plastic fragments, which had lodged in them after their canopies, made of polymethylmethacrylate (PMMA; Perspex), had shattered. He worked with the Rayner Company and Imperial Chemical Industries, both in Great Britain, to develop Perspex CQ, a more purified “clinical-quality” PMMA. He used the human lens as his model and selected similar radii of curvature to create a biconvex disc while using approximately half the thickness and weight (∼5 mm thick and 230 mg for the human lens). One of his original lenses made by Rayner, a 23.00 diopter (D), was measured at 8.5 mm in diameter and 2.4 mm thick, with a weight of 108 g. Modern analysis has demonstrated that this IOL passes modern optical bench examinations10 (Fig. 2).
|
Ridley said that the cataract operation “without a replacement lens was an incomplete, only half finished operation”11 and that he would like to be remembered as the man who cured or at least initiated the cure for aphakia. He saw aphakic vision as a highly significant but unnecessary disability. Now only a memory, at least in the modern world, aphakic vision contained many disturbing visual side effects. The roving scotoma, Jack-in-the-box phenomenon, 30% magnification, distortion, loss of side vision, and extreme spectacle dependence were disabling for many patients. Because of this, even as late as the mid-1970s, surgery was typically performed only when the patient's vision decreased to 20/70 or worse in the better eye, with that eye undergoing operation 3 days after the first eye.
In recognition of his pioneering efforts in IOL development and implantation, Mr. Ridley was knighted on February 19, 2000, by Queen Elizabeth II, 50 years after his first implantation. Warren Reese was the first American surgeon to perform the first IOL surgery in the United States at the Wills Eye Hospital, Philadelphia, in 1952. Mr. Ridley himself performed the first operation in America and gave the lenses to him after delivering a lecture at the Chicago Ophthalmological Society. Despite that early beginning, it would take more than 25 years for IOL implantation to become the dominant and standard accepted method of curing aphakia in the United States.
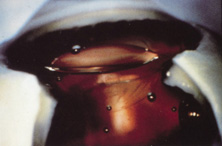
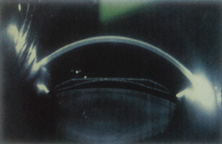
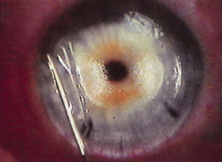
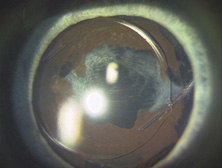
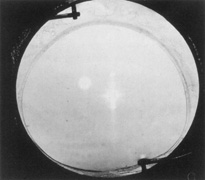
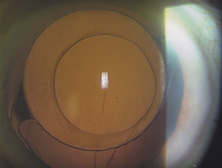
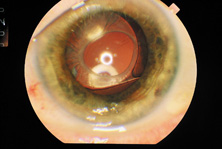
The Ridley lens was placed in the posterior chamber after ECCE (Fig. 3). The anterior capsulectomy of the day was very large, and thus zonular support was poor. Some Ridley lenses dislocated into the vitreous because of poor zonular support, and partially because of their weight, which was approximately eight times that of current IOLs.
|
Ridley's achievements were finally and belatedly celebrated in numerous tributes. His first and perhaps most-prized honor was his election to the Royal Society in 1986. Dr. Apple presented him his first University Doctorate at the Medical University of South Carolina in 1988. He received another honor at the Science Museum in London on November 29, 1999, the 50th anniversary of the first part of the first IOL implantation. In the Flight Room, with airplanes suspended overhead, Ridley was honored by fellow pioneers and colleagues from around the world, as well as by the Rayner Corporation and government representatives from the United Kingdom and United States. The same year, the American Society of Cataract and Refractive Surgery (ASCRS) honored him as one of the 10 most influential ophthalmologists of the 20th century. In 1990, he was guest of honor at the Annual Meeting of the American Academy of Ophthalmology. In recognition of his unique efforts in IOL development and implantation, Ridley was knighted on February 9, 2000, by Queen Elizabeth II. On May 25, 2001, at the age of 94 years, Sir Harold died in Salisbury, England, after a cerebral hemorrhage.
Because of the difficulty with posterior chamber IOL placement, pioneering surgeons would spend the next two decades trying to find a better place to fixate the IOL. The AC lens, pupil-fixated IOL, iris-fixated IOL, and iridocapsular IOL would be placed in large numbers, only to return to the posterior chamber in the 1970s. This made for very interesting surgical residencies during these times. We were always learning something completely new. For example, during J.A.D.'s 3-year residency starting in 1977 at the Mayo Clinic, we evolved through cryoextraction ICCE using no IOL, cryoextraction ICCE with Medallion iris-sutured IOLs, machine-assisted ECCE with iridocapsular fixation using metal and then plastic clips through the iridectomy, and machine-assisted ECCE with posterior chamber IOL implantation.
ANTERIOR CHAMBER AND PUPIL-SUPPORTED LENSES
Apple and associates have described in detail the evolution of the IOL, describing six generations. AC IOLs were generation two.12
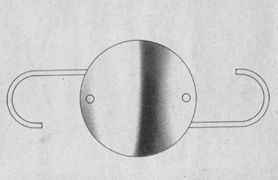
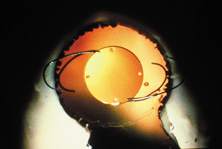
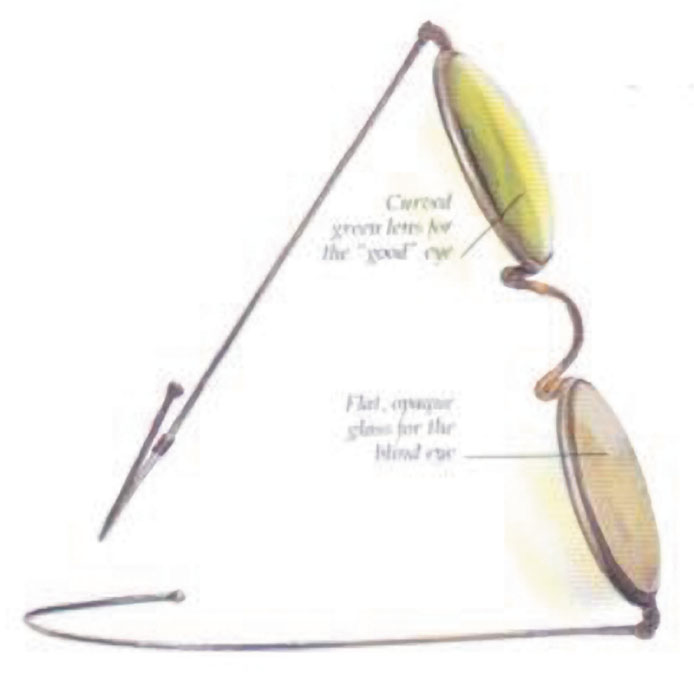
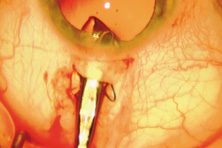
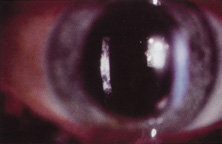
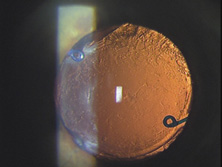
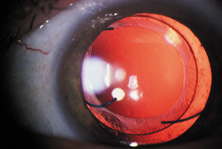
The first AC IOL was implanted by Baron of France in 1952. This lens failed primarily because of excessive anterior vaulting, which caused contact with the corneal endothelium. Mr. Ridley's good friend and long-time defender, Peter Choyce, also of the United Kingdom, was one of the developers and certainly the greatest champion of the AC lens (Fig. 4). These lenses could be placed after ECCE or ICCE procedures. His models were very successful with his last models, the Mark VIII and Mark IX, used until the mid-1970s in the United States.
|
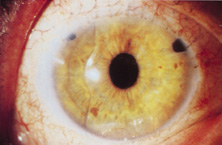
AC lenses had problems as well. Ellingson's uveitis, glaucoma, hyphema syndrome13 was associated with their use. Chronic irritation of the delicate structures of the angle caused this problem, and even without it, pain could sometimes be elicited by simply touching the eye. Later, similar rigid AC IOLs would have similar problems. Some had to do with sizing difficulties; others were problems of poor finish and the misapplication of polypropylene haptics in the AC, where they underwent ultraviolet (UV) degradation.
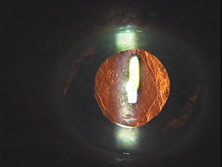
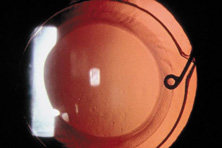
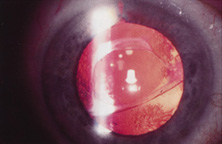
Ultimately the flexible AC IOL was developed, most commonly the Kelman Multiflex (Fig. 5). This style of IOL features rather broad area smooth footplates, which can be placed in the angle without causing the chafe and erosion that small-sized loop-shaped haptic IOLs did. The Multiflex-type lens has provided excellent performance with similar and sometimes lower long-term corneal endothelial cell loss in secondary implantation than has the sutured ciliary sulcus posterior chamber IOL.14
|
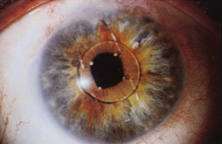
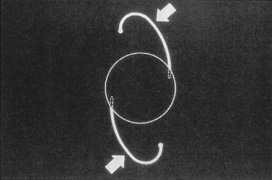
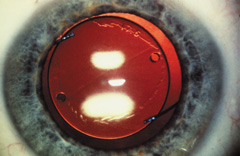
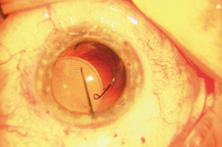
Taking another path, Cornelius Binkhorst of Holland developed a lens that involved pupil fixation with pairs of horseshoe-shaped haptics in front of and behind the iris (Fig. 6). This lens was associated with total dislocation into the AC or vitreous after pupil dilation, so miotics were many times given on a prophylactic basis indefinitely. Also, after ICCE, there was so much iridodonesis, especially with the initially used metal haptics, that the anterior aspects of the anterior haptics could touch the endothelial surface of the cornea, leading to localized corneal decompensation. Also, the weight of metal haptics could erode the iris. It was this style of lens that was implanted by early American implant surgeons Jaffe, Hirschman, Byron, and Kwitko in 1967.15
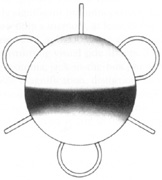
Introduced in 1967, Stanislav Fyodorov's “sputnik” IOL design achieved stabilization without larger anterior haptics (Fig. 7). In the mid-1970s, the Worst medallion IOL also featured an anterior optic with two horizontally oriented horseshoe-shaped looped posterior haptics similar to the Binkhorst structure. A Prolene suture was passed horizontally through the superior iris and then threaded between through two small holes in the superior optic. Because iris suture had to be preplaced, it always seemed to become entangled with the haptics during insertion making it difficult to place smoothly under air after ICCE, which featured a 180-degree corneal incision and an unprotected anterior hyaloid membrane. The suture was tied loosely so that the optic was secured to the superior iris. This would still allow the posterior haptics to dislocate anteriorly (embarrassingly, many times after having sex) or create a partial papillary capture, but at least it prevented total dislocation. Because surgical manipulation was expensive and carried the risk of infection, we would spend hours positioning patient's heads and bodies after pharmacologic weak dilation so that gravity would reposition the IOL. Then, when the IOL fell into position, we would reposition the patient and administer a topical miotic to capture the appropriate part of the IOL with the pupil. Aside from partial dislocation, this type of lens performed very well, but eye movement after ICCE still generated substantial irido-pseudophakodonesis.
ICCE was difficult and time-consuming and carried a high risk. Even when done well, the procedure left an eye not able to support an IOL in stable fashion. The structural diaphragm of the posterior capsule after ECCE was rediscovered and appreciated because it contained the vitreous and created compartmentalization and stabilization of the AC, iris, and posterior chamber. Iridocapsular fixation was the next step. The lenses designed for this technique had a small platinum or plastic rod attached to the superior optic that could be clipped to the superior posterior haptic through a superior peripheral iridectomy (Fig. 8). The inferior haptic would be inserted, and eventually fibrose, between a leaflet of remaining anterior capsule and the posterior capsule. When properly secured, the pupils of these eyes could be dilated without fear of superior or inferior IOL dislocation. Because of capsule fibrosis and stabilization, there was also a substantial reduction in pseudophakodonesis. Surgeons who had taken up phacoemulsification, which had been introduced in 196716 and adopted by a fair number by 1972, could enjoy the small-incision control aspect of cataract removal but still had to enlarge the incision for IOL insertion. Things were improving.
|
RETURN TO POSTERIOR CHAMBER LENSES
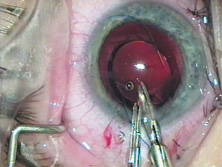
John Pearce of the United Kingdom returned implantation to the posterior chamber by developing a rigid tripod-shaped PMMA IOL designed for implantation there,17 but it was Steve Shearing who would have the greatest influence on the future development posterior chamber IOLs. In March 1977, he introduced his IOL, which featured flexible posterior haptics (Fig. 9).18 His original intention was to have the haptics placed within the capsular bag remnant. The lens had a 5-mm optic (because that was the size of the Binkhorst optic, which was already in production at the time) and an overall length of 12 mm and was flat.
|
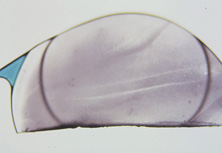
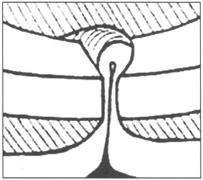
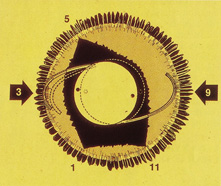
This lens design revolutionized the concept of IOL placement, but the need for a supportive capsular bag architecture was not fully appreciated, nor had the techniques to preserve it been developed. Most dislocations were incomplete (i.e., nonintravitreal). For example, the sunset syndrome (Fig. 10) resulted from occult anterior radial capsular tears (ARTs), which extended through the equator or inferior zonular disruptions and allowed the inferior haptic to sink through the defect. However, in most inferior dislocations, the IOL optic was still contained within the ciliary sulcus. The IOL's overall length was too short for symmetric sulcus fixation, so the whole IOL structure gravitated inferiorly with sulcus placement. The “windshield wiper syndrome” resulted from sulcus contact inferiorly but none superiorly. Surgeons tried to retain at least a leaflet of anterior capsule inferiorly in which to place the inferior haptic. When that was accomplished, the IOL stayed fairly well centered. However, if asymmetric placement occurred with the inferior haptic contained within the capsular bag remnant and the superior haptic loose in the ciliary sulcus, there would be opportunity for another type of decentration, the sunrise syndrome.
|
Because the capsular bag was such a hard place to find, the IOL was soon modified to include a 6-mm optic and 13-mm overall length. Even then, fixation was not perfect, and pupillary capture, just as Dr. Shearing had experienced in his fourth case, was not rare (personal communication, Steve Shearing, MD, 1998).
However, an important step had been taken, and during the next two decades, the Shearing posterior chamber IOL would evolve into three basic styles. The first was designed primarily for ciliary sulcus fixation but could also be used for capsular bag fixation. It was the result of the tremendous influence of two popular, committed, prestigious California phacoemulsification surgeons who had trained together at Duke University, Dick Kratz and Bob Sinskey. The second was the short haptic diameter modified C-loop IOL designed for capsular bag placement. The third featured larger haptic and optic diameters and was designed for surgeons still practicing planned ECCE who might place the IOL within the sulcus or asymmetrically with one haptic in the sulcus and one in the capsular bag.
The Kratz IOL was compatible with Kratz's phacoemulsification technique, which involved a prolapse of the superior nuclear pole into the iris plane for external attack phacoemulsification. In this technique, remaining anterior capsule was a problem because it could produce asymmetric placement and decentration. Therefore, anterior capsulotomies were large so that the IOL could be placed in the ciliary sulcus. Kratz developed a “tap test” that involved tapping the eye over the ciliary sulcus with a Weck sponge to see whether the IOL would move, which would indicate that the haptic was in contact with the structures of the ciliary sulcus and not hung up in the capsular bag remnant. Through the Precision Cosmet Company (Minneapolis, Minnesota), Kratz introduced a 10-degree optic posterior angulation as an effective way to prevent pupillary capture. The resistance to compression of the haptics was softened significantly by a side optic mounting modification with the overall length of 13.5 mm. Actually, before that, more than a few surgeons had been bending the Shearing haptic at the optic haptic junction to try to reduce compression resistance. Sinskey's lens was very similar and was introduced at about the same time, but by a different company, IOLAB (San German, Puerto Rico). Because of the nearly simultaneous introduction and similarity, the lens ultimately has been called the Kratz-Sinskey IOL (Fig. 11).
|
Sinskey's phacoemulsification technique was basically one-handed and allowed a more generous anterior capsular remnant to exist after nucleus removal. Symmetric capsular bag fixation was possible a fair amount of the time. Optic posterior angulation was incorporated in his lens to prevent pupillary capture, which was still possible.
The concept of intracapsular phacoemulsification and the desire to fixate the IOL within the capsular bag ultimately led to the second type of posterior chamber IOL, the minimally compression-resistant, short haptic configuration created for capsular bag fixation that exists today.19
The third style was a transitional lens that was oversized and was to be used with planned ECCE. It eventually measured 14 mm in haptic diameter and 7 mm in optic diameter. It was large and stiff in its eventual one-piece design. The problem was that after ECCE, the capsular bag did not exist in a structural sense. With broad contact, at least one haptic would be captured by some partially unrolled capsular flap remnant, usually the inferior. If it were asymmetrically placed, which was the expectation, the lens was so long that capsular fibrosis would not decenter the very large optic too much because of the contact created in the superior ciliary sulcus by the high resistance to compression. Because of their large size, these lenses were very difficult to place symmetrically within the capsular bag but are still in use today as ciliary sulcus placed secondary IOLs in patients with very large eyes.
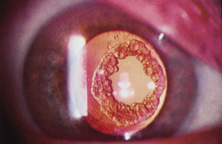
As an aside, glass IOLs were marketed for a short period. Even in aqueous, they were heavier than plastic. Glass also broke if hit by the neodymium:yttrium-aluminum-garnet (Nd:YAG) laser (Fig. 12).20 Plastic IOLs inadvertently hit by the YAG laser during capsulotomy developed small cracks or pits that did not affect vision and did not completely fracture. Glass was eventually abandoned because of its weight and YAG intolerability. The right to use polyamide framing and haptic material was purchased from Lynell Optics by STAAR Surgical (Monrovia, CA) and is in use today by that company as a haptic material combined with silicone optics.
ENDOTHELIAL CELL DAMAGE, VISCOELASTICS, ANTERIOR CAPSULAR TEARS, AND SUTURELESS CLOSURE
With the introduction of clinical corneal endothelial photography 1976, Bourne and Kaufman heightened our awareness of quantitative and qualitative endothelial damage associated with IOLs.21 In 1980, Miller and Stegmann, working with the Pharmacia Company of Uppsala, Sweden, introduced the first viscoelastic, 1% sodium hyaluronate (Healon).22,23 This not only protected the corneal endothelium during IOL implantation but also made anterior capsulotomy much easier to perform. The control of the anterior capsular surface with viscoelastic, pushing it back and making it flat, was an important aid in the prevention of unwanted ARTs during the can-opener capsulotomy process.
The number of central corneal endothelial cells after IOL implantation decrease at rates greater than those of healthy unoperated corneas24–29 (normal loss ranged from 0.3% to 1.0% per year).
The only randomized trial of lens implantation, the Oxford Cataract Treatment and Evaluation Team found a higher rate cell loss in eyes with implants than those without in the first 3 postoperative years.30 Unfortunately the type of surgical technique and IOLs (ICCE and Binkhorst 4-loop lens) are no longer being used.
A 5-year prospective study from the Mayo Clinic31 reported 23% to 28% of endothelial cell loss after cataract surgery over the 5-year postoperative period. The rate of cell loss was not found to be influenced by the surgical technique (ICCE vs. ECCE), implantation of an IOL, and type of IOL implanted (medallion iris suture IOL, transiridectomy clip implant, or posterior chamber IOL). Correlation was found between the endothelial trauma judged at surgery and the long-term endothelial cell loss. Extension of the follow-up time to 10 years32 demonstrated that the eyes continued to lose endothelial cells from the central cornea at an average rate of 2.5% per year (2.5–8.0 times the rate in healthy unoperated eyes); also in this study the type of IOL implanted did not influence the rate of cell loss. These studies had two major caveats: (a) A significant percentage of patients were lost to follow-up. (b) The study represents the early cases in the implant experience of the surgeons (1976–1982) and do not represent the currently surgical technique and IOL technology.
In the early days of the phacoemulsification the corneal cell loss rate was high because of the long phaco time and high energy that were commonly used. In recent years, damage to corneal endothelial cells during cataract extraction has been minimized as a result of better instrumentation,33 newer viscoelastic materials,34,35 and improved surgical technique such as phaco chop,36 which aims to reduce machine measured phaco time.
Several preoperative and intraoperative parameters can influence the risk for endothelial cell loss after phacoemulsification. A high nucleus grade,37 old age, long phaco time,38 and short axial length39 are associated with an increased risk for endothelial cell damage. Ravalico and coauthors40 reported that AC IOLs implantation did not appear to alter corneal endothelial function over 5.2 years of postoperative follow-up period. As a summary, it seems that the endothelial cell loss after cataract surgery is related to the surgical trauma and maybe to the absence of the crystalline lens but not to the IOL implantation.
Surgeons who performed one-handed phacoemulsification were preserving the capsular bag better than two-handers. In 1981, John Graether,41 a left-handed one-hander, designed the first one-piece all-PMMA IOL that was 12.25 mm in overall length designed specifically for implantation in the capsular bag (Fig. 13). He had invented a “collar button” retractor and described a way to use it by pushing on the superior optic-haptic junction to compress the inferior haptic and dial the IOL into the capsular bag, the method still used today.41 Later, a reduction in the amount of nuclear prolapse into the iris plane made it possible for surgeons who used the two-handed procedure to retain more anterior capsule by creating only one ART most of the time.42 Even if two superior tears were noted, posterior chamber IOLs could be placed in what was left of the capsular bag with minimal or no decentration.43 Sometimes lenses with broader haptics, as designed by Bill Simcoe, were used to bridge the gap created by ARTs in the capsular bag equator. Even though surgery and implantation had improved, significant IOL optic decentration, with its attendant optical and physical complications, was not uncommon (Fig. 14). Given the anatomy of the eye with possible multiple ARTs after iris-plane phacoemulsification or ECCE, two schools of thought developed. The first embraced larger optic and haptic diameters, which provided greater stability and increased resistance to compression and were designed in an attempt to center IOL optics in capsular bag, ciliary sulcus fixation, or asymmetric fixation and prevent pupillary capture (Fig. 15). The other school fashioned 6.0-mm optics and reduced 12.0-mm haptic diameters with a very low resistance to compression to try to keep them within the capsular bag or capsular bag remnant (Figs. 16 and 17).19 In an attempt to custom tailor IOLs to patient anatomy, J.A.D. created the “graduated length method” through the D.G.R. Company of Clearwater, Florida. This IOL line featured shorter 12.0-, 13.0-, and 14.0-mm haptic structures for small, medium, and large eyes.
|
|
Because of these compounding developments, David Apple began his important work with Randy Olson and in 1983 founded the Center for Intraocular Lens Research at the University of Utah. They were a uniquely qualified combination. Dr. Apple was board certified as both a pathologist and ophthalmologist; Dr. Olson was an academic ophthalmic surgeon. Much like Harold Ridley and Peter Choyce, they began their dedicated quest to improve the condition of pseudophakia. They solicited autopsy specimens and wrote innumerable articles about IOL design, centration, and complex IOL–ocular interactions. One of their most important articles, published in 1985, was a position article citing the advantages of capsular bag placement and recommending it over ciliary sulcus placement, still a controversial issue at the time.44 The eyes submitted to them that year demonstrated capsular bag fixation in 31%, ciliary sulcus fixation in 11%, and asymmetric bag–sulcus fixation in 58%.45 At that time, 85% of surgeons still preferred planned ECCE as their surgical technique.46
While in Utah, Apple and Olson saw the problems created by ARTs solved with the invention of capsulorrhexis, almost simultaneously reported in 1986 by four surgeons from around the world. Two presented articles at the Welch Cataract Congress in Houston that year, and two reported their techniques independently. The discovering surgeons in alphabetical order are Drs. Calvin Fercho (Welsh Cataract Congress, Houston, 1986), Howard Gimbel (video presentation at the annual meeting of the ASCRS in Boston, 1985),47 John Graether (Welch Cataract Congress, Houston, 1986),48 and Thomas Neuhann (video presentation at the meeting of the German Ophthalmological Society in Heidelberg, 1985).47 With this technique, and the creation of an approximate diameter of 5 mm, symmetric placement of any IOL could be guaranteed. It still took several years for the majority of ophthalmologists to incorporate continuous curvilinear capsulorrhexis (CCC) into their surgical routines.
In 1988 Dr. Apple relocated the laboratory to the Storm Eye Institute at the Medical University of South Carolina in Charleston. Reflecting new challenges, his laboratory name would be changed to the Center for Research on Ocular Therapeutics and Biodevices. From there, he and his staff, residents, and research fellows continued to receive autopsy specimens from around the world and eventually demonstrated, in the largest autopsy specimen study to date, a decline in asymmetric placement to only 10% of eyes with foldable lenses submitted in 1998.45 Early in his work there, Dr. Apple recruited Dr. Kensaku Miyake's retrociliary photographic analysis method to locate and analyze IOL placement within the eye (Fig. 18). With increasing video sophistication and use of the process by Dr. Apple and his colleagues, Dr. Miyake himself generously expanded the name of the procedure to be called the Miyake-Apple technique.
After cancer was diagnosed in Dr. Apple and he was successfully treated, the center was relocated to Salt Lake City in 2002, where it has been permanently designated as the David J. Apple, MD, Laboratories for Ophthalmic Devices Research.
In the days when the laboratory was in Charleston, Dr. Apple worked with industry representatives and surgeons to refine IOL design to ensure that capsular bag residence would be as consistent as possible, thereby reducing lens contact with other eye structures in both routine and complicated situations (Figs. 19 and 20). J.A.D. had the great pleasure of working with him in his laboratory to help improve an already sophisticated haptic configuration in a one-piece all-PMMA IOL. At that time, we thought that the entire haptic should be C-shaped so that even its distal end could be recruited for capsular equatorial support (Fig. 21). We studied resistance to haptic compression, attempting to make it softer and more uniform through diameter reductions from 13.0 to 11.5 mm 2.5 mm (Fig. 22). These efforts contributed to the development of the Pharmacia model 811, which, along with others of its day, may have represented the height of single-piece PMMA IOL development (Fig. 23).49
|
|
|
Inevitably, with the new technique of capsulorrhexis, the improved haptic architecture was actually not as critical as it had been earlier, when ARTs were universal. It was still helpful in difficult situations in which capsular support was anomalous (e.g., pseudoexfoliation and trauma). Coatings of heparin and Teflon were developed to increase the biocompatibility of PMMA IOLs.50 However, the benefits of the sophisticated one-piece PMMA capsular bag design would soon be replaced by the even greater benefits of foldable IOLs, the use of which would overtake PMMA in 1998.46
Sutured superior corneoscleral incisions with round PMMA IOLs were standard throughout the 1980s, until the self-sealing incision was described by Mike McFarland in 1991.51 In an attempt to integrate the smaller phacoemulsification incision and PMMA technology, ovoid posterior chamber IOLs had been produced since 1980 and reached their peak in popularity (35%) in 1991 (Fig. 24).46 But, because of their truncated edge, ovoid IOLs introduced a higher incidence of glare, streaks, and halos52 (pseudophakic dysphotopsia); for this reason, they never reached great popularity. Attempting to reduce edge glare and still maintain optic truncation, Charles Kelman created the PhacoFlex IOL. Two side portions of the optic had opaque silicone wings, which would fold on top of the PMMA central optic during insertion through a 3.5-mm incision, and then unfold in the eye. Many years later, Howard Fine would recommend using such a textured finish in plate haptic silicone IOL haptics, not for optical reasons, but to enhance capsular fixation to prevent IOL movement within the capsular bag.
FOLDABLE LENSES, CLEAR CORNEAL INCISIONS, AND TOPICAL-INTRACAMERAL ANESTHESIA
In 1984, Mazzocco et al introduced the first foldable plate haptic silicone lens produced by STAAR Surgical.53 It became known as the “Mazzocco Taco” because of the way it appeared when folded. It was delivered through an injector, whose cartridge went through a 3-mm incision allowing the IOL to unfold in the posterior chamber (Fig. 25). This changed cataract surgery forever because it made possible the full appreciation of phacoemulsification. Cataract surgery had become the first microscope-accomplished, machine-assisted “small-incision surgery” in medicine. All of the benefits of improved surgery safety, improved postoperative state quality, and shorter patient recovery were realized.
|
This IOL did not fixate very well in the sulcus, and asymmetric bag–sulcus fixation was the standard of the day. An intact or almost intact (one or two opposing ARTs) capsular bag was necessary for acceptable centration performance of this IOL. Concerned about this, the designers made the earliest models too long, so that when they were well fixated within the capsular bag they could actually wrinkle centrally because of capsule contraction, causing the “Z phenomenon.” The silicone material was hydrophobic and did not bond to the capsule during fixation, so that if asymmetric placement occurred, it could be squeezed in one direction out of the bag. Significant decentration was likely when only anterior capsular leaflets remained for fixation. Intravitreal dislocation was also possible after YAG laser capsulotomy.
Nevertheless, these IOLs became very popular, especially in the United States, because they were relatively easy to insert with an injector, could be implanted through a small incision, and were relatively inexpensive.
Next, three-piece foldable silicone optics with polypropylene haptics emerged. These IOLs centered better and could be used in cases with ARTs. Popular “small-incision” cataract surgery had finally arrived. Surgeons no longer had to enlarge the incision much after cataract removal to accommodate the IOL, so one of the main advantages of phacoemulsification could be realized. However, increased AC reaction, capsular fibrosis, and optic decentration compared with single-piece PMMA IOLs kept some surgeons from using early three-piece silicone IOLs.54
In 1992, Kimya Shimizu reintroduced a very clear corneal incision only 3 mm wide to accommodate phacoemulsification and foldable IOL insertion.55 One suture was placed. That same year, Howard Fine showed that the temporal clear corneal incision could be left unsutured and consistently perform well, with an extremely low incidence of incision complication and very little astigmatic consequence.56 Although this incision was not as strong as one created with an additional scleral shelf,57 it was strong enough for normal clinical application. Foldable silicone lenses could be placed without using scissors, cautery, or sutures.
Cataract surgery using only topical anesthesia was introduced by Richard Fichman in 1992. This was substantially improved with the addition of intracameral anesthesia by Jim Gills in 1995.58 As of 2003, the topical anesthesia technique was adopted by 61% of American surgeons (38% of surgeons performing one to five operations per month and 76% of surgeons performing more than 75 surgeries per month); 73% of surgeons used intracameral lidocaine with the technique.59
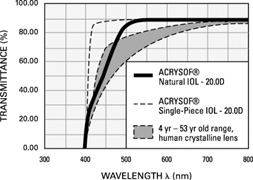
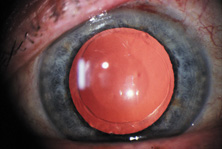
Also, in 1995, the acrylic IOL (AcrySof®, Alcon Surgical, Fort Worth, TX) was approved for use in the United States. Since its introduction, the acrylic lens has had substantial popularity. With self-sealing temporal clear corneal incisions and topical-intracameral anesthesia available, surgeons who had objected to foldable lenses made of silicone found their last obstacle to small-incision surgery removed. However, the acrylic lens had to be withdrawn temporarily because of glistenings within the optic.60 These turned out to be water vacuoles, which were associated with packaging materials. When the lens was repackaged, the glistenings were reduced.61 Through manufacturing improvement, the glistenings continue to be reduced to lower levels, but they still may be present in many patients to a trace to −1 degree, but without visual consequences.
An important part of the evolution of the IOL has been the propagation and dissemination of information as well as the continuous development of educational processes, ensuring that the latest techniques and materials were available to surgeons. The first International Intraocular Implant Club meeting was held on July 14, 1966, with Mr. Ridley presiding. The American Intraocular Implant Society was founded in 1974 by its first president, Kenneth Hoffer, MD, of Santa Monica, California. In 1985, the name was changed to the American Society of Cataract and Refractive Surgery, and in 1988 its annual meeting outgrew its traditional location, the Century Plaza Hotel in Century City, California. This organization has grown to 5000 U.S. and 2000 international members, and in 1996, it combined its journal publication with that of the European Society of Cataract and Refractive Surgery. This open climate of information sharing ultimately has benefited patients greatly.
CATARACT SURGERY 2004
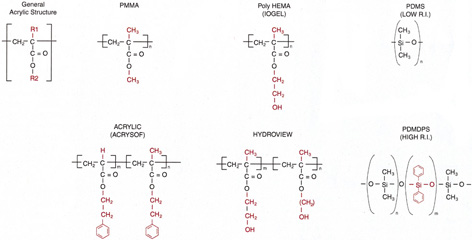
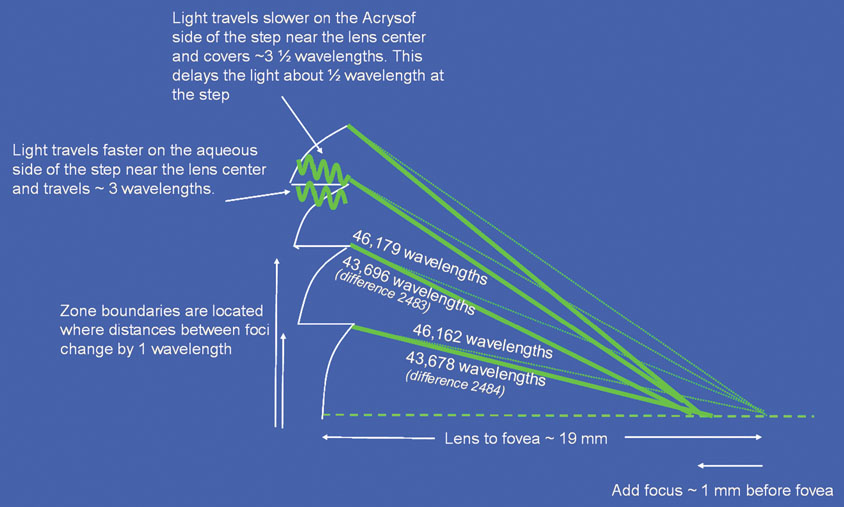
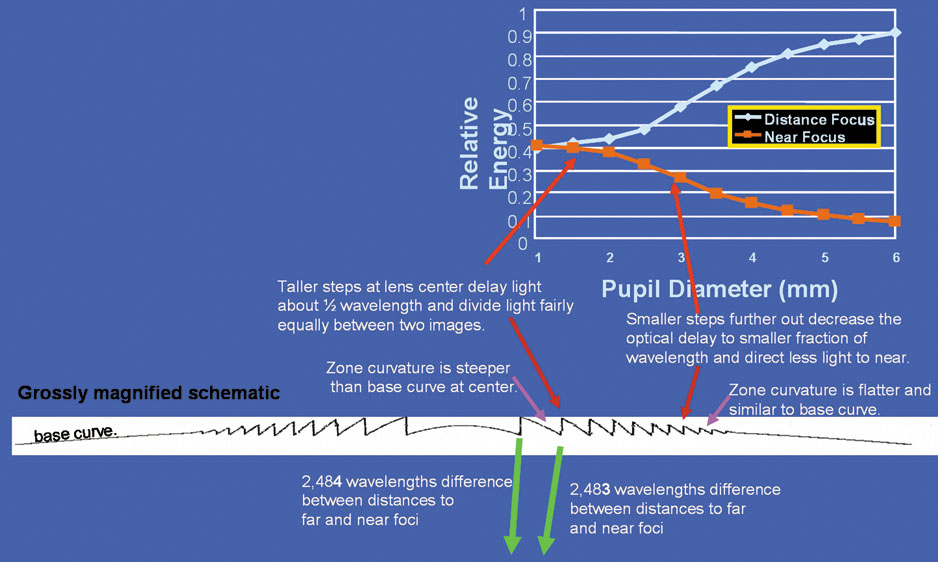
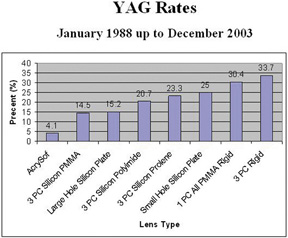
The changes in cataract removal technique and IOL implantation have been gradual, with considerable overlap of individual preferences. As can be seen in any evolutionary activity, there is never just one right answer. That is, at any one point in time, there exist multiple materials and surgical techniques that have their individual and combined inherent advantages and disadvantages. In fact, as of 2004, there were 1548 IOLs available from 33 different manufacturers.62 In 1998 many surgeons preferred PMMA (33%) and superior corneoscleral incisions, but momentum was shifting away from those methods toward the use of silicone (22%) and acrylic (42%)19 and clear corneal incisions. By 2003, PMMA had decreased to 6%, silicone had stabilized to 21%, and foldable acrylics had grown to 69%. Acrylic IOLs have evolved and are now made by manufacturers other than Alcon. The Alcon acrylics continue to evolve as well (Fig. 26).
|
Substantial industry consolidation has occurred so that, in the United States at least, there are four major manufacturers with commercially available IOLs in 2004: Alcon Surgical of Fort Worth, Texas; Advanced Medical Optics (AMO) of Irvine, California; Bausch and Lomb of Clearwater, Florida; and STARR Surgical of Monrovia, California. Although many company executives and factory representatives from earlier companies have been integrated into the contemporary organizations, gone are the days of the prominent IOL lines of the 1980s and 1990s: CooperVision, Ciba Vision, O.R.C., Cilco, IOLAB, Precision Cosmet, Lynell Optics, and Pharmacia.
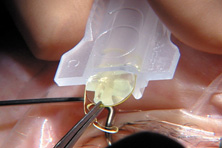
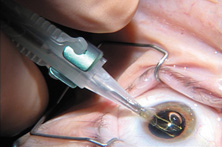
Modern lensectomy lens replacement surgery consists of removal of the nucleus, cortex, and as much remaining lens epithelium on the posterior capsule as possible while avoiding other ocular structures. It should leave a central circular anterior capsular opening so that the anterior capsule remnant can overlap the peripheral IOL optic by approximately 0.25 to 0.5 mm for a complete 360 degrees. Surgery is usually accomplished under topical-intracameral anesthesia through a temporal clear corneal incision of less than 3.0 mm in length, permitting a foldable IOL to be placed using an adjunctive viscoelastic device. If well executed with either forceps (Fig. 27) or more commonly with an injector using a disposable cartridge (Figs. 28, 29, 30), this strategy provides good IOL centration and minimal unwanted side effects regardless of the foldable IOL material or design used (Figs. 31 and 32).