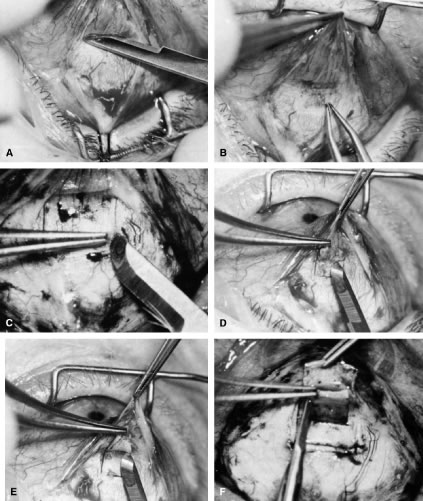
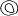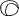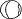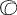| Glaucoma surgery is best performed under the operating microscope. In some
cases, high magnification is not essential. In others, such as certain
portions of guarded filtration procedure, it increases the likelihood
of a successful operation. Care should be taken to avoid photic damage
to the retina, especially in patients who have far advanced disease. The
light from the operating microscope should not focus on the posterior
pole. We usually perform most aspects of glaucoma surgery with
side illumination rather than the axial illumination from the microscope. When
axial illuminator is used, it usually is reduced in intensity, and
the cornea is covered. The surgical anatomy of the anterior chamber angle in an average eye is
shown in Figure 4A and B. Landmarks will differ markedly in eyes that have abnormalities. In the
myope, the space between the anterior limbus and the iris root is greater; in
the hyperope, it is less. An incision at the corneoscleral sulcus
may enter the anterior chamber far anterior to the trabecular meshwork
in the myope but may enter the posterior chamber in the hyperope. 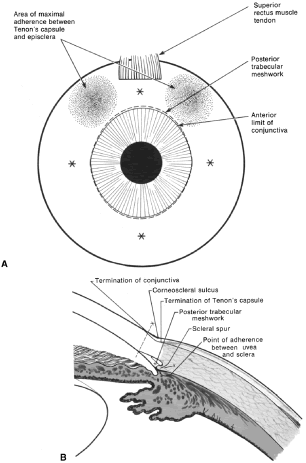 Fig. 4. A. Important anatomic landmarks on the anterior aspect of the globe. B. Schematic cross section of the limbal area at the 12 o'clock position
on the globe. The corneoscleral groove (sulcus), a landmark of paramount
importance, is located posterior to the termination of conjunctiva
and just anterior to the termination of Tenon's capsule. A perpendicular
incision (dashed line) at the corneoscleral sulcus should enter the anterior chamber just anterior
to Schlemm's canal. Normally, the uvea is adherent to the
anterior uvea in only one area, a narrow ring at the scleral spur. *, approximate
position at which anterior ciliary vessels penetrate sclera. (Spaeth GL. Glaucoma surgery. In Spaeth GL (ed). Ophthalmic Surgery: Principles
and Practice. Philadelphia: WB Saunders, 1990.) Fig. 4. A. Important anatomic landmarks on the anterior aspect of the globe. B. Schematic cross section of the limbal area at the 12 o'clock position
on the globe. The corneoscleral groove (sulcus), a landmark of paramount
importance, is located posterior to the termination of conjunctiva
and just anterior to the termination of Tenon's capsule. A perpendicular
incision (dashed line) at the corneoscleral sulcus should enter the anterior chamber just anterior
to Schlemm's canal. Normally, the uvea is adherent to the
anterior uvea in only one area, a narrow ring at the scleral spur. *, approximate
position at which anterior ciliary vessels penetrate sclera. (Spaeth GL. Glaucoma surgery. In Spaeth GL (ed). Ophthalmic Surgery: Principles
and Practice. Philadelphia: WB Saunders, 1990.)
|
Because glaucoma in infants is rare, its surgical treatment is not discussed
in detail. However, guarded filtration procedure is described later
and may be employed with satisfactory results in many patients with
congenital glaucoma (trabeculodyspenesis). PREOPERATIVE CARE The reader should refer to the earlier section of this chapter on evaluation
of the person. The goal is to move the patient from conditions auguring
failure toward those promising success. Obviously, many factors
cannot be changed. In the simplest terms, the healthier the eye and
the less likely it is that scarring will develop when the eye is injured, the
greater the chance for success. Preoperative care is discussed
in greater detail in the sections that describe the operative procedures. Many people take aspirin products routinely. Aspirin comes in many forms, and
it is part of many compounds. Because it predisposes patients to
bleeding, it should be discontinued, if prudent, 2 weeks before surgery. Most
people who are taking aspirin are taking it for relatively unimportant
indications; some simply may have heard that aspirin can help
prevent heart attacks. However, in some patients, aspirin may be an
important part of treatment, and discontinuation of the aspirin must
be coordinated with the physician who ordered the aspirin. Wherever feasible, it
should be discontinued preoperatively. Other agents also predispose patients to bleeding, for example, dipyridamole
and anticoagulants, such as dicumarol. These agents should be discontinued
an appropriate time before surgery to allow the blood to return
to a normal clotting state. Some patients may have to take heparin
instead of dicumarol at the time of surgery. This substitution must
be individualized and coordinated with the physician managing the patient's
anticoagulants. PARACENTESIS A paracentesis should be part of virtually all intraocular glaucoma surgical
procedures. This opening in the cornea facilitates management of
complications such as bleeding or flat anterior chamber; allows deepening
of the anterior chamber at the time of surgery; provides an entry
for acetylcholine or sodium hyaluronate; allows the surgeon to determine
at the time of surgery how much filtration, if any is occurring through
the guarded filtration procedure or sclerostomy; permits safe development
of a bleb at the conclusion of surgery; and allows the surgeon
to detect whether any tears or leaks are present in the conjunctival
flap. The major risk of the procedure is that it may damage the lens. This
damage can be avoided by making sure that the instrument used to
develop the paracentesis never points toward the lens; holding the instrument
parallel to the iris surface eliminates the risk of damaging
the lens. Fixation of the globe is critical. The point of fixation should be directly
in line with the intended direction of the paracentesis. If the paracentesis
is to be made at the 10 o'clock position, extending exactly
inferiorly, the sclera should be held directly superior to the 10 o'clock
position (Fig. 5). If the surgeon prefers a horizontal paracentesis, starting at the 3 o'clock
position, then the globe must be fixated precisely at the 3 o'clock
position. An instrument with fine teeth provides good fixation. Examples
are the Bonn or Barraquer-Colibri fine-toothed forceps. The tips
should be separated only slightly, approximately 1 mm, and then pressed
hard against the sclera. Conjunctiva, Tenon's capsule, and
episclera are not adequate sites for fixation. 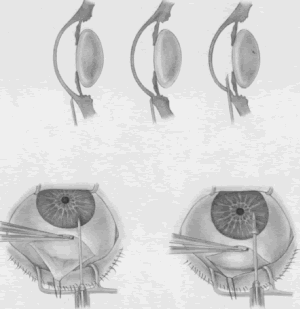 Fig. 5. Paracentesis. It is advisable to perform a keratostomy in virtually every
patient who is undergoing an intraocular glaucoma procedure. A 25-gauge, short, sharp, disposable needle is useful for this purpose and allows
easy insertion of a 30-gauge blunt irrigating needle later in the
procedure. To ensure that the needle does not damage the iris or lens, it
must be introduced in a plane parallel to the iris. Penetration
of the cornea is achieved by indentation of the cornea that is caused
by pressure on the syringe, pushing the syringe and needle against the
globe. Penetration is not achieved by angulating the needle. (Spaeth GL. Glaucoma surgery. In Spaeth GL (ed). Ophthalmic Surgery: Principles
and Practice, 2nd ed. Philadelphia: WB Saunders, 1990.) Fig. 5. Paracentesis. It is advisable to perform a keratostomy in virtually every
patient who is undergoing an intraocular glaucoma procedure. A 25-gauge, short, sharp, disposable needle is useful for this purpose and allows
easy insertion of a 30-gauge blunt irrigating needle later in the
procedure. To ensure that the needle does not damage the iris or lens, it
must be introduced in a plane parallel to the iris. Penetration
of the cornea is achieved by indentation of the cornea that is caused
by pressure on the syringe, pushing the syringe and needle against the
globe. Penetration is not achieved by angulating the needle. (Spaeth GL. Glaucoma surgery. In Spaeth GL (ed). Ophthalmic Surgery: Principles
and Practice, 2nd ed. Philadelphia: WB Saunders, 1990.)
|
We prefer to use a new, sharp, short, disposable 27-gauge needle on a 2- or 5-ml
syringe. The needle is held, bevel up, absolutely parallel to
the iris surface. In an eye with an iris bombe or a flat anterior chamber, the
needle actually may be pointing anteriorly. The tip of the
needle is placed against the cornea in the desired position, and the globe
is pulled by the fixating hand in the direction opposite to that
in which the needle is pointing. For example, if the needle is held horizontally, at
the 3 o'clock position of the left eye, then the sclera
is grasped at the 3 o'clock position and pulled laterally (temporally) (see Fig. 5). The needle tip enters the cornea. If there is normal or elevated pressure
and the chamber is deep, the needle penetrates the cornea after making
a paracentesis that is approximately 1 to 2 mm long. If the eye is
soft or the chamber is shallow, the needle remains in the cornea and
does not penetrate the anterior chamber. This position is desirable in
cases in which the cornea is thin, such as in the buphthalmic eye. If
the intracorneal track is longer than 2 mm, then the needle and syringe
are depressed back toward the apex of the orbit; they are depressed
toward the floor of the operating room (see Fig. 5, top right). The tip of the needle must not be angled toward the iris-lens; it must
be kept parallel to the iris. As the needle and syringe are pushed
toward the floor, the needle changes the curvature of the cornea, permitting
it to enter the anterior chamber when advanced. The needle is advanced
by a combination of pulling the globe and pushing the syringe. The
importance of firm fixation and of introducing the needle against
traction provided by the fixation must be stressed. Also, it is essential
that the needle not be angled toward the iris, but kept parallel
to the iris surface. Once the needle enters the anterior chamber, it is more clearly visible
than when it is intracorneal (see Fig. 5, bottom right). The tip is advanced carefully about 1 to 3 mm, until the surgeon is
sure that the endothelium has been completely penetrated. The needle is
then withdrawn. If the paracentesis has been made with a no. 25 needle, a no. 30 blunt-tipped
needle can be introduced later with ease. If the cornea is especially
thin or if the surgeon wishes the fit to be especially tight, a 30-gauge
needle should be used for the paracentesis. When re-entering the paracentesis track, the blunt needle must be directed
exactly parallel to the original track and must hug the posterior (internal, deep) aspect
of the track. Often, the neophyte struggles unnecessarily
to get the no. 30 blunt-tipped needle into the anterior chamber
through a no. 27-size paracentesis. However, when the blunt-tipped 30-gauge
needle is angled in the direction of the floor, that is, toward
the iris, and slid along the internal aspect of the paracentesis, it
enters gracefully. Alternatively, use a sharp 27-gauge needle for
the paracentesis and a sharp no. 30 needle for later entry. USE OF SLIP KNOTS AND RELEASABLE SUTURES The use of a slip knot is helpful in achieving the desired level of tightness
of the scleral flap in a guarded filtration procedure. Slips knots
also have the advantage of being easier to bury than the usual surgeon's
knot. They cannot be used to close incisions under tension, but
are appropriate for almost all other situations. We use them routinely. Figure 6 illustrates the method of tying slip knots. See p. 24 for a discussion
of releasable sutures (Fig. 7). 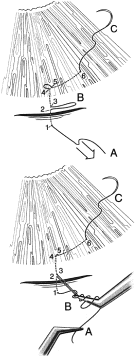 Fig. 6. Tying a slip knot. The suture needle enters at 1, crosses the incision, and
exits at 2. It then re-enters the tissue at 3, a distance of about
a 0.25 mm from 2, and in the same direction as the suture is moving. The
suture then passes underneath to where the conjunctiva is adherent, such
as at the corneoscleral sulcus. The surgeon must be meticulously
careful to make sure that the needle is held in a way so that it is
advanced in the same plane as the internal and external surfaces and
so that it does not exit too soon through the conjunctiva. The needle
exits at 4, about 1 to 2 mm in the cornea anterior to the adherence of
the conjunctiva at the corneoscleral sulcus. The needle re-enters 0.5 to 1 mm
away from point 4, specifically at point 5, and is passed intracorneally
a distance of about 3 mm, exiting at 6. At this point, then, the
leading end of the suture is at C, and there is a potential loop
between 4 and 5, a definite loop between 2 and 3 at B, and the trailing
end of the suture is at A. Fig. 6. Tying a slip knot. The suture needle enters at 1, crosses the incision, and
exits at 2. It then re-enters the tissue at 3, a distance of about
a 0.25 mm from 2, and in the same direction as the suture is moving. The
suture then passes underneath to where the conjunctiva is adherent, such
as at the corneoscleral sulcus. The surgeon must be meticulously
careful to make sure that the needle is held in a way so that it is
advanced in the same plane as the internal and external surfaces and
so that it does not exit too soon through the conjunctiva. The needle
exits at 4, about 1 to 2 mm in the cornea anterior to the adherence of
the conjunctiva at the corneoscleral sulcus. The needle re-enters 0.5 to 1 mm
away from point 4, specifically at point 5, and is passed intracorneally
a distance of about 3 mm, exiting at 6. At this point, then, the
leading end of the suture is at C, and there is a potential loop
between 4 and 5, a definite loop between 2 and 3 at B, and the trailing
end of the suture is at A.
|
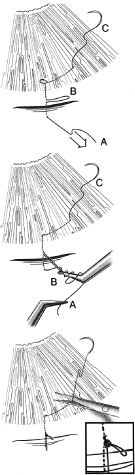 Fig. 7. Tying a releasable suture. The releasable knot is tied by placing three
or four throws around a needle-holder of the suture end that extends
from A to 1. The forceps around which those throws have been passed then
grasps loop B. The end A is then pulled towards the cornea, and loop
B is pulled posteriorly away from the cornea, creating a slip knot. Loop
B should be approximately 1 to 4 mm long. The end C is then very
gently pulled so that the loop between 4 and 5 becomes flush with the
cornea. The end of the suture exiting the cornea is cut with a Vannas
scissors, and the end of the suture knot tied over the incision is gently
cut with the Vannas scissors to make sure that the slip knot is not
disrupted by the ends being pulled at the time of the suture cutting. Fig. 7. Tying a releasable suture. The releasable knot is tied by placing three
or four throws around a needle-holder of the suture end that extends
from A to 1. The forceps around which those throws have been passed then
grasps loop B. The end A is then pulled towards the cornea, and loop
B is pulled posteriorly away from the cornea, creating a slip knot. Loop
B should be approximately 1 to 4 mm long. The end C is then very
gently pulled so that the loop between 4 and 5 becomes flush with the
cornea. The end of the suture exiting the cornea is cut with a Vannas
scissors, and the end of the suture knot tied over the incision is gently
cut with the Vannas scissors to make sure that the slip knot is not
disrupted by the ends being pulled at the time of the suture cutting.
|
IRIDECTOMY Iridectomy deserves separate treatment in this chapter because, with rare
exceptions, its basic purpose is different from that of all other glaucoma
surgery. Iridectomy often is performed not to lower IOP but to
correct an anatomic aberration, narrowness of the anterior chamber angle (Fig. 8). It is important to explain to the patient why the iridectomy is recommended. Patients
usually conclude that glaucoma surgery has as its purpose
the lowering of IOP, and unless enlightened to the contrary, most
patients anticipate that IOP will be lower after iridectomy. 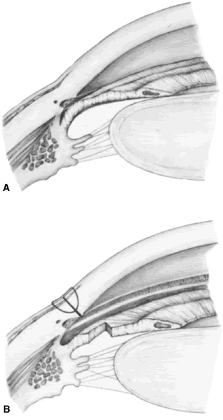 Fig. 8. Effect of iridectomy on the anterior chamber angle. The elimination of
pupillary block allows aqueous to pass without resistance from the posterior
to the anterior chamber (A), eliminating the gradient in pressure that is responsible for anterior
bowing of the peripheral iris (B). When iridectomy is not followed by deepening of the peripheral anterior
chamber, it is not likely that it will be effective in preventing
primary angle-closure glaucoma. (Spaeth GL. Glaucoma surgery. In Spaeth GL (ed). Ophthalmic Surgery: Principles
and Practice. Philadelphia: WB Saunders, 1990.) Fig. 8. Effect of iridectomy on the anterior chamber angle. The elimination of
pupillary block allows aqueous to pass without resistance from the posterior
to the anterior chamber (A), eliminating the gradient in pressure that is responsible for anterior
bowing of the peripheral iris (B). When iridectomy is not followed by deepening of the peripheral anterior
chamber, it is not likely that it will be effective in preventing
primary angle-closure glaucoma. (Spaeth GL. Glaucoma surgery. In Spaeth GL (ed). Ophthalmic Surgery: Principles
and Practice. Philadelphia: WB Saunders, 1990.)
|
Diagnosis and Classification An extensive description of the diagnosis and classification of the angle-closure
glaucomas cannot be given here.1,43 However, the essential component in the diagnosis of the angle-closure
glaucomas, gonioscopy, must be mentioned. Differentiation between optical
contact and actual adhesion between the iris and the cornea cannot
be made without the use of indentation gonioscopy; therefore, the correct
diagnosis of the angle-closure glaucomas demands the appropriate
use of a gonioscopic lens that can be used in indentation gonioscopy8,44 (Fig. 9). We prefer the Zeiss four-mirror lens on an Unger handle. For the diagnosis
of angle-closure glaucoma to be certain, the ophthalmologist must
be certain that the symptoms could only be the result of angle closure
and that the anterior chamber angle actually has closed. Thus, the
search for peripheral anterior synechiae, characteristically between
the 10 and 2 o'clock positions of the eye, assumes great significance. 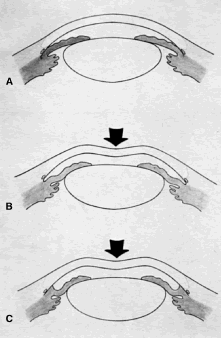 Fig. 9. Indentation gonioscopy. A. The angle appears closed. However, the observer cannot determine whether
this appearance is due to mere contacts between the iris and cornea
or to actual adhesion. B. The goniolens has been pressed against the central cornea, displacing
aqueous into the periphery and showing that the angle is open. C. Indentation gonioscopy displaces the iris posteriorly, showing peripheral
anterior synechiae. (Schwartz LW. Diagnostic evaluation of the patient. In Spaeth GL (ed). Early
Primary Open-Angle Glaucoma: Diagnosis and Management. Boston: Little, Brown & Co, 1979.) Fig. 9. Indentation gonioscopy. A. The angle appears closed. However, the observer cannot determine whether
this appearance is due to mere contacts between the iris and cornea
or to actual adhesion. B. The goniolens has been pressed against the central cornea, displacing
aqueous into the periphery and showing that the angle is open. C. Indentation gonioscopy displaces the iris posteriorly, showing peripheral
anterior synechiae. (Schwartz LW. Diagnostic evaluation of the patient. In Spaeth GL (ed). Early
Primary Open-Angle Glaucoma: Diagnosis and Management. Boston: Little, Brown & Co, 1979.)
|
Peripheral iridectomy is described in moderate detail because it is our
opinion that the general ophthalmologist should still be fully competent
in performing a standard surgical iridectomy. Nd:YAG laser iridotomy
is clearly the procedure of choice in patients needing an iridotomy. However, it
is not always possible. In patients in whom the cornea does
not become sufficiently clear to allow a laser iridotomy, an incisional
surgical iridectomy becomes necessary. In addition, incisional iridectomy
may be necessary in some patients with a diagnosis of pupillary
block glaucoma in whom a persistent, patent peripheral iridotomy cannot
be achieved with laser techniques. Preoperative Care Most attacks of acute primary angle-closure glaucoma can be treated medically. The
longer the attack, the higher the pressure, the more the eye
has been damaged, and the less likely the patient is to be harmed by
the medications used to treat the attack, the more vigorous the treatment
should be. When the attack is brief, the inflammatory signs minimal, and
the pressure relatively low, pilocarpine 1% every 5 minutes for
four doses often is adequate. Apraclonidine 1% often is helpful in
treating patients with high IOP or in cases in which lowering IOP is not
expected to be accomplished with the use of pilocarpine alone. When
treatment must be maximal, the following routine may be followed: pilocarpine 1% every 5 minutes for four doses; timolol 0.5% immediately and
again in 1 hour; apraclonidine 1% immediately; acetazolamide 500 mg
intravenously; and an osmotic agent (isosorbide, 30 mg/kg body weight
in routine cases; mannitol 20%, 70 ml/kg body weight intravenously in
the nauseated patient who can tolerate a large sudden increase in blood
volume; or anhydrase glycerol orally, 1 ml/kg body weight in the patient
who can take oral medication but is likely to have urinary retention). Topical
steroids are appropriate if the eye is inflamed. Occasionally, forceful
compression of the anterior chamber with an instrument
such as the Zeiss four-mirror gonioprism can push the angle open and
help to end the angle-closure attack. Once the attack has been stopped, it
is helpful to allow the eye to quiet before proceeding with the
iridectomy. Weak pilocarpine administration, such as 1% two or three times
a day and aqueous suppressants, such as timolol and acetazolamide, should
be continued until the time of surgery. If surgery is indicated
on the fellow eye, it may be appropriate to perform an iridectomy on
that eye while waiting for the involved eye to quiet. Pilocarpine should
be used with caution in both the involved eye and the fellow eye
because it can predispose the eye to angle closure by increasing the degree
of papillary block or causing the lens-iris diaphragm to move anteriorly. Occasionally, it is impossible to break an attack. In such cases, it is
advisable to proceed with surgery promptly, despite the presence of high
IOP. Here, a retrobulbar injection of anesthetic agent is appropriate
and may help lower the IOP. If unsuccessful, the pressure will be
brought down easily by paracentesis at the time of surgery. When a peripheral iridectomy is performed, the pupil should be as small
as possible. The use of mild pilocarpine is an easy, effective approach. In
cases in which the IOP remains elevated or the sphincter is nonfunctional, however, reducing the size of the pupil may be difficult. Multiple
instillations of pilocarpine are not recommended in such cases. Usually, they
only make the patient systemically ill and the eye inflamed; they
do not reduce the size of the pupil. If the sphincter is
functional, the pupil will contract quickly once the IOP has been lowered. On
the other hand, if the surgeon is planning to perform a sector
iridectomy, it is best to have the pupil dilated as widely as possible. Such
a sector iridectomy probably is the preferred procedure in patients
with severe iris atrophy, a dilated fixed pupil, or a cataract that
is limited to the visual axis. Operative Technique Adequate anesthesia for surgical iridectomy performed with a blade is provided
by topical administration of an agent such as proparacaine 0.5% eye
drops. Tetracaine also is effective, but it appears to have a slightly
more irritating effect on the corneal epithelium. Proparacaine 0.5% given
approximately every 30 seconds for ten doses 10 minutes before
the procedure and then followed by about five instillations after
the eye has been prepared and draped, immediately before the surgery, almost
always gives satisfactory anesthesia. It is even possible to place
a superior rectus bridal suture without causing undue discomfort. In most cases, it is preferable to perform a facial nerve block with a
modified O'Brien block.45 This approach allows easier and more comfortable placement and retention
of the speculum. Because the procedure usually lasts only about 5 to 15 minutes, a
short-acting injection anesthetic, such as lidocaine, is
appropriate. The relevant anatomic considerations are shown in Figure 4A and B. The operative technique is shown in Figure 10. The iridectomy should be performed so that the only instruments that
enter the anterior chamber are the needle that is used to develop a paracentesis
track and the tip of the blade that is used to make the corneoscleral
incision. The advantages of the paracentesis so greatly outweigh
the minute risks associated with it that we believe that it should
be performed routinely. The technique is described in detail earlier
in this chapter and is illustrated in Figure 5. When properly performed, this procedure is virtually without risk, even
in patients with extremely shallow or flat anterior chambers. Without
such an opening into the anterior chamber, the integrity of the incision
cannot be tested, blood in the anterior chamber cannot easily be
irrigated away, and chamber deepening cannot readily be performed. 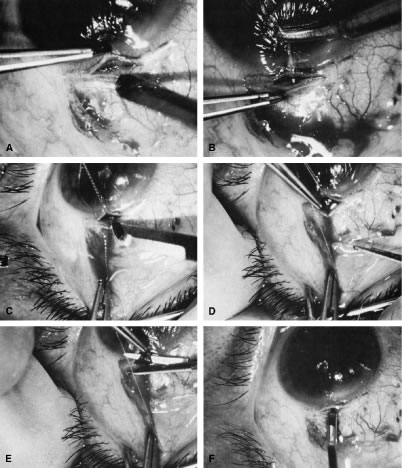 Fig. 10. Peripheral iridectomy with the use of a preplaced suture to retract the
edges of the incision. A. An incision is made through two thirds of the thickness of the sclera
directly at the corneoscleral sulcus. B. A 9-0 white virgin silk suture is placed so that it will be able to be
retracted from the depths of the incision. C. The suture is looped and used to retract the edges of the incision superiorly
and inferiorly. The incision is completed, permitting prolapse
of a small knuckle of iris. D. The iris is grasped with a fine-toothed forceps. E. The iris is pulled over the blade of the DeWecker scissors; after the
position of the iris is noted, the blades are closed and the tissue is
excised. F. The tip of an irrigator is placed just inside the incision, with care
taken to ensure that it does not enter the anterior chamber. Remnants
of the pigment epithelium are flushed away, and the iris is permitted
to return to its proper position so that the pupil is completely round. (Spaeth GL. Glaucoma surgery. In Spaeth GL (ed). Ophthalmic Surgery: Principles
and Practice. Philadelphia: WB Saunders, 1990.) Fig. 10. Peripheral iridectomy with the use of a preplaced suture to retract the
edges of the incision. A. An incision is made through two thirds of the thickness of the sclera
directly at the corneoscleral sulcus. B. A 9-0 white virgin silk suture is placed so that it will be able to be
retracted from the depths of the incision. C. The suture is looped and used to retract the edges of the incision superiorly
and inferiorly. The incision is completed, permitting prolapse
of a small knuckle of iris. D. The iris is grasped with a fine-toothed forceps. E. The iris is pulled over the blade of the DeWecker scissors; after the
position of the iris is noted, the blades are closed and the tissue is
excised. F. The tip of an irrigator is placed just inside the incision, with care
taken to ensure that it does not enter the anterior chamber. Remnants
of the pigment epithelium are flushed away, and the iris is permitted
to return to its proper position so that the pupil is completely round. (Spaeth GL. Glaucoma surgery. In Spaeth GL (ed). Ophthalmic Surgery: Principles
and Practice. Philadelphia: WB Saunders, 1990.)
|
To prevent the tip of the knife used to develop the corneoscleral incision
from damaging the iris or lens, it is preferable to use a broad blade, such
as the no. 67 Beaver blade. Multiple small scratches are made, with
the surgeon verifying that the depth of the incision is uniform
from end to end. Some surgeons prefer an anteriorly shelved incision
because it tends to close more easily; in the past, many surgeons did
not place a suture through this type of incision. However, a perpendicular
incision allows the iris to prolapse more readily, gives better
visualization, and facilitates the procedure. The availability of fine
suture material allows tight closure of the incision with minimal irritation
or difficulty. The scissors used to perform the iridectomy should
be sharp and should be tested immediately before the procedure to
ensure their proper operating condition. We prefer the use of a preplaced suture, as seen in Figure 10. This approach provides a clearer view of the process of creating the
corneal incision, it aids in prolapsing the iris, and it allows for immediate
closure with a suture that the surgeon knows is perfectly placed. Any
of a number of sutures is satisfactory. Absorbable polyglactin [Vicryl (Ethicon, Somerville, NJ)] works well and has the advantage
of not requiring removal. A 9-0 nylon suture also is satisfactory; it
is thick enough to be used to retract the tissue yet fine enough
to be well tolerated. The small fornix-based flap can be closed by stretching it toward one side, especially
if a radial relaxing incision approximately 2 mm long
and extending from the limbus has been performed (Fig. 11). This closure can be done with the same suture used to close the corneoscleral
incision, or it can be coapted with the wet-field cautery. 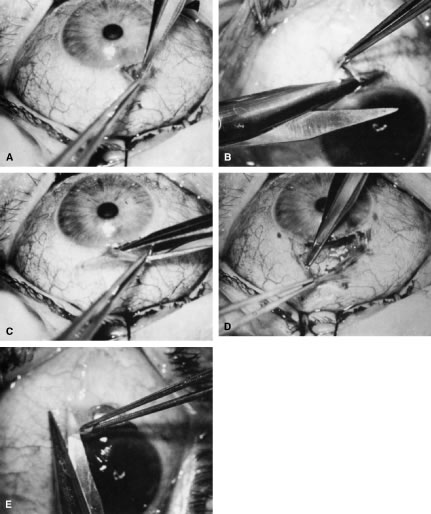 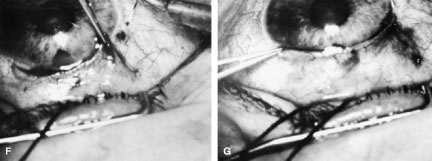 Fig. 11. Fornix-based flap procedure. A. With a scissors or knife, the conjunctiva is incised as close to the limbus
as possible. B. The incision is widened. The surgeon uses forceps to stretch the tissue. The
scissors cut inferiorly so that no remnant of conjunctiva is left
on the globe. C. Tissue is separated from the globe by inserting the scissors with the
tips closed and then spreading them bluntly. D. Blunt dissection is continued until the sclera is cleaned adequately. Bleeding
from the cut conjunctival vessels is almost inevitable and usually
exceeds the bleeding that occurs when a limbus-based flap is raised. E. A radial cut at the edge of the peritomy will improve visualization of
the sclera and permit a neat closure of the conjunctiva. F. The cut edge of a fornix-based flap is pulled inferiorly and secured with
a 10-0 nylon purse-string suture. G. With large incisions, it usually is necessary to suture both edges to
ensure tight closure. (Spaeth GL. Glaucoma surgery. In Spaeth GL (ed). Ophthalmic Surgery: Principles
and Practice. Philadelphia: WB Saunders, 1990.) Fig. 11. Fornix-based flap procedure. A. With a scissors or knife, the conjunctiva is incised as close to the limbus
as possible. B. The incision is widened. The surgeon uses forceps to stretch the tissue. The
scissors cut inferiorly so that no remnant of conjunctiva is left
on the globe. C. Tissue is separated from the globe by inserting the scissors with the
tips closed and then spreading them bluntly. D. Blunt dissection is continued until the sclera is cleaned adequately. Bleeding
from the cut conjunctival vessels is almost inevitable and usually
exceeds the bleeding that occurs when a limbus-based flap is raised. E. A radial cut at the edge of the peritomy will improve visualization of
the sclera and permit a neat closure of the conjunctiva. F. The cut edge of a fornix-based flap is pulled inferiorly and secured with
a 10-0 nylon purse-string suture. G. With large incisions, it usually is necessary to suture both edges to
ensure tight closure. (Spaeth GL. Glaucoma surgery. In Spaeth GL (ed). Ophthalmic Surgery: Principles
and Practice. Philadelphia: WB Saunders, 1990.)
|
If the scissors performing the iridectomy are held as shown in Figure 12 the iridectomy will tend to be broad based but will remain basal, reducing
the chance that the patient will have a disturbing sense of double
vision postoperatively. It is important to stress that in a properly
performed iridectomy, it is not necessary to insert the iridectomy forceps
into the anterior chamber. To do so unjustifiably increases the
risk of damage to the cornea, lens, and zonules. If a fine-toothed forceps
such as a Bonn forceps is employed, the surgeon must be careful to
pull the iris out of the anterior chamber far enough to ensure that
the iridectomy will he penetrating. A nontoothed forceps, such as a McPherson
tying forceps, offers the relative advantage of providing a less
secure grip on the iris, requiring the surgeon to grasp more tissue. The
disadvantage, however, is that control of the tissue is less certain, and
the forceps must be inserted further into the incision. 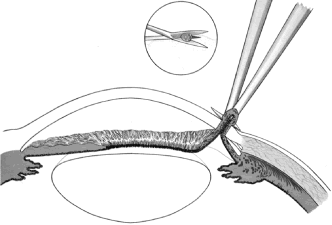 Fig. 12. Proper technique for performing iridectomy. (Spaeth GL. Glaucoma Surgery. In Spaeth GL (ed). Ophthalmic Surgery: Principles
and Practice. Philadelphia: WB Saunders, 1990.) Fig. 12. Proper technique for performing iridectomy. (Spaeth GL. Glaucoma Surgery. In Spaeth GL (ed). Ophthalmic Surgery: Principles
and Practice. Philadelphia: WB Saunders, 1990.)
|
After the iridectomy is complete, the iris usually will return spontaneously
into the anterior chamber unless it has suffered damage as a result
of high pressure (as occurs in severe attacks of angle closure glaucoma). In
patients with a reactive sphincter, it usually is necessary
only to release the iris from the grasp of the incision. The iris can
be stroked into position with the use of a blunt instrument on the corneal
surface. Attempting to reposit the iris by directing a stream of
irrigating solution into the incision is not recommended; the solution
may enter the posterior chamber, forcing even more iris out of the incision. If
the surgeon has difficulty restoring the iris to its proper
position, injection through the previously placed paracentesis track
of freshly mixed acetylcholine usually will solve the problem. In the
eye with a dilated fixed pupil and a flaccid iris, it often is preferable
to perform a sector iridectomy. Once the pupil is entirely round, the suture placed previously in the corneoscleral
incision can be pulled up and secured promptly. Injecting
balanced salt or acetylcholine solution through the previously placed
paracentesis can deepen the chamber. In patients who have had recent
angle-closure attacks and in whom peripheral anterior synechiae may be
newly developed, forceful deepening of the anterior chamber at this point
may tear open the synechiae and restore normal function of the anterior
chamber. The procedure of chamber deepening must be done cautiously, with
careful monitoring of IOP. A gonioprism, such as the Zeiss
four-mirror lens, can be used with an operating microscope to monitor
the effect on anterior chamber angle. At the close of the procedure, the pupil should be round, the chamber deep, and
the incision sufficiently closed that there is no leakage. The
IOP should be approximately 15 to 30 mm Hg, being raised to that level
by injection through the keratostomy. Such a pressure may help prevent
subsequent choroidal detachment and allows the integrity of the incision
to be tested. If the corneoscleral incision has been properly placed
and sutured, there should be minimal transient astigmatism. Vision
should return to the preoperative level within several days. If the eye is inflamed, it usually is helpful to instill a short-acting
cycloplegic agent such as cyclopentolate. Furthermore, the surgeon may
give a subconjunctival injection of corticosteroid, although it rarely
is necessary to do so. An antibiotic ointment and a light patch usually
are applied until the facial nerve block has worn off. If there is a reasonable chance that the patient will need a filtration
procedure at a later date, then the surgical procedure is modified to
perform the iridectomy through clear cornea, rather than under a fornix-based
flap. The technique is almost exactly the same, with the obvious
exception that the incision is placed through clear cornea. Postoperative Care In almost all instances, iridectomy can be performed as an outpatient procedure. However, when
associated with severe pain or when the attack
of angle closure has been difficult to stop and has required extensive
usage of carbonic anhydrase inhibitors and osmotic agents, admission
of the patient to the hospital may be justified. Especially in older
and infirm patients, the general state of health must be monitored carefully. It
is important to verify that the patient has not become overly
dehydrated or experienced an attack of congestive failure. The eye patch usually is removed from the treated eye as soon as the facial
nerve block has worn off. If the procedure is done on an outpatient
basis, a patch and shield may be left in place until the patient returns
the next day for the first postoperative visit. A drop containing
a corticosteroid can be used topically three or four times a day until
the inflammatory response has disappeared, usually 4 to 7 days. Careful attention must be paid to the state of the pupil. The development
of posterior synechiae is an important and usually unnecessary complication. Although
it is not clear that posterior synechiae predispose
the patient to the development of cataract, the presence of such adhesions
makes extracapsular cataract extraction more difficult. Because
many patients with angle-closure glaucoma will later have cataract extraction, the
development of such adhesions may jeopardize the long-term
visual result. Furthermore, inability of the pupil to dilate in the
dark may limit the patient's visual function, especially in conjunction
with a developing cataract. In most cases, dilating drops are used
routinely until the inflammatory response has worn off. One acceptable
program is to use tropicamide 1% at sufficient intervals to ensure
that the pupil is dilated adequately for a period of approximately 1 week. The
medication can be continued at bedtime for 2 to 3 weeks, or
even longer, until it is certain that the inflammatory response has disappeared. Many
patients are given pilocarpine preoperatively. There
is a tendency to continue the administration of pilocarpine postoperatively, especially
when control of IOP has been difficult. This practice
should be discouraged. Pilocarpine will predispose patients to posterior
synechiae and should be avoided if possible. The drug of choice for
pressure control usually is topical epinephrine, a β-blocker, or
epinephrine plus a β-blocker. If these two agents are not adequate, a
carbonic anhydrase inhibitor can be used until the eye is entirely
quiet, at which time pilocarpine can be reinstituted. In such cases, however, care
should be taken to dilate the pupil periodically to prevent
the development of posterior synechiae. Some surgeons may be reluctant to use dilating drops for fear of inducing
angle closure. This possibility exists, but it is uncommon. Furthermore, it
is preferable to know immediately whether the iridectomy has
eliminated the possibility of angle closure caused by papillary dilatation. Therefore, this
concern is an additional reason to use cycloplegic
agents in the immediate postoperative period. Because of the possibility of an increase in IOP after dilatation, however, IOP
should be checked carefully after the use of the cycloplegic
agent. In addition, IOP should be monitored carefully during the initial
postoperative period. Pressure spikes are not uncommon. These spikes
are of little concern if the optic nerve is healthy. However, in patients
with advanced glaucomatous nerve damage, such pressure spikes can
be devastating; they should be avoided if possible. Postoperative evaluations ordinarily are made on the first postoperative
day, after 1 week, and after 1 month. If a problem with the control
of IOP is suspected, visual fields and disc photographs should be obtained. Complications The most serious complication after iridectomy is malignant glaucoma (aqueous
misdirection, ciliary block glaucoma). Eyes predisposed to this
complication are those with small anterior segments, typically seen in
patients with marked hyperopia or small globes. Furthermore, there is
some indication that eyes with high IOP preoperatively may be predisposed
as well. Patients who have had an attack of malignant glaucoma in
one eye are likely to have the same complication in the second eye. GUARDED FILTRATION PROCEDURES (TRABECULECTOMY) What traditionally has been called a trabeculectomy has become the standard
filtration type of surgery for most cases of open-angle glaucoma. The name is a generic one, describing a wide variety of surgical methods, some
substantively different from others.46–50 A goal of the original procedure was to allow filtration out of the cut
ends of Schlemm's canal. In most cases, this mechanism does not
appear to be the actual reason for the decrease in IOP that usually follows. Another
theoretic explanation for its pressure-lowering effect, direct
access of aqueous humor to the collector channels, bypassing diseased
trabecular meshwork or Schlemm's canal, may be the actual
mechanism for pressure reduction in a few cases. Exploration of the operative
site does not disclose any area of gross filtration in some patients
who have effective pressure lowering after surgery. It is likely
that in such cases, aqueous humor exits into the collector channels
or perhaps through the thinned scleral tissue. In most cases, the mechanism
responsible for lowering IOP after guarded filtration procedure
is a through-and-through fistula connecting the anterior chamber with
the subconjunctival space. As such, the word trabeculectomy is a misnomer. Complete
success is possible without removing trabecular tissue. Lamellar
sclerokeratectomy would be a more accurately descriptive title, and
it has been suggested in the past. However, the term is cumbersome
and to some extent, inaccurate, because sclera is not always removed. Because
the term trabeculectomy is a significant misnomer, we rarely
use it in this chapter. The procedure performed currently functions
as a filtration procedure; consequently, it is appropriately called
a filtration procedure. When it is performed so that the filtering area
is at least partially protected in an attempt to control the amount
of filtration, the term guarded filtration procedure is appropriate and is used in this chapter. Consideration of how the operation actually works is not of purely academic
interest; it materially affects how the surgery is performed and
influences the final result. Patients with gross fistulas with filtering
blebs resembling those seen with operations that were popular in the
past, such as the corneoscleral trephine, usually achieve lower IOP
than patients in whom the filtering bleb is thicker, more posterior, and
less cystic. Furthermore, the thinner, polycystic bleb probably lasts
longer, providing more years of low IOP. Consequently, if the surgeon
wants to obtain IOP that is as low as possible, the guarded filtration
procedure should be designed to produce gross filtration. Such a goal
has its costs, however. A general principle of glaucoma surgery is that every millimeter of pressure-lowering
effect has a price. This principle applies to guarded filtration
procedure; the complication rate with procedures designed to
produce gross filtration and lower IOP is greater than that with procedures
that are more completely guarded and in which a higher final IOP
is considered satisfactory, as discussed in detail in the first section
of this chapter. The surgeon must carefully consider the major goal
of the surgery being planned. For example, for the patient who has uncontrollable
angle-closure glaucoma and is predisposed to flat chamber
or even malignant glaucoma, the surgeon probably will fashion the scleral
flap and suture it in place so that leakage is minimal. On the other
hand, for a patient with high myopia with glaucoma that is progressive
at a pressure level of 14 mm Hg, the surgeon probably will want
to make the flap thin or ensure that there is leakage around the edge
of the flap, or both, to have the greatest likelihood of gross filtration
with a low final IOP. Factors predisposing patients to the final level
of IOP are shown in Tables 14, 17, and 18. As shown in these tables, the more the surgical procedure mimics the classic
Elliot corneoscleral trephine, the more reasonable to expect a final
low IOP. Also, there is more likelihood of flat anterior chamber, choroidal
detachment, cataract development, and a thin cystic bleb predisposing
to endophthalmitis. Several modifications have been suggested for achieving a low final IOP
with a reduced incidence of side effects. These include the placement
of sutures so that they can be released easily in the postoperative period27,51–53 and the transconjunctival cutting of sutures postoperatively with a laser.54–57 These techniques are discussed later in this chapter. In routine cases, we prefer a limbus-based flap (Fig. 13). The development of this type of flap is only slightly more difficult
than fashioning a fornix-based flap (see Fig. 11), but its advantages are significant. Compared with procedures performed
with a fornix-based flap, the limbus-based operation is more likely
to eliminate problems with postoperative leakage through the conjunctiva. In
contrast, leakage at the cut edge of the conjunctiva of a fornix-based
flap is common in the immediate postoperative period. Furthermore, cooperation
under the limbus-based flap tends to be easier than
with the procedure performed with the fornix-based flap. In addition, and
of great importance, localized deformation of the wound in the immediate
postoperative period for the purpose of encouraging filtration
is performed more easily and safely when the radial grooves are covered
with a limbus-based rather than a fornix-based flap. Because we often
use this procedure [the Carlo Traverso maneuver (CTM)],58 the security afforded by the limbus-based flap is welcome. Thus, in most
cases, the limbus-based flap is preferred. An exception is the case
in which previous filtration surgery has failed and in which development
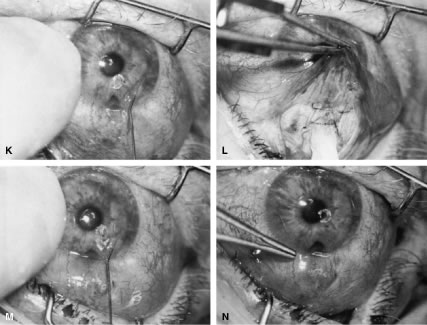
of a limbus-based flap appears unjustifiably difficult and risky. 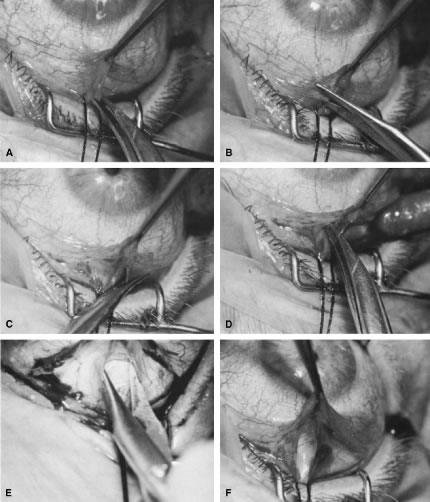 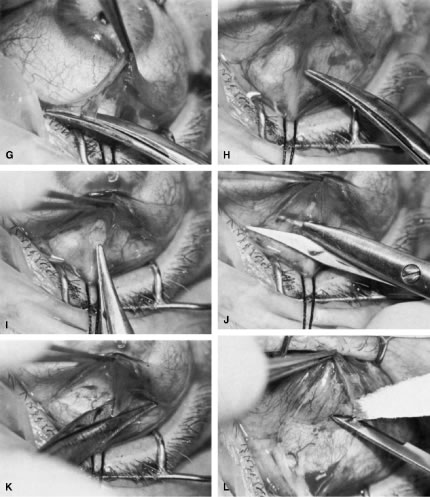  Fig. 13. Limbus-based flap procedure. A. Conjunctiva is lifted away from the globe, stretched, and incised adjacent
to the superior rectus muscle bridle suture. B. The incision in the conjunctiva is extended nasally. C. The conjunctival incision is extended temporally. D. Tenon's capsule is lifted up, away from the globe, and incised with
the scissors held obliquely to avoid cutting into the underlying superior
rectus muscle. E. The incision in Tenon's capsule is spread bluntly. F. The superior rectus muscle can be seen through the buttonhole in Tenon's
capsule. Bleeding should be minimal; if it occurs, it should be
controlled promptly with cautery. G. Tenon's capsule is incised nasally and temporally. H. The connective tissue overlying the superior rectus muscle is seen easily
after Tenon's capsule has been incised. This tissue usually is
highly vascular in the area directly at the base of the superior rectus
muscle. I. Episclera is buttonholed approximately 4 mm posterior to the limbus, showing
the underlying sclera. J. One blade of the scissors is insinuated between the sclera and the episclera, and
the episclera is incised nasally. K. The episclera is incised temporally. The plane of the scissors is flush
with the sclera. L. Remaining adhesions between the episclera and sclera are dissected in
a semisharp fashion with the no. 67 Beaver blade, which is pushed at right
angles to the cutting axis. M. Tenon's capsule is closed in a separate layer with an 8-0 absorbable
suture. N. The superior edge of Tenon's capsule tends to retract up under the
lid. It can be hooked over the needle and pulled inferiorly. Sutures
are locked. O. After Tenon's capsule is closed, the needle is placed from the underneath
side to the superficial side of the conjunctiva and exteriorized
so that it can be used to close the conjunctiva. P. The conjunctiva is closed with closely spaced running, unlocked sutures. The
final suture is tied securely. Q. After the needle has passed through the tissue, it is lifted away from
the globe firmly. The underlying tissue is stretched. A blunt forceps
is used to grasp this underlying tissue firmly, as close to the needle
as possible. This maneuver will hold the needle firmly in place, permitting
the surgeon to release the end of the needle containing the suture
without having to change the position of the needle. R. The needle can be regrasped toward the cutting end so that it is held
in proper position for placement of the next suture. (Spaeth GL. Glaucoma surgery. In Spaeth GL (ed). Ophthalmic Surgery: Principles
and Practice. Philadelphia: WB Saunders, 1990.) Fig. 13. Limbus-based flap procedure. A. Conjunctiva is lifted away from the globe, stretched, and incised adjacent
to the superior rectus muscle bridle suture. B. The incision in the conjunctiva is extended nasally. C. The conjunctival incision is extended temporally. D. Tenon's capsule is lifted up, away from the globe, and incised with
the scissors held obliquely to avoid cutting into the underlying superior
rectus muscle. E. The incision in Tenon's capsule is spread bluntly. F. The superior rectus muscle can be seen through the buttonhole in Tenon's
capsule. Bleeding should be minimal; if it occurs, it should be
controlled promptly with cautery. G. Tenon's capsule is incised nasally and temporally. H. The connective tissue overlying the superior rectus muscle is seen easily
after Tenon's capsule has been incised. This tissue usually is
highly vascular in the area directly at the base of the superior rectus
muscle. I. Episclera is buttonholed approximately 4 mm posterior to the limbus, showing
the underlying sclera. J. One blade of the scissors is insinuated between the sclera and the episclera, and
the episclera is incised nasally. K. The episclera is incised temporally. The plane of the scissors is flush
with the sclera. L. Remaining adhesions between the episclera and sclera are dissected in
a semisharp fashion with the no. 67 Beaver blade, which is pushed at right
angles to the cutting axis. M. Tenon's capsule is closed in a separate layer with an 8-0 absorbable
suture. N. The superior edge of Tenon's capsule tends to retract up under the
lid. It can be hooked over the needle and pulled inferiorly. Sutures
are locked. O. After Tenon's capsule is closed, the needle is placed from the underneath
side to the superficial side of the conjunctiva and exteriorized
so that it can be used to close the conjunctiva. P. The conjunctiva is closed with closely spaced running, unlocked sutures. The
final suture is tied securely. Q. After the needle has passed through the tissue, it is lifted away from
the globe firmly. The underlying tissue is stretched. A blunt forceps
is used to grasp this underlying tissue firmly, as close to the needle
as possible. This maneuver will hold the needle firmly in place, permitting
the surgeon to release the end of the needle containing the suture
without having to change the position of the needle. R. The needle can be regrasped toward the cutting end so that it is held
in proper position for placement of the next suture. (Spaeth GL. Glaucoma surgery. In Spaeth GL (ed). Ophthalmic Surgery: Principles
and Practice. Philadelphia: WB Saunders, 1990.)
|
Preoperative Care A careful history should be taken to determine whether systemic medical
issues may affect the operative or postoperative course. For example, breathing
problems and spinal arthritis may prevent the patient from
lying completely flat. Chronic coughing or constipation with straining
may increase the risk of postoperative suprachoroidal hemorrhage. Other
relevant information includes what allergies are present and if the
patient has a proclivity to excessive bleeding. Bleeding studies, such
as prothrombin time, platelet count, and, in some cases, bleeding time, are
appropriate in certain cases. Patients who are taking aspirin
should not do so at least a week preoperatively, if possible. Similarly, anticoagulants
should be discontinued long enough preoperatively to
allow the coagulation characteristics to return to satisfactory levels. The eye should be as quiet as possible. Long-acting miotics, such as echothiophate, should
be discontinued at least 3 weeks before surgery. If
possible, pilocarpine or other short-acting miotics also should be discontinued
long enough before surgery to allow their effect to wear off. Any
signs of medication allergy may warrant discontinuation of the
offending agent and sometimes preoperative topical steroids to quiet
the inflammation, reducing intraoperative bleeding and postoperative scarring. The purpose of the surgery must be clear to the patient and the surgeon (see Table 1). Full discussion of the reasons for considering the surgery is essential, including
alternative therapies and complications. Patients must
understand that the usual goal of surgery is not improved visual acuity; visual
acuity probably will be different and may be worse after the
operation. Postoperative care must be discussed before surgery, with
patients having a clear understanding of the need for continuing, careful
monitoring of their condition. If it will be difficult for the patient
to return to the operating surgeon, other arrangements that are satisfactory
to both patient and surgeon must be made before the operation. Before the patient is sedated or anesthetized, the surgeon should again
ensure that the patient's expectations and anticipations are realistic. Anesthesia Depending on the patient, surgeon, and procedure, anesthesia may range
from general endotracheal anesthesia to topical agents alone. General
anesthesia is reserved for patients unable to cooperate and hold still
for the duration of the procedure. Retrobulbar or peribulbar injection
of short- and medium-acting anesthetics, such as lidocaine and mepivacaine, with
or without hyaluronidase are usually adequate. Some surgeons
do not include hyaluronidase in the retrobulbar block because it may
make the subconjunctival tissues boggy, complicating the surgery and
making evaluation of the postoperative bleb more difficult. A facial
block, such as a modified O'Brien facial nerve block,45 with approximately 10 ml of a short-acting agent, such as lidocaine or
mepivacaine, may reduce lid squeezing. Most patients have only light
sedation, such as 4 to 6 mg intravenous diazepam (Valium). In many cases, no
sedation is necessary. Surgeons anticipating shorter, routine cases
with cooperative patients may be comfortable with topical anesthesia, such
as lidocaine jelly and topical tetracaine. If an iridectomy
is planned, topical anesthesia may be supplemented with intracameral (nonpreserved) lidocaine 1%. Intracameral lidocaine may result in dilation
of pupil, necessitating intracameral miotics before the iridectomy
to prevent accidentally large iridectomies. We prefer to perform guarded
filtration procedures with a modified O'Brien facial nerve blocky
with approximately 10 ml of a short-acting agent, such as lidocaine or
mepivacaine, in addition to a retrobulbar block with the same agent. Hyaluronidase
is not included in the retrobulbar block because it tends
to make the subconjunctival tissues boggy, complicating the surgery
and making evaluation of the postoperative bleb more difficult. Most patients
have only light sedation, such as 4 to 6 mg intravenous diazepam. In
many cases, no sedation is necessary. Many patients are older, and
in some, the only eye with useful vision is having surgery; therefore, the
sooner the patient can be completely ambulatory after surgery, the
better. In addition, in most cases, the goal of surgery is to develop
a functional fistula, so it probably is helpful to remove the patch
as soon as possible after surgery to encourage blinking and automassage. In
some instances, especially with topical anesthesia, it is not
necessary to apply a patch at all. In addition, rapid ambulation helps
to ensure that the patient maintains a posture that prevents blood
from settling in the area of the procedure. The use of short-acting agents
and minimal sedation encourages prompt return to full normal function, an
important consideration in the older person with sensory deprivation. Operative Technique The limbus-based conjunctival flap should be developed as far superiorly
in the superior fornix as possible (Figs. 13 and 14). The conjunctival incision should be sufficiently far posteriorly that
it involves the thick tissue overlying the superior rectus muscle; if
it is made more anteriorly, the likelihood of leakage through the incision
increases. This leakage is especially a problem when agents specifically
used to combat postsurgical scarring are employed. Toothed forceps, or
even serrated forceps with indentations that are sharp enough
to cut the conjunctiva, should not be employed. On the other hand, a
common error is to forget the normal elasticity of the conjunctiva and
consequently to neglect to stretch the tissue sufficiently to permit
clear identification of the edges of the incisions. This problem is
a special concern with Tenon's capsule, which tends to retract out
of the operative field, and may be overlooked unless specific attention
is paid to bringing it into view. 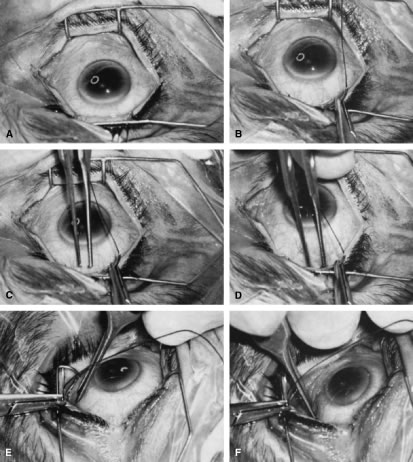 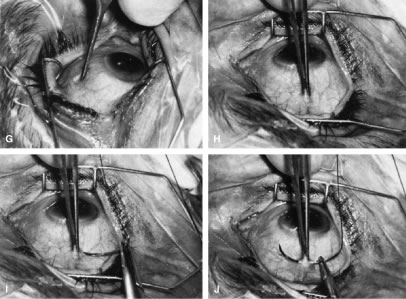 Fig. 14. Placement of a superior rectus muscle bridle suture. A. The eye with a Barraquer-Colibri speculum in place. The superior rectus
muscle is well hidden by the upper lid (lower portion of photograph). B. The needle of a single-armed 4-0 black silk suture is used to retract
the upper lid, allowing better visualization of the area in which the
superior rectus suture is to be placed. C. The tips of a Lister forceps are held tangential to the superior portion
of the globe, approximately 2 mm apart. D. The forceps are moved superiorly so that the tips extend past the insertion
of the superior rectus muscle, about 12 mm superior to the lumbus. E. Side view showing the lid held superiorly by the needle of the 4-0 black
silk suture and the Lister forceps in its initial position. F. The handle of the forceps is rotated superiorly while the tips are pressed
firmly against the globe. The indentation of the globe is caused
by the pressure of the forceps. When the forceps have been rotated to
the position shown, the tips are closed around the superior rectus muscle. G. After the muscle has been grasped firmly, the forceps are again rotated
inferiorly and the globe is pulled inferiorly. H. Surgeon's view of the proper grasp of the superior rectus muscle. I. The needle of the 4-0 black silk suture is placed under the superior rectus
muscle not through its belly. J. Proper position of the needle, deep to the forceps and between the globe
and the superior rectus muscle belly. (Spaeth GL. Glaucoma surgery. In Spaeth GL (ed). Ophthalmic Surgery: Principles
and Practice. Philadelphia: WB Saunders, 1990.) Fig. 14. Placement of a superior rectus muscle bridle suture. A. The eye with a Barraquer-Colibri speculum in place. The superior rectus
muscle is well hidden by the upper lid (lower portion of photograph). B. The needle of a single-armed 4-0 black silk suture is used to retract
the upper lid, allowing better visualization of the area in which the
superior rectus suture is to be placed. C. The tips of a Lister forceps are held tangential to the superior portion
of the globe, approximately 2 mm apart. D. The forceps are moved superiorly so that the tips extend past the insertion
of the superior rectus muscle, about 12 mm superior to the lumbus. E. Side view showing the lid held superiorly by the needle of the 4-0 black
silk suture and the Lister forceps in its initial position. F. The handle of the forceps is rotated superiorly while the tips are pressed
firmly against the globe. The indentation of the globe is caused
by the pressure of the forceps. When the forceps have been rotated to
the position shown, the tips are closed around the superior rectus muscle. G. After the muscle has been grasped firmly, the forceps are again rotated
inferiorly and the globe is pulled inferiorly. H. Surgeon's view of the proper grasp of the superior rectus muscle. I. The needle of the 4-0 black silk suture is placed under the superior rectus
muscle not through its belly. J. Proper position of the needle, deep to the forceps and between the globe
and the superior rectus muscle belly. (Spaeth GL. Glaucoma surgery. In Spaeth GL (ed). Ophthalmic Surgery: Principles
and Practice. Philadelphia: WB Saunders, 1990.)
|
To facilitate a posterior incision, a superior rectus bridle suture may
be placed and gently affixed to the drape. A second alternative is the
corneal traction suture. A 7-0 Vicryl suture is placed through the peripheral
cornea, approximately 0.5 to 1.0 mm from the limbus. The suture
should be placed at least ½, but not full thickness, with plane
of the spatulated needle parallel to the surface of the cornea in
midbite, so that the cutting sides of the suture do not create tracks
that may facilitate cheesewiring through the cornea. The eye is then
rotated inferiorly, the suture entry and exit from the cornea checked
for leakage indicative of an inadvertent full thickness passage, and the
suture affixed to the drape with two pieces of tape, folding the suture
of the first to prevent slippage. Sterile adhesive medication labels
work well. The incision in the conjunctiva and Tenon's capsule should be long
enough to permit easy retraction of the tissue toward the limbus, providing
good visibility. A 10-mm incision is average. It is better to have
the incision longer than shorter. If the initial incision is made directly over the insertion of the superior
rectus muscle, it should not be carried deep into the tissue, because
the muscle could be cut. Rather, the edge of Tenon's capsule
and conjunctival tissue should be held firmly, and the underlying tissue
separated with blunt dissection. The scissors should be tilted anteriorly
toward the limbus to avoid the underlying muscle and the rich
vascular network surrounding it. The blunt dissection is continued until
Tenon's capsule is completely incised. A second approach is to make the incision in the supertemporal or supernasal
quadrant, 8 mm or more posterior to the limbus, through conjunctiva
and Tenon's capsule down to bare sclera. This approach avoids
the risk of immediate damage to the muscle with bleeding. The scissors
are then used bluntly spread and dissect the plane between Tenon's
capsule and the sclera horizontally, parallel to the limbus, over
the superior rectus. One blade of the scissors is then placed above the
conjunctiva, and one beneath Tenon's and the wound enlarge parallel
to the limbus. As the incision is enlarged, traction on the internal
blade of the scissors away from the globe stretches the tissues over
that blade, allowing careful inspection. Before making this enlargement
of the wound, the surgeon must ascertain that the superior rectus
is not within the tissue to be cut by the scissors. Tenon's capsule, with its conjunctiva, is spread anteriorly over the
cornea to give the best view possible of the corneal scleral sulcus. In
almost all instances, there is a thin layer of episclera remaining. This
layer is grasped and elevated forcibly, permitting development
of a 2 × 2-mm buttonhole, through which the bare sclera is easily
visible. This episcleral tissue is markedly adherent. The buttonhole
should be extended approximately 5 mm temporally and laterally. The
forceps are used to grasp the edge of this deep episcleral tissue, and
pull it anteriorly and inferiorly, so the surgeon can clean down to bare
sclera anteriorly to the corneoscleral sulcus. The sulcus is not readily
visible until the episcleral tissue has been reflected from it. In
fact, it probably is the single most important landmark with regard
to filtration surgery. As seen in Figure 4, the conjunctiva inserts just anteriorly to the corneoscleral sulcus. Dissection
to that point can be carried out with relative ease, and with
meticulous technique, even 1 mm or so anterior to the groove. More
anterior to that, conjunctiva has metamorphosed into the epithelium of
the cornea, and attempts to develop a conjunctival flap over the cornea
itself are doomed to failure. Furthermore, the trabecular meshwork
usually lies near the corneoscleral sulcus. In large eyes, which are common
in myopes, a perpendicular incision just anterior to the corneoscleral
sulcus would enter the anterior chamber well anterior to the iris
root. In contrast, in small eyes, which are found in most hyperopes, an
incision at the same point also would enter the anterior chamber
but just in front of the iris root. When these eyes have extensive peripheral
synechiae, an incision made with a sharp blade through the corneoscleral
sulcus will penetrate into the posterior chamber, which obviously
is not the location for most glaucoma procedures. Consequently, in
small eyes with small anterior segments, especially in association
with angle closure, the surgeon must place the corneoscleral incision
more anteriorly than usual and be certain that the incision actually
is entering the anterior chamber. In such cases, it may be necessary to
shelve the incision anteriorly. This problem clearly affects patients
with acute or chronic primary angle-closure glaucoma. We prefer to clean the conjunctiva meticulously until no fibers are crossing
the corneoscleral sulcus. The most effective method is to use a
no. 67 Beaver blade held at a 45-degree angle from the direction of the
incision. Only the pointed tip is used to cut the tissue (see Fig. 13A). The dissection is continued anteriorly until the conjunctiva can no
longer be cleaned further anteriorly. The corneoscleral sulcus should
be free of fibers for a width of approximately 4 to 5 mm. Partial cleaning of the area posterior to the corneoscleral sulcus can
be accomplished in a variety of ways, including grasping the Tenon's
episclera and pulling it anteriorly and inferiorly, scraping the surface
of the sclera in a semiblunt fashion with the edge of the blade, and
pushing the tissue with the tip of a Weck-cell sponge (Xomed-Treace, Jacksonville, FL) or
similar dry sponge. Such techniques almost always
are only preparatory, and they do not clean the sulcus adequately. We
emphasize this point because a frequent cause of failure of filtration
procedures is blockage of the site of attempted filtration as the
result of an excessively posterior incision, and the usual cause for
an excessively posterior incision is failure to reflect the conjunctiva-Tenon's
episclera sufficiently anteriorly. Some surgeons excise Tenon's capsule, especially where it appears
to be redundant or particularly profuse. This practice is thought to increase
the rate of success and to result in a thinner, more cystic conjunctival
filtration bleb. Few studies have evaluated the advantages
and disadvantages of this technique. However, it has the disadvantage
of predisposition to a thinner, more fragile covering to the filtering
bleb, with a greater incidence of postoperative complications, and it
does not appear to result in lower IOP.59,60 A fornix-based approach, beginning with a peritomy, may also be used. The
conjunctiva can be cleaned from the limbus by making an incision directly
at the limbus at approximately the 11 o'clock position and performing
a peritomy clockwise to approximately the 1 o'clock position (see Fig. 11). The addition of a relaxing incision at the 11 o'clock position allows
better visibility and improves the closure. The limbal area is cleaned
meticulously, and the conjunctival-Tenon's tissue is undermined
to facilitate final closure of the fornix-based flap. The corneal epithelium
directly at the limbus between the 11 and 1 o'clock positions
should be removed 1 mm anterior to the limbus. Wet-field cautery is an
excellent instrument for this. Care should be taken to test the power
level of the cautery on the sclera or gently at the limbus first, to
prevent unintended thermal damage to the cornea. High cautery settings
may thus create astigmatism, but this usually reverses in days to weeks
during the postoperative period. After the limbal area has been meticulously cleaned, either with a fornix-based
or a limbus-based flap, light cautery outlines the position of
the scleral flap (Fig. 15B). The shape of the flap is unimportant but in the routine case. We prefer
one that is approximately 2 mm wide circumferentially and 3 mm long
radially. This rectangular flap is easy to dissect and involves relatively
little scleral tissue. A no. 67 Beaver blade is used to incise the sclera in the area outlined
by the cautery. This incision should be at least half and preferably
two thirds the thickness of the sclera. Magnification of the operating
microscope usually is increased during the creation of the scleral flap. If the surgeon is right handed, the right-hand posterior corner of the
scleral flap is grasped with a forceps such as the Pierce-Hoskins forceps. This
instrument has indentations but no teeth and, therefore, is
not likely to penetrate or tear the scleral flap. Even in cases in which
the surgeon wishes to develop a thin scleral flap, it is preferable
to start the dissection with a thick flap, at least one half the scleral
thickness (see Fig. 13C). As the surgeon proceeds, the flap can be thinned to the proper thickness. The no. 67 Beaver blade may be used to dissect the flap. The knife should
be held as parallel to the brow as possible, because this position
facilitates dissection of the flap (see Fig. 15D). Finer, sharper “superblades” are useful when making very
small scleral flaps. The thickness of the flap must be monitored carefully as it is developed. The
surgeon should be careful never to dissect blindly but always must
see the tip of the knife. Flipping the flap forward and back, so that
the surgeon sees the outside and then the inside, can help in estimating
the thickness of the sclera. The assistant should be diligent in sponging to keep the view of the dissection
as clear as possible. We prefer to keep the scleral flap dry
rather than to irrigate it; irrigation tends to hide the landmarks at
the depth of the flap. Firm traction should be placed on the scleral flap that is being developed
so that cutting is performed against traction. The flap is extended
for at least 1 mm and preferably 2 mm anterior to the junction of the
white sclera with a clear cornea (see Fig. 15E). Once the flap has been developed, and before the anterior chamber is
entered, a paracentesis is formed with a short, sharp, disposable 25-gauge
needle (see Fig. 5). We believe that the placement of this paracentesis track is vitally
important. An alternative method is a stab type, uniplanar, temporal paracentesis. The
stab-type paracentesis tends to leak more as balanced salt solution
is instilled, which may make evaluation of fistula outflow more difficult. However, this
paracentesis is more easily accessed, both intraoperatively
and postoperatively and may be helpful if postoperative anterior
chamber reformation is necessary. The anterior chamber is entered by incising through the sclera in a radial
fashion, along the right-hand line of radially placed cautery. Multiple
light scratches are placed, and the blade is kept vertical so that
entry will be as close as possible to the anterior extent of the scleral
flap. A sharp blade such as a diamond knife or superblade can be
employed, but unless used with a guard, these blades are likely to penetrate
into the iris. These sharper, more pointed blades make it possible
to enter more anteriorly, which is a real advantage. But they must
be used cautiously to avoid damage to the iris and lens, with subsequent
development of cataract. The incision is made long enough so that its posterior edge is just posterior
to the junction between the white sclera and the clear cornea. If
the posterior extent of the incision is not long enough at the time
of entry, the incision is lengthened. Vannas scissors are placed into
the anterior chamber, with the surgeon making certain that the tip neither
penetrates into the iris nor splits the sclera. This entry is assisted
by lifting the area to be excised with a fine forceps, such as
a Pierce-Hoskins forceps, and depressing the bed of the sclera with the
edge of the Vannas scissors. The scissors must not be directed in an
angled fashion so that they extend through the iris into the lens and
underlying zonules. After the radial groove has been made, the block must be excised. There
are advantages to cutting the anterior aspect of the block first, and
advantages to cutting the posterior edge of the block first. To some
extent, the method that is used depends on each eye and what occurs at
the time of the surgery. Once the Vannas scissors is placed so that one
blade is definitely in the anterior chamber, it can be moved anteriorly
as far as possible so that it pushes firmly against the base of the
scleral flap, where it is attached to the cornea. In the right eye, it
usually is easier to use the right hand to hold the Vannas scissors. The
left hand is used to fix the sclera at the posterior edge of the
bed. The left hand pulls the globe superiorly in the direction of the
brow, and the right hand pushes the Vannas scissors inferiorly toward
the nose. The goal is to ensure that the section is placed as far anteriorly
as possible, so that no ledge of cornea will be left. The blade
of the Vannas scissors should be held exactly perpendicular to the
cornea so that the cut is not shelved. The outer blade should be directly
over the inner blade, as shown in Figure 15G. Once the anterior aspect of the block has been cut, the fixation on the
posterior edge of the bed is released. The assistant who is holding
the scleral flap anteriorly to ensure adequate visualization continues
to hold the flap firmly. The left hand holds the anterior edge of the
block and pulls it anteriorly (inferiorly) toward the nose. The Vannas
scissors move posteriorly (superiorly) toward the brow, and the posterior
edge of the block is incised. Again, the surgeon holds the scissors
absolutely vertically so that the sclera will not be cut. Just as
the anterior aspect of the block was incised all the way to the radial
groove, the posterior block is incised all the way to the nasal radial
groove. The surgeon changes hands, holding the Pierce Hoskins forceps in the right
hand and the Vannas scissors in the left hand. The block is held with
the right hand and amputated with the Vannas scissors, leaving the
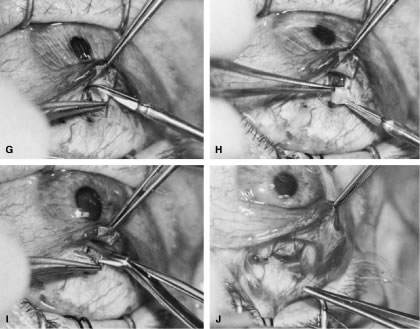
desired amount of ledge, usually 0.25 to 0.5 mm. When the block is removed, it should be inspected (see Fig. 15H). If it is not exactly rectangular with edges that are completely unshelved, then
it has not been taken properly. The surgeon should note the
error so that it can be corrected at the next surgical procedure. The
corneosclera to be excised does not include sclera posterior to the
scleral spur. Thus, there is no chance of performing a cyclodialysis and
much less of a chance of injuring the large vessels that form such
a rich plexus where the ciliary body attaches to the scleral spur. A second approach to anterior chamber entry and ostomy creation is to use
a sharp blade to make an incision parallel to the iris at the far anterior
extent flap bed, under the flap. Keeping the blade parallel to
iris, entering similar to the creation of the phaco-tunnel, reduces the
risk of damage to the lens and iris. A sclerocorneal punch, such as
a Kelly-Descemet punch, is then introduced parallel to the iris, under
the flap, through the incision. The punch is then rotated vertically
so that it is perpendicular to the bed of the flap. The punch is then
used to make several bites, usually near the temporal side of the flap
bed, to create an ostomy. After each bite the punch is removed and the
excised piece is wiped off with a sponge, and the punch reintroduced. Experience
with the punch and care not to use excessive posterior pressure
allows for the gentle creation of an ostomy of the desired dimensions
with sharp, vertical edges. How much ledge the surgeon wishes to leave and how thin the surgeon wishes
to fashion the scleral flap should have been determined carefully
before surgery. These decisions will depend on the desired postoperative
result (see Table 16). The thinner the flap, the lower the pressure; the smaller the ledge, the
greater the likelihood of postoperative flat anterior chamber. An iridectomy is performed (see Fig. 15I). To ensure that the iris tissue does not plug the guarded filtration
procedure site, the iridectomy should be large enough so that no edge
of the iris is visible through the area of excised sclera. The scleral
flap is sutured in place with 10-0 nylon. The suture is placed at a 45-degree
angle to both the posterior and the radial edges, so that as
the suture is tightened, the scleral flap will return to its proper position. An
additional suture is placed at the other posterior corner (see Fig. 15J). Scleral flap sutures should be ½ to 2/3 thickness through the peripheral ½ mm of the flap. This reduces
full-thickness holes through the flap that may allow excessive leakage. Longer
bites on the scleral side allow easier suture isolation and
viewing for possible postoperative laser suture lysis. Unless it is clear that there has been much scleral contraction and that
the scleral flap has not closed adequately, at this point, the anterior
chamber is filled with balanced salt solution through the previously
placed paracentesis (see Fig. 15K). The surgeon monitors carefully the IOP with a finger placed on the cornea. If
the anterior chamber cannot be formed readily, then additional
sutures are placed until that can be achieved (see Fig. 15L). If the anterior chamber forms readily and the globe becomes firm, the
surgeon tests the area of the procedure with sponges to determine whether
fluid actually can leak through the radial edges (see Fig. 15L). Leakage through the posterior edge of the flap is undesirable because
that portion almost always will scar closed, at which time the procedure
will fail. Leakage, minimal or marked, through the radial incisions
is the surgeon's goal. The amount of leakage is adjusted appropriately
by applying cautery to shrink the edges of the radial incision
or by placing additional sutures. It has not been determined whether the actual amount of leakage of aqueous
humor through the sclera affects the final level of IOP. However, it
certainly affects the immediate postoperative course, and it probably
affects the final IOP. When leakage is marked, so that the anterior
chamber collapses spontaneously, the eye is predisposed to flat anterior
chamber and hypotony, two undesirable postoperative complications. When
there is no leakage, the eye is predisposed to excessive elevation
of IOP postoperatively; although this elevation is an undesirable complication, in
almost all cases, it can be handled by removing a releasable
suture or cutting a laserable suture. We prefer to close the scleral
flap sufficiently tight so that when balanced salt solution is injected
through the paracentesis, the IOP will be raised to approximately 30 to 40 mm
Hg. After the injection is stopped, the IOP ideally should
fall gradually to approximately 10 to 20 mm Hg and remain in that
range. If the scleral flap has been fashioned improperly so that it is too thin
and contains tears, it may not be possible to close the flap tightly
enough to maintain the desired level of IOP. In such cases, a viscoelastic
material may be injected into the anterior chamber. However, we
prefer not to use viscoelastic materials routinely. In practice, this
method rarely is employed but may be used particularly in cases with extremely
high preoperative pressures, requiring higher early postoperative
pressures to reduce the risk of complications. If the surgeon does not believe that sutures will be able to be released
with a laser, externalized releasable sutures should be used (see Fig. 7). If the pupil can be dilated, atropine 1% is instilled at this point and
repeatedly to ensure that it is dilated at the conclusion of the operation. If a fornix-based flap was developed at the start of the guarded filtration
procedure, the corner at the 11 o'clock position is pulled forcibly
counterclockwise so that the cut edge of the conjunctiva completely
covers the area of the scleral flap. This corner is sutured into the
episclera immediately at the limbus, with care taken to avoid the episcleral
blood vessels. A purse-string technique is used to ensure a watertight
closure. A 10-0 nylon suture can be used. Some surgeons prefer
to use an absorbable suture such as 9-0 PDS, but these sutures are more
likely to produce a foreign body sensation in the postoperative period. The
relaxing incision is closed with a running suture so that the
conjunctiva is pulled tightly over the sclera and the incision is watertight. A
running mattress suture also may be used but usually is unnecessary. If
a mattress suture is used, it must be placed so that the
conjunctiva is stretched firmly to prevent leakage. Some surgeons prefer
to close with two “wing-type” sutures, one at either side, placed
just anterior to the limbus with the tissue stretched firmly
between them. Some surgeons pass these sutures twice through the conjunctiva
and cornea to reduce early suture slippage. A purse string may
be used to close any “dog-ears” at the lateral edges. If a limbus-based flap has been performed, the incision is closed in two
layers. Tenon's capsule is sutured to Tenon's capsule, with
care taken to avoid penetrating the conjunctiva. This closure is facilitated
if the surgeon starts at the right-hand side of the incision
and proceeds clockwise, locking the running sutures. Four to six locked
sutures usually are adequate. The suture is exteriorized through the
apex of the conjunctival incision, and the conjunctiva is closed in a
counterclockwise fashion with multiple running sutures. The surgeon should
be careful to approximate the edges of the conjunctiva together
without including the underlying Tenon's capsule. The sutures are
placed approximately 1 mm apart to ensure a watertight closure. We prefer to use a soft suture such as 8-0 Vicryl on a vascular needle. Other
appropriate suture materials are 9-0 Vicryl or 9-0 PDS. Another
option is 10-0 nylon, but this material is technically more difficult
to use, tends to be more difficult to pull up tightly, and predisposes
to leaking incisions. Silk suture should be avoided because this material
predisposes to fistulas. After the conjunctival flap has been closed, a 30-gauge blunt needle on
a bottle of balanced salt solution is placed through the paracentesis
into the anterior chamber, and balanced salt solution is irrigated into
the anterior chamber (see Fig. 15M). As this irrigation is done, the surgeon's index finger should monitor
the firmness of the pressure of the globe. The IOP should not rise
above 40 mm Hg as the irrigation is performed. The IOP, however, should
rise, and if some firmness does not develop in the eye, say a pressure
of 10 mm Hg, additional sutures should be placed. As the saline
is injected, a bleb should develop overlying the scleral flap (see Fig. 15M). This bleb should continue to enlarge as the surgeon continues to irrigate
into the anterior chamber. At the end of the procedure, the eye
should have a well-closed conjunctival flap without extruding Tenon's
capsule and without leakage; a totally formed anterior chamber; an
adequately dilated, round pupil; and a satisfactory conjunctival bleb (see Fig. 15N). If the eye does not look right at the end of the surgery, the result
probably will not be as good as the surgeon and the patient would like. In some cases, subconjunctival betamethasone 3 mg may be injected deep
into the inferior fornix. Caution should be used to ensure that it does
not dissect up underneath the conjunctival flap superiorly. Modifications of Technique The surgeon should alter the technique according to the desired goal (see Table 16). A surgeon who is considering releasing sutures postoperatively by burning
them with a laser, usually will place a 10-0 nylon suture in the middle
of the posterior edge of the scleral flap. This approach will permit
release of the sutures on either corner of the scleral flap with a
laser while maintaining the integrity of the incision and preventing
excessive leakage. In some cases, the surgeon may believe that it will not be feasible to
release sutures with the laser. When the conjunctiva is thick or scarred, or
when there may be subconjunctival bleeding, it may be impossible
to see the sutures clearly enough so that they can be released with
a laser. In some cases, the tissue may be too thick to permit laser cutting, and
releasable sutures are recommended. If the surgeon wishes to
obtain low IOP postoperatively, the guarded filtration procedure can
be changed to a full-thickness procedure by removing all of the sutures
in the sclera flap. If that is the surgeon's goal and there is
a reasonable likelihood that the sutures will not be able to be cut with
a laser postoperatively, then all of the sutures should be placed
in a releasable fashion. Because suture release is often important to
the success of the surgery and because it is not always possible to cut
sutures with a laser, we prefer releasable sutures. One technique for using releasable sutures is shown in Figure 7. The basic principle is to place the suture so that the ends are exteriorized, permitting
easy release postoperatively. USE OF AGENTS THAT ALTER INFLAMMATION AND WOUND HEALING Probably the most significant change in the technique of glaucoma surgery
in the past 10 years has been the introduction of agents that strongly
affect the way wounds heal.28–34,61–76 It has been known for many years that postoperative scarring is one of
the major causes of unsuccessful filtration procedures. Efforts to alter
postoperative inflammation use a variety of techniques, including
corticosteroids and nonsteroidal anti-inflammatory agents, irradiation, and
agents such as heparin. However, the introduction of 5-FU by Heuer
and co-workers30 at the Bascom Palmer Eye Institute represented a major step forward. The
carefully done collaborative study sponsored by the National Institutes
of Health established without doubt that 5-FU administered postoperatively
results in lower IOP by promoting filtration. Since then, many
other agents have been studied, including weaker and less dangerous
substances, such as trifluridine, which was shown by Katz and co-workers74 to produce a small decrease in IOP in comparison with untreated cases, and
more potent agents, such as mitomycin, which was studied by Chen
in Taiwan and Skuta and colleagues76 at the University of Michigan, and others.69,70,75 Indications (see Table 19) This discussion is limited to three agents—corticosteroids, 5-FU, and
mitomycin—not because these are the only agents, or the best
ones, but because they represent a spectrum offering the ophthalmologist
a wide choice and because they are the most extensively studied agents. Nonsteroidal
anti-inflammatory agents applied topically do not
appear to have a beneficial effect on glaucoma filtering procedures and
are not indicated in the postoperative care of patients who undergo
glaucoma surgery. Because 5-FU and mitomycin result in lower IOP in many patients and because
there is current interest in achieving lower IOP than in the past, many
surgeons tend to conclude that 5-FU or mitomycin should be used
in association with most, if not all, glaucoma surgery. Some surgeons
recommend these agents not just in cases in which a less than satisfactory
result is expected, but in all cases, including primary cases. This
practice is, in our opinion, unwise. The lowering of IOP achieved
by 5-FU and mitomycin comes at a cost. The short-term inconvenience or
short-term side effects are less of a concern than the long-term effect
on the eye and, most importantly, the patient. The goal of glaucoma surgery, as discussed earlier, is not to lower IOP
but to help restore the wholeness of the patient. When antimetabolites
result in low IOP, they do so by encouraging filtering blabs that are
thin, ischemic, and highly elevated. Corneoscleral trephines and other
similar full-thickness procedures tended to produce this type of bleb, and
this tendency led surgeons such as Cairns46 and Watson50 to develop alternative methods of surgery. The blebs occur in patients who undergo full-thickness filtration procedures
and in those in whom antimetabolites are used. These blebs predispose
patients to endophthalmitis, approximately 1% per year when the
bleb is located superiorly. In addition, eyes with high, thin blebs leak
aqueous through the bleb, with resulting unpredictably low pressures. The
Seidel test originally was developed to determine whether a bleb
was working properly, that is, leaking aqueous through the conjunctiva. Blebs
that occur after antimetabolite administration often have positive
Seidel test results. This problem predisposes patients to endophthalmitis
and a variety of other problems. Hypotonous eyes have unstable
refraction, which results in disturbing changes in vision. In addition, especially
in younger patients, macular edema develops in a significant
percentage of eyes with IOP of approximately 6 mm Hg or when IOP
is lowered 6 mm Hg or more.27,50–52 This hypotony-macular edema syndrome may occur with IOP as high as 12 mm
Hg, although it usually is limited to eyes with much lower IOP. Among
the most serious problems facing patients who have high, excessively
filtering, thin blebs, and the type of bleb that results from 5-FU and
mitomycin administration are photophobia and discomfort. For example, the
pressure in the eye is 6 mm Hg, and, although the surgeon may be
delighted, the patient is miserable. These problems are especially common
when the filtering blebs are not underneath the upper lid. In such
cases, the development of a dellen adjacent to the bleb is routine
and usually is associated with significant disability. Surgeons who use antimetabolites in glaucoma surgery also should discuss
the potential risks with the operating room and nursing committees in
their institutions. At the Wills Eye Hospital, these agents are treated
in the same manner as any carcinogen: Any material with which they
come in contact is handled as if it is radioactively contaminated and
is discarded in containers designed for radioactively contaminated materials. Antimetabolites The more potent the antifibrosis agent, the greater the potential for both
help and harm.27,28 Although 5-FU is less effective than mitomycin in developing successful
filtering blebs, the long-term side effects of 5-FU, both ocular and
systemic, also are less serious than those of mitomycin. Similarly, 5-FU
is more effective and has fewer side effects than topical corticosteroids. Consequently, there is a theoretic justification in matching
the type and dose of antimetabolites (wound healing suppressants) with
the anticipated likelihood of failure of the procedure. The greater the
likelihood of failure, the more potent the agent to be used. Tables 17 and 18 list some of the factors that predispose to success and to failure. The optimal dose has not been established with certainty. These considerations
are covered more fully under the discussion of each agent. Topical Corticosteroids The use of topical corticosteroids is an essential part of almost all glaucoma
surgery. Inflammation must be prevented as much as possible in
all glaucoma surgery, whether it is laser iridectomy or a Molteno shunt
procedure. The dose-response relationships of topical steroids have
not been studied extensively in glaucoma surgery, but there is little
evidence that administration of topical corticosteroids in doses that
exceed the equivalent of prednisolone acetate 0.1% four times daily is
significantly beneficial. The response appears to be biphasic. When
inflammation appears to be significant, or the predisposition to inflammation
is great, administration of corticosteroid eye drops as frequently
as every hour may be indicated for several days. However, the propensity
of eyes with open-angle glaucoma, as well as several other types
of glaucoma, to have a steroid-induced rise in IOP should be recalled, and
the complicating nature of this rise in pressure on the postoperative
course kept in mind. In addition, topical corticosteroids can
produce cataract. The effect of fluorometholone and some of the newer congeners of fluorometholone, which
appear to have less propensity to cause elevation of
IOP, have not been studied adequately. They may offer a theoretical advantage, but
the current drugs of choice are dexamethasone phosphate 0.1%, prednisolone
phosphate 1%, or prednisolone acetate 1%. A satisfactory
routine for most patients who have undergone uncomplicated filtration
procedures is prednisolone acetate 1% four times daily for 2 weeks, three
times daily for 1 week, twice daily for 1 week, and then once
daily for 1 week (Table 22). Table 22. Typical Postoperative Schedule*
| Treatment | Daily Frequency | Postoperative Period Employed |
| Medication |
| Prednisolone acetate 1% | 8 | Day 1 |
| | 4 | Days 2–7 |
| | 3 | Days 8–14 |
| | 2 | Days 15–21 |
| | 1 | Days 22–28 |
| Atropine 1% | 4 | Days 1–7 |
| | 2 | Days 8–14 |
| Short-acting cycloplegic† | Once at bedtime | Days 15–28 |
| Phenylephrine 2.5% | 4 | Days 1–4 |
| Broad-spectrum antibiotic | 4 | Days 1–7 |
| Eye patch | | Remove by 4–24 hours |
| Eye shield | | Remove by 4–24 hours, then wear at bedtime for 1 month |
| Instruction |
| Activity of patient | | No strain until intraocular pressure is above 6 mm, then no limitation |
| Evaluation |
| Amount of discomfort‡ | | Days 1–3, mild ache |
| Visual acuity measurement | | Days 1–3, severe blur |
| | | Days 3–7, moderate blur |
| | | Days 8–28, trouble reading |
| Medical evaluation§ | | Day 1 |
| | | Day 2 |
| | | Days 5–10 |
| | | Days 25–35 |
| | | Days 50–60 |
| Repeat visual field examination* | | 3 months |
| | | 12 months |
*This schedule is a guideline only. Actual treatment will vary in relation
to many factors, such as access to care, state of the eye, and general
health.
†For example, tropicamide or cyclopentolate.
‡Onset of severe pain is an indication of a complication.
§Examination should include estimate of anterior chamber depth, visual
acuity, and ophthalmoscopy.
Subconjunctival corticosteroids may cause subconjunctival bleeding. Their
routine use can be neither recommended nor discouraged because they
have not been studied adequately. They appear unnecessary for routine
cases. However, repository steroids are contraindicated because they
may cause an intractable elevation of IOP, requiring surgical removal
of the steroid. Some glaucoma surgeons have used systemic corticosteroids for many years. These
agents have significant systemic risks, and their use in routine
cases cannot be recommended on the basis of clinical studies. We use
oral steroids in our surgical patients extremely rarely. 5-Fluorouracil The optimal schedule for using 5-FU has not been determined. Furthermore, although
it is apparent that the agent works more effectively in some
patients than in others, it is not known how to identify patients in
whom 5-FU has its greatest effect. It is reasonable to assume that the
patients with the greatest likelihood of surgical failure are those
in whom the drug must be used most vigorously. Related factors are listed
in Table 19. There are two major methods of application: intraoperative and postoperative. INTRAOPERATIVE USE. Major advantages of the intraoperative use of 5-FU include its ease of
administration, the avoidance of postoperative injections,62,65,66,81 and a significant decrease in side effects, especially corneal side effects, that
are associated with postoperative injections. The main disadvantage
is the increased seriousness of a complication, such as a leaking
conjunctiva. A limbus-based flap should be used when 5-FU is given intraoperatively, because
it is difficult to close fornix-based flaps in a way that ensures
that they will not leak. The incision in the conjunctiva should be
made as high in the superior cul-de-sac as possible, over the insertion
of the superior rectus muscle. Conjunctival incisions that are made
preparatory to limbus-based flap procedures for any type of filtration
procedure should be placed in this area. When an antimetabolite is
not used, the surgeon has a greater margin of error because of the tendency
of the eye to heal itself. The surgeon does not have this luxury
when using an antimetabolite. All personnel who use 5-FU or mitomycin
should wear disposable gloves and other appropriate equipment. A fluid-retaining sponge, such as a Weck-cell sponge, is fashioned to be
approximately 3 × 3 mm in length and width, and about 0.5 mm in
depth. The sponge is soaked in 0.1 to 0.2 ml of 5-FU that has been withdrawn
directly from an undiluted vial of 5-FU. The 5-FU concentration
should be 50 mg/ml. The sponge is placed on the sclera over the area
of the scleral flap. The sponge is left in position for 5 minutes. The
conjunctiva is pulled over the sponge so that the Tenon's capsule
side of the conjunctiva is in contact with the sponge impregnated
with the 5-FU. The sponge is removed with a forceps that can be passed
off of the tray, and the sponge is discarded in a container designed
for disposal of toxic material. Fluffs are placed around the eye to absorb
the irrigating solution, and the eye is irrigated with 5 to 15 ml
of balanced salt solution. The fluffs and other contaminated materials
are discarded in the special container. The nurse dispensing the 5-FU
and the assistant picking up the fluffs wear disposable gloves before
irrigation is done. After the 5-FU has been irrigated and the eye cleaned
completely, the disposable gloves are discarded, and the procedure
continues. Some surgeons prefer to make the radial and posterior scleral grooves first, and
to develop the scleral flap before applying the 5-FU. This approach
has the advantage of delivering a more concentrated dose of 5-FU
deep into the sclera. Its disadvantage is that the material cannot
be used if the flap is made improperly so that the anterior chamber is
entered. The scleral flap usually is fashioned so that it is at least
one third of the thickness of the sclera. Thinner flaps tend to leak
excessively, predisposing patients to unwanted flat anterior chambers. It
is inadvisable to use 5-FU if there is a possibility that the agent
can get into the anterior chamber. Releasable or laserable sutures, or both, can be employed. However, the
time at which sutures are cut differs from the suture cutting time when
an antimetabolite is not used. The effect of suture cutting in eyes
that have been treated with 5-FU tends to be more dramatic than in cases
in which the antimetabolite is not used. Furthermore, it is possible
to cut the sutures as late as 1 month postoperatively and still have
a significant effect. This situation never occurs in patients who have
not been treated with antimetabolites and whose sutures must be cut
within 10 days or, in some cases, as early as 5 days, if cutting the
sutures is to have a significant effect. The amount of flow through the
scleral flap is checked carefully, as it is in all patients who undergo
guarded filtration procedures. However, here, it is doubly important. If
the anterior chamber cannot be reformed easily when balanced salt
solution is injected through a paracentesis track into the anterior
chamber, additional sutures are placed until the goal can be achieved. At the end of the operation, as with all filtering procedures, balanced
salt solution is injected through the paracentesis track. The injection
is continued until the bleb becomes greatly elevated, approximately 180 degrees
superiorly. After the bleb is elevated, sponges are used
to test the incision and inspect the conjunctiva to ensure that there
is no leakage. If the surgeon is not completely confident about the surgical
technique or is relatively inexperienced in using 5-FU or performing
guarded filtration procedures with antimetabolites, it is best to
paint the conjunctiva and incision with fluorescein and examine the
patient's eye under a blue light to determine whether there is any
leak age. Leakage must be corrected (see discussion of complications). An additional technique, used now by some of us, is to raise a bleb inferotemporally
about 3 to 5 mm posterior to the limbus with a highly cohesive
viscoelastic material. The 5-FU is then placed by inserting the
no. 30 needle on the syringe containing 0.3 ml 5-FU through the bleb
of viscoelastic and advancing the needle cautiously towards the nasal
aspect of the globe, injecting the 5-FU slowly. This develops an area
of conjunctival elevation in front of the needle tip, helping avoid penetrating
the conjunctiva. The remaining contents of the syringe are then
injected, making a bleb inferonasally. POSTOPERATIVE USE. Initially 5-FU was used as an adjunct to glaucoma surgery by subconjunctival
administration during the postoperative period.28,30–34,63,64,68,71–73 The initial dose increased the success rate significantly, but it was
associated with significant side effects, most of which were related to
the cornea. Attention to the method of administration can reduce the
side effects dramatically. Using a 30-gauge needle and making sure that
it is introduced for a distance of at least 1 cm is important; after
the injection, the eye is irrigated copiously. In addition, in many
cases, fewer injections are needed than were used in the initial study. Before administering 5-FU, disposable gloves are placed on both hands. The 5-FU
is drawn directly from the vial of 5-FU as it comes from the
pharmacy. The vial contains 10 ml of 5-FU 50 mg/ml. The conjunctiva is
anesthetized by multiple instillations of topical anesthetic or, preferably, by
the placement of a pledget of anesthetic that remains in the
inferior cul-de-sac for 5 minutes. The 5-FU 0.1 ml is drawn directly
from the vial into a tuberculin syringe. The needle is removed and replaced
with a short, sharp, disposable 30-gauge needle. With the patient
seated comfortably at the slit lamp, and the eye anesthetized, the
needle is placed in the subconjunctival tissue, usually in the inferior
cul-de-sac, and advanced so that the tip is at least 1 cm away from
the point of entry. The further the needle can be introduced under the
conjunctiva, the more likely it is that the 5-FU will not leak out from
the needle puncture site. This leakage from the needle puncture site
is the major cause for corneal complications that are related to the
use of 5-FU when it is given by injection. The frequency of injection of 5-FU varies with the need to suppress postoperative
inflammatory response. The patient is examined carefully after
the surgery to verify that there is no buttonhole, tear in the conjunctiva, or
other contraindication to the use of the 5-FU. If there is
no contraindication, then the injections may be given on the first postoperative
day and then for a total of 5 to 10 injections. One satisfactory
routine is to give the injections every other day for 2 weeks, although
some prefer to give them daily for 1 week. It is not known which
routine works best. If corneal decompensation occurs, the injections
usually are stopped until the cornea has cleared, although the corneal
changes are not an absolute contraindication to the continuing use
of the 5-FU. Mitomycin Mitomycin is given intraoperatively in the same manner as 5-FU.34,75,76,82–84 The major differences involve dose and time of administration. Because
they are the group most likely to have hypotonus maculopathy, mitomycin
is used with particular caution in young myopes. However, when the
surgeon is convinced that a filtration procedure is the optimal approach, and
a good limbus-based conjunctival flap can be developed, mitomycin
may be appropriate. However, its ability to cause serious ocular damage
and its uncertain intraocular effects must be kept in mind.77–80 We perform a limbus-based flap when using mitomycin. All personnel using mitomycin should double glove. Concentrations of 0.2 to 0.5 mg/ml
have been used. When a concentration of 0.3 mg/ml is used, we
usually leave the sponge on the sclera for 2 to 4 minutes. When
a concentration of 0.2 ml is used, some surgeons leave the sponge in
place for a longer period (4 to 5 minutes). The sponge is placed over
the area where the scleral flap for the guarded filtration procedure is
to be prepared, although some surgeons prefer to develop the flap before
applying the mitomycin. The conjunctiva is pulled back over the sponge
so that the surface adjacent to the globe is in contact with the
mitomycin. The tissue is held so that the cut edge of the conjunctiva-Tenon's
flap does not come in contact with the mitomycin. The sponge
is held in place for 1 to 4 minutes. After the desired time, the
sponge is removed and discarded in a special container for toxic waste. The
eye is irrigated with 15 to 30 ml of balanced salt solution. Some
surgeons prefer to use larger volumes. The irrigation fluid is collected
carefully on fluffs, and these are discarded in the toxic waste container. The
drapes are drained of all residual fluid as well. Postoperative Care After a guarded filtration procedure, the pupil should be kept adequately
dilated, inflammation minimized, and the eye protected from direct
internal or external pressure. One regimen is atropine 1% four times daily
and a potent corticosteroid, such as prednisolone acetate 1%, four
times daily. A typical postoperative schedule is shown in Table 22. The goal is to minimize inflammation, check for infection and other complications, and
maintain the state of the eye, including IOP, in a condition
that is expected to ensure long-term success. If the scleral flap has been difficult to close or if there is concern
about excessive filtration or leakage from the conjunctiva at the end
of the procedure, a light patch may be left in place for approximately 24 hours
before the first dressing is applied. At the first postoperative evaluation, the surgeon asks the patient about
pain and its nature. The eye may ache slightly, but severe pain usually
is an indication of a complication. The sudden, immediate onset of
excruciating pain, sometimes after coughing, sneezing, or straining
at stool, almost always is pathognomonic of an expulsive, suprachoroidal
hemorrhage. More gradual development of severe, deep pain may signal
an endophthalmitis. Rapidly rising IOP also can cause pain of varying
degree and severity. Also included in the postoperative evaluation should be an estimate of
acuity, IOP, nature of the bleb, character of the anterior chamber (depth
and reaction), shape of the iris, size of the pupil, and funduscopy. Some
postoperative complications have specific findings, such as the
sudden onset of excruciating pain that characterizes a suprachoroidal
expulsive hemorrhage or the pooling of aqueous humor behind the lens
or vitreous face in malignant glaucoma (aqueous misdirection syndrome). However, in
most cases, the combination of the level of IOP, the appearance
of the bleb, and the depth of the anterior chamber gives the
most valuable information. If IOP is high, the bleb is flat, and the chamber is deep, it is clear
that adequate filtration is not occurring, and the CTM should be applied.58 If the CTM chamber is excessively shallow, the eye is soft, and the bleb
is extremely high, excessive filtration probably is occurring. The
surgeon can apply cycloplegic agents in the maximum tolerated dosage, including
repeat instillations of atropine and phenylephrine. The four major factors that we watch postoperatively are the height of
the bleb, the level of IOP, the depth of the anterior chamber, and the
size of the pupil. These factors usually make it possible to determine
whether there is pupillary block, aqueous misdirection, excessive filtration, or
inadequate filtration and to allow the surgeon to take the
appropriate steps (Table 23). Table 23. Diagnosis of Postoperative Complications of Glaucoma Filtering
Surgery
| Condition | Intraocular Pressure | Bleb | Anterior Chamber | Other Findings | Time of Appearance |
| No filtration | High | None | Deep | May have pain | Day 1–3 |
| Inadequate filtration | High | Low | Deep | May have mild pain | Day 1–7 |
| Excessive filtration | Less than 10 mm Hg | High | Normal, flat | Negative Seidel's test result | Day 1–4 |
| Inadequate aqueous filtration | Less than 5 mm Hg | Low and getting lower | Shallow, flat (I–III) until sclerostomy closes, than deep | Inflamed eye | Day 2–7 |
| Leaking conjunctiva with excess filtration | 0–10 mm Hg | Low | Shallow, flat | Positive Seidel's test result | Day 1–7 |
| Excessive filtration with developing inadequate aqueous production | Less than 5 mm Hg and falling | Low or none | Flattening | Increasingly inflamed eye | Day 3–7 |
| Aqueous misdirection syndrome (malignant glaucoma) | Usually high | None | Shallow | Fluorescein pools behind vitreous face | Day 1–7 |
| Ciliary body detachment | 0–10 mm Hg | None to high | Shallow, flat | Detachment may be visible ophthalmoscopically, acuity may be reduced focally | Day 1–10 |
| Pupillary block | | | | | |
| Early in association with excess filtration | 5–15 mm Hg | Moderate | Shallow | Posterior synechiae | Day 1–7 |
| In association with inadequate aqueous production | 0–10 mm Hg | None | Shallow, flat | Posterior synechiae | Day 4–10 |
| After closure of sclerostomy | High | None | Shallow or normal | Posterior synechiae | Day 14-∞ |
| Suprachoroidal expulsive hemorrhage | 5–60 mm Hg | High | Flat, normal | Sudden onset of severe pain, acuity poor | Day 1–10 |
| Encapsulated bleb | 20–40 mm Hg | Dense, dome shaped, high | Deep | Usually comfortable | Day 14–30 |
| Failure of filtration | High | Low or absent | Deep | Gradual onset of pain | Day 20–late |
| Endophthalmitis | Usually elevated | Varies, may be blebitis | Deep, inflamed | Vitreous hazy, acuity poor | Day 1–4 or late |
| Blebitis | Varies | Milky, with pseudohypopyan | Deep, quiet | No pain, clear vitreous | Late | Once the anterior chamber is maintaining a well-formed condition, long-acting
cycloplegic agents, such as atropine, can be discontinued. As soon as the intraocular inflammation begins to resolve, the topical
steroids can be tapered, and, in most cases, they should be discontinued
within 3 weeks of surgery. Longer usage predisposes patients to cataract
and steroid-induced glaucoma. Systemic steroids are used by some
surgeons, and they may increase the success rate arid tend to produce
an eye with a thin, polycystic bleb and a low final IOP. The complications
of systemic steroids, however, are significant, and must be taken
into account. One of the most helpful maneuvers after a guarded filtration procedure
is the CTM,58 which is shown in Figure 16. This technique of applying pressure in a localized fashion directly over
the radial edge of the scleral flap is appropriate for guarded filtration
procedures that are performed so that the scleral flap overlies
a small edge of underlying cornea and sclera. Localized pressure at
the radial edge of the flap deforms the edge, disrupting the union between
the overlying scleral flap and the underlying corneosclera. Thus, gentle
pressure will encourage aqueous to flow through the radial edge
of the scleral flap unlike the technique that has been used for generations
to encourage aqueous to flow out of a sclerostomy.54 This technique involves pressing oh the globe, which increases the IOP
and causes the underlying sclera and cornea to be pushed up against the
overlying scleral flap, making the apposition between these two tissues
even tighter. Thus, this technique does not work well in patients
who have had a guarded filtration procedure and may be more likely to
force iris into the internal ostomy opening. 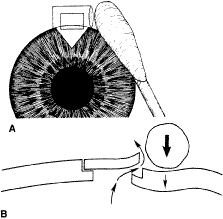 Fig. 16. The Carlo Traverso maneuver. Shortly after a guarded filtration procedure, the
pressure may become elevated. Usually, this elevation is due to
failure of filtration through the sclerostomy. This elevation can be
corrected easily by depressing the sclera just temporal to the tempororadial
groove or nasal to the nasoradial groove. This depression should
be gentle and focal, and a sterile applicator should be used. This
maneuver can be repeated many times during the first 2 weeks postoperatively. A. Anterior view. B. Cross section of superior view. Fig. 16. The Carlo Traverso maneuver. Shortly after a guarded filtration procedure, the
pressure may become elevated. Usually, this elevation is due to
failure of filtration through the sclerostomy. This elevation can be
corrected easily by depressing the sclera just temporal to the tempororadial
groove or nasal to the nasoradial groove. This depression should
be gentle and focal, and a sterile applicator should be used. This
maneuver can be repeated many times during the first 2 weeks postoperatively. A. Anterior view. B. Cross section of superior view.
|
The CTM is performed by anesthetizing the eye well with several instillations
of proparacaine 0.5%.58 A sterile, cotton-tipped applicator is soaked with the anesthetic agent. With
the patient observed at the slit lamp, the upper lid is lifted, the
patient is asked to look down, and gentle pressure is applied with
the tip of the applicator directly over one of the radial grooves of
the scleral flap. This maneuver should be associated with immediate
shallowing of the anterior chamber, development of a high bleb, and fall
of IOP. If these changes do not occur immediately, the applicator is
placed over the other radial incision, and pressure applied. It may
be necessary to try several locations until the spot is found where pressure
will cause deformation of the incision arid result in encouraging
filtration. The CTM should be done gently. In our hands, it has not been associated
with complications. It may be repeated three or four times daily for
the first 3 or 4 days to encourage the development of a patent fistula. After 2 weeks
or so, it probably will not have a permanent effect on
the outcome of surgery. We often use the CTM in the early postoperative period. However, we do
not believe in long-term continued use of massage or pressure of any kind. In
our opinion, this pressure is traumatizing to the eye and rarely
results in an improvement in the long-term result. When CTM does not re-establish satisfactory filtration or when the IOP
remains higher than the surgeon has hoped and filtration is inadequate, it
often is helpful to release one of the 10-0 nylon sutures in the
scleral flap by burning the suture transconjunctivally with an argon laser. A
small lens is used to flatten the conjunctiva. We use the lens
designed by Hoskins.54 The edge of the Zeiss four-mirror lens also may be used. The Hoskins lens
compresses the conjunctiva, making a suture in the sclera easily visible. The
suture can be burned with a spot size of 50 μm, an exposure
time of 0.1 second, and a power of 300 to 700 mW, using the blue
or green light of the argon laser. Depending on the laser and the surgeon's
technique, more or less power may be necessary. In most cases, one
or two applications with a laser will burn through the suture, allowing
it to retract and releasing traction on the incision. In many
instances, a bleb develops immediately, and IOP falls. Several sutures
may need to be released to obtain adequate filtration. As mentioned
earlier, when the surgeon plans to release sutures with a laser, a 10-0 nylon
suture can be placed in the middle of the posterior edge of
the flap so that there is no excessive filtration when sutures are released. In
the presence of subconjunctival blood, there is a significant
risk of conjunctival burns or holes. Use of the red krypton laser reduces
absorption by blood and increases the success of suture lysis in
the presence of subconjunctival blood. The management of complications
is covered in the third section of this chapter. TRABECULOTOMY Trabeculotomy is useful for managing congenital glaucoma where Schlemm's
canal can be identified. Although goniotomy is the procedure of
choice in infants or children with congenital glaucoma in whom the cornea
is sufficiently clear to allow the surgeon to see into the anterior
chamber angle, trabeculotomy is a satisfactory second choice, and it
is the preferred procedure where the cornea is hazy. It is of value
in the secondary glaucomas as well, especially the open-angle inflammatory
glaucomas, such as those seen in association with Still's disease. The procedure demands high magnification; it is best done with an operating
microscope. The initial portion of the operation is virtually identical to a guarded
filtration procedure (Fig. 17; see also Fig. 15). A fornix-based or limbus-based conjunctival flap is developed at the 12 o'clock
position. A scleral flap approximately 4 × 4 mm is fashioned, hinged
at the limbus (see Fig. 13A). This flap, however, differs from a guarded filtration procedure flap
in that it should be thicker; whereas a trabeculotomy flap ordinarily
is one third to one fourth the thickness of the sclera, when performing
a trabeculotomy, the scleral flap should be two thirds the thickness
of the sclera. This difference in approach facilitates the identification
of Schlemm's canal. Because leakage of aqueous through the
sclera is not the mechanism by which trabeculotomy works, a thicker flap
does not predispose the patient to failure of the procedure. 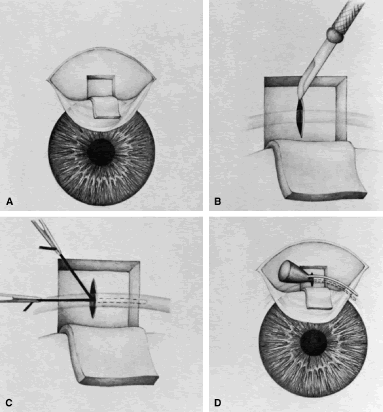 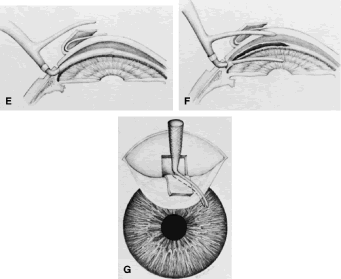 Fig. 17. Trabeculotomy. A. After development of a limbus-based conjunctival flap, a small scleral
flap approximately two thirds of the thickness of the sclera is made. B. The underlying sclera is incised, with care taken to watch for leakage
of aqueous and search for Schlemm'canal. C. A suture is threaded into Schlemm's canal, and its position is verified. D. The McPherson's trabeculotome is threaded into Schlemm's canal. E. Gonioscopic view showing the trabeculotome in the proper position. F. With the plane of the trabeculotome parallel to the iris, the handle is
rotated so that the tip is torn through the trabecula into the anterior
chamber. G. After the tip has entered the anterior chamber, the trabeculotome is withdrawn
and the chamber reformed. (Spaeth GL. Glaucoma surgery. In Spaeth GL (ed). Ophthalmic Surgery: Principles
and Practice. Philadelphia: WB Saunders, 1990.) Fig. 17. Trabeculotomy. A. After development of a limbus-based conjunctival flap, a small scleral
flap approximately two thirds of the thickness of the sclera is made. B. The underlying sclera is incised, with care taken to watch for leakage
of aqueous and search for Schlemm'canal. C. A suture is threaded into Schlemm's canal, and its position is verified. D. The McPherson's trabeculotome is threaded into Schlemm's canal. E. Gonioscopic view showing the trabeculotome in the proper position. F. With the plane of the trabeculotome parallel to the iris, the handle is
rotated so that the tip is torn through the trabecula into the anterior
chamber. G. After the tip has entered the anterior chamber, the trabeculotome is withdrawn
and the chamber reformed. (Spaeth GL. Glaucoma surgery. In Spaeth GL (ed). Ophthalmic Surgery: Principles
and Practice. Philadelphia: WB Saunders, 1990.)
|
A paracentesis track should be placed before performing the trabeculotomy (see Fig. 5). This maneuver permits the flushing of excess blood from the chamber, either
at the time of initial surgery or later. A paracentesis also provides
a useful method of testing the tightness of the closure of the
scleral flap. Once the flap has been developed, the microscope magnification is increased, and
the region straddling the transition between sclera and cornea
is inspected carefully. In many cases, a thin gray line just at the
transition between white sclera and clear cornea can be visualized; this
line indicates the area of Schlemm's canal. A radial cut, placed
in the center of the bed and extending about 1 mm into clear cornea
and 1 mm into opaque sclera, is placed, using a blade such as a no. 67 disposable
Beaver knife (see Fig. 17B). This incision is made very delicately and superficially. After each
partial incision, the tissue is pushed to the side by the blade, and the
depth of the incision is inspected carefully as the surgeon searches
for Schlemm's canal. When the canal is opened, there usually is
seepage of aqueous humor out of the cut wall. Once the canal has been identified, a firm dark suture such as 5-0 nylon
is threaded carefully into the canal for 5 to 10 mm (see Fig. 17C). The visible portion of the suture extending out from the canal is pulled
posteriorly away from the limbus and released. If the suture is within
Schlemm's canal, when it is released, it will spring back into
its original position, parallel with Schlemm's canal. If the
suture has penetrated Schlemm's canal and is in the anterior chamber, it
will not spring back when released. Once the canal has been identified, a Harm's or McPherson's trabeculotome
is threaded into the canal for at least 5 mm and, if possible, for
the complete length of the trabeculotome blade (see Fig. 17D and E). There should be minimal resistance as the trabeculotome is advanced. If
the surgeon must press forcibly, it is virtually certain that the
probe is not in the canal. After the probe has been introduced as far as possible into Schlemm's
canal, the handle of the probe is twisted between the fingers so that
the blade rotates into the anterior chamber (see Fig. 17F and G). As it does, it tears an opening through the trabecular meshwork. The
blade should be rotated so that it does not scrape along the corneal
endothelium or tear Descemet's membrane. The surgeon feels the resistance
of the internal wall of the trabecular meshwork. If the resistance
is great, making forward advance of the probe difficult, the blade
probably is intrascleral and not in Schlemm's canal. Tearing open
the canal almost always is accompanied by mild bleeding. Once the trabeculotomy has been performed in one direction, the blade is
withdrawn, and its fellow probe is inserted in the opposite direction, in
the other side of the cut edge of Schlemm's canal. A similar
trabeculotomy is performed. No iridectomy is necessary. An alternative method of performing trabeculotomy is to use a 5-0 proline
suture threaded into Schlemm's canal. The initial stages of the
procedure are performed as described. A 5-0 proline suture, approximately 3 in. long, is
prepared by lightly heating one end with a cautery, causing
the end to develop a rounded, umbrella shape. The opening of
Schlemm's canal is identified with certainty, as described earlier. The
rounded end of the proline suture is then threaded into the cut
end of Schlemm's canal and gradually, cautiously advanced. In
most cases it is possible to advance 360 degrees, at which point the rounded
end will exit from the other cut end of Schlemm's canal. At
that point the right end is pulled towards the left, and the left end
pulled towards the right, so that the thread is pulled taut, tearing
open Schlemm's canal 360 degrees. In some cases the thread cannot
be advanced the full circumference. In such a case a similar scleral
flap can be developed 180 degrees from the first scleral flap, and the
cut end of the suture retrieved at that point. This can then be pulled
taut in a similar fashion, performing a 180-degree trabeculotomy, or
a second suture can be introduced at the superior cut edge of Schlemm's
canal and run through Schlemm's canal down to the new opening, permitting
a 360-degree trabeculotomy. This method of performing
a trabeculotomy is our preference. The anterior chamber is irrigated through the previously placed paracentesis
with balanced salt solution to remove any excess blood. The scleral
flap is closed with two 10-0 nylon sutures at each posterior corner
of the flap. The chamber is filled with balanced salt solution through
the paracentesis track, raising the pressure in the eye to 25 to 40 mm
Hg. The high IOP pressure serves several purposes: (1) it tests the
adequacy of closure of the scleral flap; (2) it helps to prevent choroidal
effusion or hemorrhage; and (3) it prevents retrograde bleeding
from the episcleral veins into Schlemm's canal, and thence into
the anterior chamber. Postoperatively, anti-inflammatory agents are appropriate; prednisolone
acetate 1% four times daily for 1 to 3 weeks usually is adequate. Atropine
is not employed. Some authors advocate weak pilocarpine for a few
weeks. CYCLODIALYSIS In the past, cyclodialysis was a widely used glaucoma procedure. However, it
has fallen out of favor. Nevertheless, it has a relatively satisfactory
risk/benefit ratio, and ophthalmologists performing surgery on
patients with glaucoma should know how to perform the procedure so that
they can employ it when indicated. The major disadvantage of cyclodialysis is its low success rate. Even in
the most favorable cases, cyclodialysis results in a permanent cleft
and in success in fewer than 50% of patients. When it works, it tends
to be very long lasting in its effect and to result in a low IOP, often 8 to 15 mm
Hg. An additional problem that has limited its use is its
tendency to produce cataract in phakic patients. We believe that this
complication may have been exaggerated; with careful microsurgical technique, such
a complication is rare. Because of the concern regarding
the development of cataract, most surgeons have limited cyclodialysis
to aphakic patients, especially those who have had intracapsular cataract
extraction. Unfortunately, these patients are not the best candidates
for cyclodialysis, because traction by the zonules on the ciliary
body may be of some help in maintaining an open cleft. Indications The classic indication for cyclodialysis is noninflammatory open-angle
glaucoma in an aphakic or pseudophakic patient. However, the procedure
also may be used with caution in phakic patients. Cyclodialysis is not likely to succeed in any of the secondary glaucomas, including
secondary angle-closure glaucoma. Furthermore, there seems
to be a tendency for it to work better in the deep-chambered rather
than the shallow-chambered eye. No special preoperative care is required. The surgeon must be especially
careful to ask the patient about the use of medications that predispose
to bleeding (such as aspirin and dipyridamole) or any other systemic
conditions that may make bleeding more likely. If possible, such proclivities
to bleeding should be corrected before surgery. The patient
should undergo gonioscopy preoperatively. The surgeon should avoid vascularized
areas and, if possible, peripheral anterior synechiae. Operative Technique A facial nerve block arid a retrobulbar block with a short-acting agent, such
as mepivacaine, provide adequate anesthesia. The site where the
cyclodialysis is to be performed should be determined preoperatively
at the time of gonioscopy. If there is no contraindication, the most propitious
position is supertemporally; the superior position is favored
because blood is less likely to occlude clefts developed superiorly, and
the temporal approach is preferred because it is easier. The conjunctiva
is incised approximately 5 mm posterior to the limbus. The incision
should be long enough to permit cleaning an area of sclera approximately 4 × 4 mm, with its center 4 to 5 mm posterior to the limbus (Fig. 18). The episclera is cleaned from the sclera. Cautery is applied to the
sclera to make an oval ring approximately 5 mm long in a circumferential
direction and 2 mm radially (see Fig. 18). The sclera is incised deeply but not penetrated. The incision should
be approximately 4 mm long and should stay within the cauterized area. If
more bleeding occurs, cautery is applied meticulously to ensure that
there is no bleeding whatsoever. A paracentesis track is developed, using
a short, sharp 25-gauge disposable needle (see Figs. 1 and 18). There is some advantage in placing this track directly opposite the
area where the cyclodialysis will be performed. Thus, if the cleft is
to be developed at the 11 o'clock position, the paracentesis should be
performed at the 5 o'clock position. This placement permits easier flushing
of blood out of the cyclodialysis cleft and may allow the surgeon
to open the cleft directly at the end of the procedure or later. The
patency of the paracentesis track is tested with a blunt 30-gauge needle
attached to a 2-ml syringe filled with air. 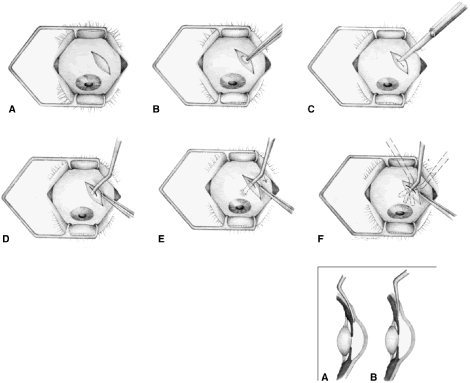 Fig. 18. Cyclodialysis. A. An incision is made in the conjunctiva approximately 5 to 6 mm posterior
to the limbus. B. Cautery is applied in the shape of an oval doughnut 5 mm long and 3 mm
wide. C. An incision 4 mm long is made in the center of the oval. A traction suture
may be placed. D. The tip of a cyclodialysis spatula is introduced into the space between
the choroid and the sclera. E. As the tip is moved anteriorly, the heel of the spatula is depressed, and
the tip is lifted up toward the microscope. A small bulge in the sclera
results from the firm pressure employed. Inset. A. As soon as the tip is seen in the anterior chamber, it is retracted slightly
and depressed posteriorly to avoid tearing Descemet's membrane. Inset. B. After the tip is in the anterior chamber, it is advanced anteriorly in
a plane parallel to the iris. F. The spatula is swept to both sides. The pupil is observed carefully as
the tip is moved posteriorly. The anterior ciliary vessels are meticulously
avoided. Not shown is a keratostomy; this procedure is essential
before the cyclodialysis can be performed. Keratostomy should be made 180-degrees
opposite from the intended site of the cyclodialysis to
permit irrigation of fluid into the cyclodialysis cleft. Air should be
injected immediately after cyclodialysis to raise the intraocular pressure
to approximately 30 mm Hg to keep the cleft well open and tamponade
any bleeding. (Spaeth GL. Glaucoma surgery. In Spaeth GL (ed). Ophthalmic Surgery: Principles
and Practice. Philadelphia: WB Saunders, 1990.) Fig. 18. Cyclodialysis. A. An incision is made in the conjunctiva approximately 5 to 6 mm posterior
to the limbus. B. Cautery is applied in the shape of an oval doughnut 5 mm long and 3 mm
wide. C. An incision 4 mm long is made in the center of the oval. A traction suture
may be placed. D. The tip of a cyclodialysis spatula is introduced into the space between
the choroid and the sclera. E. As the tip is moved anteriorly, the heel of the spatula is depressed, and
the tip is lifted up toward the microscope. A small bulge in the sclera
results from the firm pressure employed. Inset. A. As soon as the tip is seen in the anterior chamber, it is retracted slightly
and depressed posteriorly to avoid tearing Descemet's membrane. Inset. B. After the tip is in the anterior chamber, it is advanced anteriorly in
a plane parallel to the iris. F. The spatula is swept to both sides. The pupil is observed carefully as
the tip is moved posteriorly. The anterior ciliary vessels are meticulously
avoided. Not shown is a keratostomy; this procedure is essential
before the cyclodialysis can be performed. Keratostomy should be made 180-degrees
opposite from the intended site of the cyclodialysis to
permit irrigation of fluid into the cyclodialysis cleft. Air should be
injected immediately after cyclodialysis to raise the intraocular pressure
to approximately 30 mm Hg to keep the cleft well open and tamponade
any bleeding. (Spaeth GL. Glaucoma surgery. In Spaeth GL (ed). Ophthalmic Surgery: Principles
and Practice. Philadelphia: WB Saunders, 1990.)
|
The incision through the sclera is completed with a curved, dull instrument, such
as a Beaver no. 67 blade. It is important to ensure that the
incision is made completely through the sclera; when thinned markedly, the
sclera is transparent, and the inexperienced surgeon may be misled
by the black color, concluding incorrectly that the incision is deep
enough. It is helpful to extract both edges of the incision, allowing
the textured choroid to prolapse slightly up into the incision. The anterior edge of the incision is grasped with a forceps that will allow
excellent fixation, such as a 0.12 Castroviejo or a Bonn forceps. A
Barraquer's sweep or a cyclodialysis spatula is introduced carefully
into the incision, with the surgeon taking care that the tip does
not penetrate or engage the choroid (see Fig. 18). If a cyclodialysis spatula is used, the tip should be inspected to ensure
that it is not sharp and that it is without a burr. As soon as the
tip of the spatula has been introduced between the choroid and the
sclera, the handle is rotated; the heel of the spatula does not move, but
the tip is lifted firmly against the sclera (see Fig. 18). This maneuver should be done with sufficient vigor so that the sclera
is deformed slightly because the tip of the spatula pushes it up, away
from the choroid. The entire spatula is lifted up toward the microscope
so that the major vector of force is up toward the microscope, against
the internal wall of the sclera. With the surgeon holding the anterior
lip of the sclera firmly with the fixation forceps, the spatula
is moved anteriorly toward the limbus and the upward force against the
sclera is maintained. There should be no resistance to this forward
movement. If there is resistance, then the spatula must be in the sclera
and not between the choroid and sclera. Although occasional vessels
from the choroid penetrate the sclera, the only position in which the
uvea actually is adherent to the sclera is at the scleral spur. In most
cases, when the tip of the spatula reaches this junction, the surgeon
can feel the resistance to anterior movement. It is especially important
at that point to continue to lift the spatula as it is advanced
anteriorly so that the tip will hug the sclera as closely as possible
and not penetrate into the underlying ciliary body. After the spatula has advanced past the ciliary body, it suddenly meets
no resistance (see Fig. 18). It is advanced slowly arid cautiously until it is just visible at the
extreme periphery of the cornea. At that point, it must not be advanced
further without release of the upward force, or Descemet's membrane
will be torn off of the posterior surface of the cornea. The spatula
is retracted slightly, perhaps 0.5 mm, and the tip is rotated away
from the sclera. Upward force against the sclera, however, is maintained. This
force is now exerted by the force of the spatula just anterior
to the heel not by the tip. After the tip of the spatula has been
advanced anterior to the scleral spur and has been rotated inferiorly, away
from the posterior surface of the endothelium, the cyclodialysis
is performed by twisting the handle of the spatula between the fingers
so that the axis of the handle does not move and the tip moves laterally
and then posteriorly (see Fig. 18E). The spatula should continue to be lifted against the sclera during this
maneuver so that as the tip moves posteriorly, it hugs the inner wall
of the sclera, separating the uvea from the sclera exactly where the
two tissues join. After the handle is rotated in one direction so that
the tip has passed posterior to the scleral spur (reaching a point
where a straight line would lie on the sclera if it were extended circumferentially
from the cyclodialysis incision in the sclera), the handle
is rotated in the other direction so that the tip returns to its former
position, passes that position, and disinserts the ciliary body
from the sclera on the opposite side. The initial incision in the sclera
should be placed so that these rotations can be made without the spatula
damaging the blood vessels that penetrate the sclera at the 12, 3, 6, and 9 o'clock
positions. Avoidance of these vessels is vital to
the success of the procedure. The spatula is removed, and the anterior chamber is filled rapidly with
air through the previously placed paracentesis (see Fig. 18). A finger should monitor the IOP as the anterior chamber is filled, to
permit development of proper IOP. This pressure should be firm, between 30 and 50 mm
Hg, high enough to tamponade any bleeding. If the IOP
is raised too high, the choroid can be prolapsed through the scleral
incision. The scleral incision is closed with a relatively heavy suture, such as 7-0 polyglactin, with
care taken not to allow the tip of the needle to
damage the underlying choroid. A 30-gauge blunt needle is connected to a small (approximately 50 ml) compressible
bottle of irrigating solution such as balanced salt solution. Approximately 1 to 2 ml of the solution is squeezed from the bottle, and, as
the squeezing is continued, the tip of the blunt needle is
introduced into the anterior chamber. Pressure on the bottle is then released, allowing
material to be sucked back into the irrigating bottle. The
tip of the blunt needle is placed in the air bubble, and the air
is evacuated from the eye with ease and safety. If the bottle is held
vertically, the air will rise to the top of the bottle. Further pressure
on the bottle will introduce saline rather than air back into the
anterior chamber. In this way, most of the air is removed, and the chamber
is filled with saline. If there has been extensive bleeding, the blood in the anterior chamber
is removed as completely as possible by this maneuver. A fairly vigorous
stream can be directed into the cyclodialysis cleft. At the end of the procedure, the eye should have moderately firm pressure (15 to 30 mm
Hg), a deep chamber, a small pupil to prevent any air
from moving into the posterior chamber, and little or no blood in the
anterior chamber. When it is possible to achieve this outcome, the incidence
of success is high. Postoperatively, the patient is maintained on strong miotics, such as echothiophate (0.125% twice
daily in blue-eyed patients and 0.25% twice
daily in brown-eyed patients). It is not unusual for the pressure to
be elevated on the first or second postoperative day. Presumably this
elevation is related to blood or fibrin plugging the cleft; as this material
clears, the pressure spontaneously falls to a low level. If the
procedure has been performed in an inferior quadrant, it is helpful to
position the patient postoperatively so that blood will not settle into
the cleft. If the IOP has not fallen within 3 days, the operation
probably will fail. If a successful cleft is achieved, approximately 1 month after surgery, the
echothiophate can be reduced to a less concentrated and less frequent
dose. In most cases, 0.125% once daily is adequate to maintain patency
of the cleft. Topical corticosteroids help suppress the inevitable
inflammation associated with surgery. The anterior chamber of the treated eye temporarily becomes shallower immediately
after surgery. In some cases, this change may be a factor of
papillary block induced by the strong miotic, especially if there is
significant inflammation. In most cases, however, it probably is caused
by a rotation of the iris root anteriorly resulting from a ciliary
body detachment. Failure of the cleft to develop is the major problem with cyclodialysis. Factors
predisposing patients to failure of the cleft to develop include
bleeding in the area of the cleft, air in the posterior chamber, papillary
block, marked inflammation, and the use of cycloplegics. Bleeding is the major complication. Bleeding may occur in the form of an
expulsive hemorrhage at the time of surgery. If this type of hemorrhage
occurs, the scleral incision should be closed immediately, and hypotensive
agents, such as intravenous acetazolamide and mannitol, administered. The
suprachoroidal hemorrhage should not be drained at the time
of the initial surgery. Slight amounts of blood in the anterior chamber
almost invariably are absorbed. However, blood in the anterior chamber
predisposes patients to failure of the cleft; if this failure occurs
before the blood is absorbed and the pressure rises markedly, blood
staining of the cornea may develop. Months or even years after a successful cyclodialysis, the cleft may close
suddenly; as a consequence, the IOP rises precipitously. This condition
typically is associated with severe pain resembling that of an acute
angle-closure glaucoma. If the condition has been recognized promptly, use
of strong miotics (such as echothiophate 0.25%) usually can
reopen the cleft. Consequently, patients should be informed about the
occurrence of cleft closure and told that they should seek ophthalmologic
care immediately if they have suggestive symptoms. Various methods have been used to try to maintain patency of the cleft, including
the use of sodium hyaluronate at the time of surgery. In our
opinion, none has proven useful. For reasons that are not clear, sodium
hyaluronate injected into the anterior chamber tends to cause the
procedure to fail. CYCLODESTRUCTIVE PROCEDURES The goal of cyclodestructive procedures is similar to that for most glaucoma
procedures, specifically, lowering IOP. The mechanism by which cyclodestructive
procedures work, however, differs. Rather than improve
aqueous outflow, these operations reduce aqueous inflow. Exactly how
they decrease aqueous outflow is not certain; they appear to damage or
kill secretory epithelial cells of the ciliary body. They damage the
ciliary body and cause immediate uveitis; this inflammation tends to be
long lasting and may be permanent. Indications Although these procedures are grouped for discussion, they differ significantly
in their degree of safety and effectiveness, and possibly in
their mechanism of action as well (Table 24). Furthermore, there are undoubtedly reasons for preferring one procedure
over another in a particular case. Because the surgical techniques
are not uniform and because few comparative studies have been performed, there
is little consensus as to which procedure is preferred in a
particular case. Table 24. Differences Between Cyclodestructive Procedures
| | Cyclophotocoagulation |
| | Cyclocryotherapy | Transscleral | Contact | Endoscopic | Therapeutic Ultrasound |
| Cost | Low | Moderate | High | High | High |
| Convenience | High | High | Moderate | Low | Moderate |
| Special equipment | No | Yes | Yes | Yes | Yes |
| Availability | Great | Fair | Poor | Low | Low |
| Portability of equipment | Easy | Fair | Fair | No | No |
| Experience in technique | Most | Great | Moderate | Small | Small |
| Morbidity | High | Low | Low | Low | Moderate |
| Ease of performance | Easy | Easy | Easy | Hard | Hard |
| Effectiveness | Great | Good | Good | Good | Fair |
| Serious Complications | Frequent | -5%–10% | -5%–10% | Uncommon | Common |
| Postoperative inflammation | | | | | |
| External | Marked | Mild | Mild | Moderate | Marked |
| Internal | Marked | Moderate | Moderate | Moderate | Mild | The general indication for a cyclodestructive procedure is the generic
need for most glaucomatous procedures, specifically, the need to lower
IOP surgically. Such procedures may be especially suited to cases in
which there is a major advantage in not having to perform intraocular
surgery, especially in eyes that are predisposed to suprachoroidal hemorrhage (Table 25). In addition, certain types of cyclodestructive procedures are remarkably
convenient to the patient, requiring no hospitalization and little
special equipment and allowing the patient to obtain care quickly and
relatively inexpensively. The cyclodestructive procedures are especially
useful in patients in whom visual prognosis is poor and who, for
whatever reason, are not considered appropriate for hospitalization (see Table 25). For example, the older patient who has mild Alzheimer's disease
and a painful eye related to pseudophakic secondary glaucoma is not a
good candidate for hospitalization or intraocular surgery; nevertheless, a
cyclophotocoagulation could be done with minimal disruption of the
patient's life and with fair anticipation of benefit. Similarly, a
patient who has advanced primary open-angle glaucoma and has lost
central vision from macular degeneration would be a suitable candidate
for cyclophotocoagulation because one of the major concerns of this
procedure, specifically, loss of central visual acuity, would no longer
be a problem. Table 25. Indications for Cyclodestructive Procedures*
Need to lower intraocular pressure and one or more of the following conditions: Poor macular function in the eye under consideration
Anticipated problem from performing intraocular surgery
Predisposition
Suprachoroidal expulsive hemorrhage
Anticipated inability to develop satisfactory conjunctival flap for filtration
procedure
Anticipated problem with hospitalization
Inconvenience to patient related to hospitalization
Patient fearful of cutting surgery
Concern regarding cost of other forms of surgery
Not neovascular glaucoma *Where available, cyclophotocoagulation is the desired cyclodestructive
procedure of choice. It carries significantly less morbidity and a lower
rate of complication than cyclocryotherapy. Other reasons for preferring
one procedure over another can be concluded from the information
in Table 24.
CYCLOCRYOTHERAPY Cyclocryotherapy has the major advantage of being readily available and
inexpensive. Because of its significant disadvantages, however, it is
not used widely by all ophthalmologists. Problems associated with it
are listed in Table 26, roughly in their order of frequency. Table 26. Complications of Cyclocryotherapy
Inflammation
Rise of intraocular pressure Pain
Secondary to uveitis
Secondary to suprachoroidal hemorrhage
Reduced vision
Uveitis
Hypotony, macular edema
Intraocular hemorrhage
Intraocular bleeding
Hyphema
Suprachoroidal hemorrhage
Cataract
Hypotony
Excessive tissue destruction
Ciliary and choroidal detachment
Phthisis bulbi
Anterior segment necrosis
Scleral staphyloma
Sympathetic ophthalmia
The most common indication for cyclocryotherapy is an aphakic glaucoma
that is unresponsive to medical therapy, especially when the eye has an
associated problem such as inflammation or neovascular changes. It rarely
is used in phakic patients because it is highly cataractogenic. Cyclocryotherapy has been advocated as appropriate treatment for patients
with blind, painful eyes and elevated IOP. In our opinion this treatment
rarely is justified. It is unusual for an eye to be persistently
painful on the basis of elevated IOP itself. A sudden rise of IOP usually
causes pain, but when the pressure remains elevated for months, the
pressure itself rarely is responsible for discomfort. Hence, treatment
primarily designed to lower chronically elevated IOP is not entirely
rational. In addition, most of these patients obtain pain relief from
measures directed at limiting inflammation, such as topical atropine
and corticosteroids, whereas cyclocryotherapy increases inflammation. Operative Technique The cyclocryotherapy procedure itself is painful and requires effective
anesthesia. A retrobulbar injection of bupivacaine 0.75% containing hyaluronidase
almost always is adequate. Because the position of the ciliary body relative to the limbus varies
from eye to eye, an effort should be made to locate the ciliary body before
the procedure is done. This position can be approximated in most
cases simply by inspection; transillumination is helpful when landmarks
are less clear. Applications should be centered over the ciliary body; if this site is
close to the limbus, it is better to place the applications slightly more
posteriorly to ensure that the iceball does not extend into the cornea, where
it could damage the corneal endothelial cells (Fig. 19). 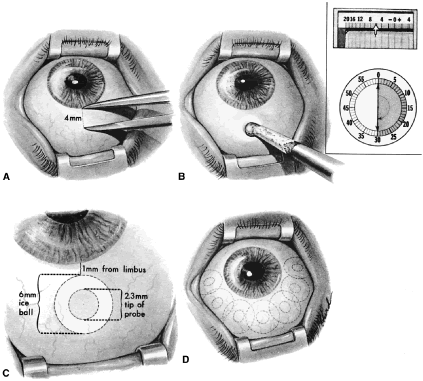 Fig. 19. Cyclocryotherapy. A. Calipers mark a point on the bulbar conjunctiva 4 mm from the limbus. B. A Linde unit 2.3-mm probe straddles the mark made in A. Inset. Probe temperature is lowered to -60°C and maintained in contact with
sclera for 30 seconds after a 6-mm iceball forms. C. The 2.3-mm probe creates and maintains a 6-mm iceball at -60°C. A 1-mm
clear area is left proximal to the limbus to prevent freezing the
cornea. D. A probe is applied to the superior half of the globe so that an area of
approximately 200 degrees is treated by overlapping iceballs. Fig. 19. Cyclocryotherapy. A. Calipers mark a point on the bulbar conjunctiva 4 mm from the limbus. B. A Linde unit 2.3-mm probe straddles the mark made in A. Inset. Probe temperature is lowered to -60°C and maintained in contact with
sclera for 30 seconds after a 6-mm iceball forms. C. The 2.3-mm probe creates and maintains a 6-mm iceball at -60°C. A 1-mm
clear area is left proximal to the limbus to prevent freezing the
cornea. D. A probe is applied to the superior half of the globe so that an area of
approximately 200 degrees is treated by overlapping iceballs.
|
The intensity of treatment should vary with the condition of the patient. Associated
factors are shown in Table 27. In most cases, we use six to eight applications spread over the inferior
two quadrants, avoiding the 3 and 9 o'clock positions. If IOP does
not fall adequately within 1 week, the procedure is repeated, treating
the two quadrants between the 12 and 6 o'clock positions and avoiding
the 3 o'clock position. If pressure is still not lowered adequately, a
third treatment is placed in the superior two quadrants. Table 27. Factors that Influence the Intensity of Treatment with Cyclocryotherapy
| Favoring Less Treatment | Favoring More Treatment |
| Age younger than 10 years or older than 60 years | Blue eye |
| | Need to lower pressure markedly |
| Previous cyclodestructive procedure, especially if effective | Need to lower intraocular pressure with one treatment |
| Only eye | No effect on intraocular pressure from previous treatment | It is important to recognize that cyclocryotherapy often is followed by
a prompt rise in IOP.85–88 This increase is rapid, occurring within the first hour, and is clinically
significant. This rise in IOP is important. Many of the eyes chosen
for cyclocryotherapy have far advanced glaucomatous nerve damage; a
sudden rise in pressure may cause these eyes to lose the small amount
of visual function present before the cryotherapy. Unless there is a
specific contraindication, a carbonic anhydrase inhibitor and an osmotic
agent should be given in full doses when cryotherapy is performed. One
acceptable regimen is to give a 500-mg sequel of acetazolamide and
a full dose of an osmotic agent such as mannitol 20%, 7 ml/kg body weight. The
IOP should be monitored carefully, especially for the first 4 hours. In
some cases, a paracentesis may be indicated. Postoperative care is directed toward limiting the inflammation and controlling
the pressure. Inflammation is severe and prolonged. In almost
all cases, atropine 1% four times daily is appropriate. This dosage can
be tapered as the eye quiets and becomes more comfortable. It may be
helpful to continue the administration of atropine once daily for several
months. Corticosteroids should be given subconjunctivally at the
time of the operation. One suitable dose is betamethasone, 3 mg. We do
not employ depot steroids because they may cause a persistent elevation
of IOP and actually may have to be removed at a later date. Topical
corticosteroids should be employed vigorously and for a prolonged duration. A
four-times-a-day dose usually is adequate in the initial period, with
a potent agent such as prednisolone acetate. After 2 to 3 weeks, it
may be appropriate to start tapering the frequency of administration. Often, continuing
steroids for many months on an every other-day
dose is helpful. IOP must be monitored carefully in the immediate postoperative period. The
pressure usually starts to fall by about 24 hours and seems to reach
its lowest point several days after cyclocryotherapy has been performed. Because
the response of IOP to treatment is so unpredictable, pressure
must be measured frequently, and glaucoma therapy adjusted appropriately. Obviously, pilocarpine or other miotics should be discontinued
at the time of the surgery and not resumed until inflammation is minimal
and certainly not until the atropine has been discontinued. The most effective agents to control IOP in most of the secondary glaucomas
usually are carbonic anhydrase inhibitors and β-blockers. These
two classes of drugs usually are chosen to control IOP after cyclocryotherapy. When
cyclocryotherapy is effective, however, these agents
should not be employed, because they may produce marked hypotony and choroidal
detachment. In most cases, all glaucoma medications are discontinued
at the time of cyclocryotherapy. CYCLOPHOTOCOAGULATION The application of light energy to the area of the ciliary body for the
purpose of lowering IOP can be accomplished by applying light directly
to the ciliary processes, either through the pupil or endoscopically
or with a transscleral approach. Transpupillary photocoagulation of the
ciliary processes can be employed only in cases in which visualization
of the ciliary processes through the pupil is possible; the technique
is of such limited application that it will not be discussed further
here. Further, the success rate has not been high. Endoscopic photocoagulation
has the advantage of allowing direct, well-controlled application
of light to all of the ciliary processes. Shields and associates89 reported preliminary good results. The operation clearly is a major one
that demands sophisticated equipment and a prolonged hospital stay. To perform cyclophotocoagulation endoscopically, however, is to lose the
major advantages of cyclophotocoagulation: convenience and the ability
to provide treatment in an outpatient setting, without the need to
open the globe. Endoscopic photocoagulation should be limited to cases
in which other surgery, such as vitrectomy, is being performed. Transscleral photocoagulation has been practiced for more than 10 years.90–97 Originally, a ruby laser was employed, whereas now, the usual technique
involves the use of an Nd:YAG laser or preferably a diode laser. Indications for cyclophotocoagulation are essentially the same as for cyclocryotherapy. Because
cyclophotocoagulation appears to have less effect
on central visual acuity, to have fewer side effects, to be more
controllable, and to be less likely to cause phthisis bulbi, it is strongly
preferred over cyclocryotherapy. Complications are essentially the
same as for cyclocryotherapy (see Table 26). Cyclophotocoagulation may cause sympathetic ophthalmia, but this relationship
has not been well established. The eye must be deeply anesthetized. A short-acting agent, such as lidocaine, is
appropriate in most cases; if the surgeon is concerned that
the patient will not tolerate postoperative discomfort well, then bupivacaine
can be employed; 3 to 5 ml is adequate. One method of performing cyclophotocoagulation is as follows: the Nd:YAG
laser is used in a free-running, not a Q-switched, mode. Thirty-two
applications are placed over the ciliary body, avoiding the 12, 3, 6, and 9 o'clock
positions. Therefore, eight applications are placed supertemporally, inferotemporally, inferonasally, and superonasally. The exposure
time is 20 msec, and the size of the spot at the point of focus
is 70 μm. The focus is retrofocused 8 or 9 mm posteriorly, which
should result in maximum energy approximately at the levels of the ciliary
processes. The aiming beam is focused on the external scleral surface, and
the laser beam is activated. The patient will experience pain
unless the retrobulbar block has achieved adequate anesthesia. The power setting should be between 4 and 8 J. Higher powers will have
a greater effect on IOP, as well as a higher complication rate. The use
of a diode laser appears to be associated with a lower rate of complication. The
procedure is performed essentially in the same manner as
with a Nd:YAG laser. Postoperative treatment is similar to that for cyclocryotherapy. IOP should
be monitored carefully immediately after the treatment. Pressure
spikes are not rare, especially with higher powers. The management of
IOP is similar to that employed with cyclocryotherapy. Postoperatively, anti-inflammatory
treatment with topical corticosteroids is used. If
IOP is reduced sufficiently, miotics may be discontinued and atrophic
administered (in patients who are not predisposed to primary angle closure). The
previously employed medical therapy is continued until the
effect of the cyclophotocoagulation becomes apparent. Therapy can be
tapered or continued as appropriate. If the decrease in IOP is inadequate
after 1 week, the Nd:YAG cyclophotocoagulation can be repeated. In
unusual circumstances, it can be repeated sooner. The number of times that the treatment can safely be repeated has not been
determined. However, patients appear to tolerate the procedure well, and
we have treated some patients with five or more sessions without
apparent complications. It is not rare for the effect of cyclophotocoagulation to diminish with
time. When this decrease occurs and there appears to be a continuing
need to control IOP, a repeat cyclophotocoagulation may be the treatment
of choice. When the procedure does not appear to have been effective
initially, however, we usually perform only one repeat procedure. If
IOP has not been lowered satisfactorily after two cyclophotocoagulation
procedures, it usually is preferable to consider another procedure. NONPENETRATING FILTRATION SURGERY The appeal of successful bleb-less glaucoma surgery remains overwhelming. Nonpenetrating
filtration surgery (NPFS) is another step in the evolution
of safer filtration surgery. Even thirty years ago, Hans Goldmann98 said: “Success in establishing aqueous drainage without the formation
of an avascular bleb is an important advance in glaucoma surgery.” At
that time, Goldmann and others, thought Cairns'new trabeculectomy
procedure would enhance flow into Schlemm's canal, limiting
bleb formation. Their assumption was erroneous, for it became obvious
that bleb formation was necessary for long-term pressure reduction.99 However, trabeculectomy rapidly became popular as a new form of guarded
filtration surgery,100 a major improvement over full-thickness procedures. Over a 30-year period, most
nonfistulous filtration procedures for adult glaucomas remain
only in textbooks101–103 and have not gained widespread acceptance. At the beginning of the new
millennium, Goldmann's dream continues as the surgical manipulation
of Schlemm's canal has evolved into something new, NPFS. NPFS is an attempt to establish long-lasting filtration without entering
the anterior chamber at the subscleral surgical site. The two emerging
forms of NPFS include deep sclerectomy with collagen implant (DSCI) and
deep sclerectomy with viscocanalostomy (DSVC). With the advent of
these new procedures, filtration nomenclature requires refinement as
proposed in Table 28. It is important to remember that these procedures are in evolution and
refinements are necessary. The most significant obstacle for viscocanalostomy
is the need for a device to prevent closure of the ostia of
Schlemm's canal. Table 28. Penetrating and Nonpenetrating Outflow Procedures
| (External Filtration (extraocular) | Internal Filtration (intraocular) |
| Penetrating (PEF) | Penetrating (PIF) |
| Trabeculectomy | Trabeculotomy ab externo |
| Drainage implants | Goniotomy |
| Nonpenetrating (NPEF) | Nonpenetrating (NPIF) |
| Deep sclerectomy with collagen implant (DSCI) | Deep sclerectomy with Viscocanalostomy (DSVC) |
| Deep sclerectomy (DS) | Laser trabeculoplasty |
PEF, penetrating with external filtration.
NPEF, nonpenetrating with external filtration.
PIF, penetrating with internal filtration.
NPIF, nonpenetrating with internal filtration.
The surgical reduction of IOP is accomplished by either the external (extraocular) or
internal (intraocular) enhancement of outflow. External
filtration is achieved by either penetrating (PEF) or nonpenetrating (NPEF) means, similarly, internal filtration may be penetrating (PIF) or
nonpenetrating (NPIF). By definition, penetrating procedures such as
trabeculectomy enter the anterior chamber at the subscleral incision
site. External filtration leads to bleb formation. The newer nonpenetrating
procedures do not violate the anterior chamber, leaving natural
barrier tissue resistance to outflow. These procedures are still in evolution. The
older term of ab externo is no longer completely sufficient
because it does not specify anterior chamber entry.
Both nonpenetrating procedures are designed to decrease outflow resistance
not eliminate it, as may uncontrollably occur with standard filtration
surgery. In generic terms, NPFS consists of fashioning dual scleral
flaps (Fig. 20) with removal of the deep flap (deep sclerectomy) leaving an internal
limiting membrane and an intrascleral aqueous reservoir. More specifically, the
intrascleral space and ostia of Schlemm's canal are filled
with viscoelastic in viscocanalostomy or a collagen wick placed in
the intrascleral space as in DSCI.104 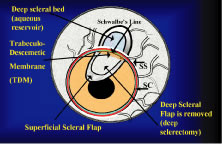 Fig. 20. Nonpenetrating filtration surgery. Nonpenetrating filtration surgery consists
of fashioning dual scleral flaps, a superficial and deep flap. The
superficial flap is typically 300 microns thick and must be dissected
at least 1 mm into clear cornea. Fashioning of the deep flap (600 microns
thick) reveals the underlying limbal anatomy with the circumferential
white fibers of the scleral spur (SS) and more anteriorly, the
blue zone with its corneal limit of Schwalbe's line. Schlemm's
canal is located directly anterior to the SS. The trabecular meshwork
is found anterior to Schlemm's canal and extends to Schwalbe's
line. The trabeculodescemetic membrane (TDM) extends from Schwalbe's
line anteriorly into clear cornea for 1 mm. The deep flap is
removed at the time of surgery creating a potential space that functions
as a collecting cavern for the potential egress of aqueous. Filtration
occurs mainly internally through Schlemm's canal (SC) with
successful deep sclerectomy with viscocanalostomy (DSVC), and externally
into the subconjunctival space with deep sclerectomy with collagen
implant (DSCI). The superficial flap guards externally and the TDM guards
internally from overfiltration. Fig. 20. Nonpenetrating filtration surgery. Nonpenetrating filtration surgery consists
of fashioning dual scleral flaps, a superficial and deep flap. The
superficial flap is typically 300 microns thick and must be dissected
at least 1 mm into clear cornea. Fashioning of the deep flap (600 microns
thick) reveals the underlying limbal anatomy with the circumferential
white fibers of the scleral spur (SS) and more anteriorly, the
blue zone with its corneal limit of Schwalbe's line. Schlemm's
canal is located directly anterior to the SS. The trabecular meshwork
is found anterior to Schlemm's canal and extends to Schwalbe's
line. The trabeculodescemetic membrane (TDM) extends from Schwalbe's
line anteriorly into clear cornea for 1 mm. The deep flap is
removed at the time of surgery creating a potential space that functions
as a collecting cavern for the potential egress of aqueous. Filtration
occurs mainly internally through Schlemm's canal (SC) with
successful deep sclerectomy with viscocanalostomy (DSVC), and externally
into the subconjunctival space with deep sclerectomy with collagen
implant (DSCI). The superficial flap guards externally and the TDM guards
internally from overfiltration.
|
Prospective studies comparing DSVC to standard filtration surgery reveal
greater pressure reduction with trabeculectomy.105 Thus, patients with severe glaucomatous damage, especially exposed to
long-term topical therapy, are more likely to benefit from standard filtration
techniques. Another study comparing deep sclerectomy with and
without collagen implant to trabeculectomy found comparable results between
the procedures.106 How does NPFS fit into our incisional options? At this time, it is difficult
to sort out the best candidates for NPFS. Each new case adds a piece
to the puzzle of how these procedures truly work and who will most
likely benefit. Results from a black African population are encouraging107 but have not been reproduced in other patient populations. Can we directly
extrapolate the same technique to different populations with dissimilar
types of glaucoma, years of topical therapy, and variable outflow
pathology? A recent study reveals NPFS in patients with primary open-angle
glaucoma was more effective in patients without prior medical
therapy than in patients on years of topical medical therapy.108 Glaucoma medications may cause changes in the conjunctiva and episcleral
that lead to failure in any type of filtration procedure,109 possibly more detrimental for nonpenetrating procedures. The learning curve for NPFS procedures is very steep, much like trabeculotomy
in an infant eye. A review of outflow anatomy is crucial before
nonpenetrating procedures (Fig. 21). DSCI and DSVC are headed down two competing pathways, one with internal
filtration and the other with external filtration. External filtration
as seen in DSCI still produces a bleb. Bleb formation with viscocanalostomy
is minimal although microcysts may form above the 300-micron
scleral flap. The proposed mechanisms of IOP reduction for NPFS are
seen in Figure 22. Direct intraoperative evidence for enhancement of outflow facility can
be seen during DSVC. Observation of this phenomenon has caused a renaissance
in outflow surgery (Fig. 23).  Fig. 21. Normal outflow system. A much broader appreciation of limbal anatomy is
necessary for deep sclerectomy procedures than for standard trabeculectomy. To
understand how nonpenetrating filtration surgery works, a review
of outflow anatomy is essential. Normal outflow resistance is approximately
distributed in the following fashion: 60% in the trabecular
meshwork (TM) and juxtacanalicular (JC) tissue, 25% in the canal and
immediate collector systems, and 15% in the scleral venous collector system (VP). The
TM is 750 μ in meridional width, Schlemm's canal (SC) is 36 mm
in circumference with an average width of 300 μ, and
roughly 30 collector channels connect the canal to the venous plexus (VP). Approximately 6 to 8 aqueous veins (AV) exit Schlemm's
canal and bypass the collector channels directly connecting into the venous
plexus. Fig. 21. Normal outflow system. A much broader appreciation of limbal anatomy is
necessary for deep sclerectomy procedures than for standard trabeculectomy. To
understand how nonpenetrating filtration surgery works, a review
of outflow anatomy is essential. Normal outflow resistance is approximately
distributed in the following fashion: 60% in the trabecular
meshwork (TM) and juxtacanalicular (JC) tissue, 25% in the canal and
immediate collector systems, and 15% in the scleral venous collector system (VP). The
TM is 750 μ in meridional width, Schlemm's canal (SC) is 36 mm
in circumference with an average width of 300 μ, and
roughly 30 collector channels connect the canal to the venous plexus (VP). Approximately 6 to 8 aqueous veins (AV) exit Schlemm's
canal and bypass the collector channels directly connecting into the venous
plexus.
|
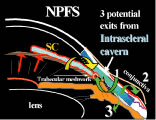 Fig. 22. Proposed mechanism of pressure reduction after nonpenetrating filtration
surgery. The red arrows indicate inhibition of aqueous flow at the trabecular
meshwork. The yellow arrow designates the passage of aqueous
from the anterior chamber through the gossamer thin trabeculodescemet's
membrane into the intrascleral cavern (blue area). The potential sites for egress of aqueous include Schlemm's canal
and its collector system (green arrow 1), transscleral flow (green arrow 2), and uveoscleral flow (green arrow 3). Fig. 22. Proposed mechanism of pressure reduction after nonpenetrating filtration
surgery. The red arrows indicate inhibition of aqueous flow at the trabecular
meshwork. The yellow arrow designates the passage of aqueous
from the anterior chamber through the gossamer thin trabeculodescemet's
membrane into the intrascleral cavern (blue area). The potential sites for egress of aqueous include Schlemm's canal
and its collector system (green arrow 1), transscleral flow (green arrow 2), and uveoscleral flow (green arrow 3).
|
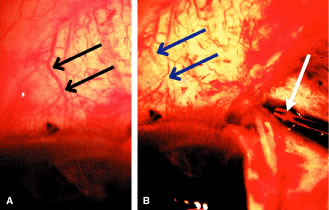 Fig. 23. Laminar flow sign. Nonpenetrating filtration surgery is a systematic layer
by layer dismantling of the iridocorneal outflow system. A. Appearance of episclera before injecting balanced salt solution (BSS) into
the ostium of Schlemm's canal. The episcleral venous system
is easily seen, with particular reference to a large scleral collector (black arrows). B. Evidence of enhancement of outflow can be found by simultaneously observing
the episclera vessels and carefully injecting BSS into the canal (white arrow). A rapid blanching of vessels is easily seen. This “white out” phenomena
or laminar flow sign (blue arrows) is a sure sign of correct anatomic location. Observation of this phenomenon
increases awareness of one possible mechanism of postoperative
pressure reduction. Fig. 23. Laminar flow sign. Nonpenetrating filtration surgery is a systematic layer
by layer dismantling of the iridocorneal outflow system. A. Appearance of episclera before injecting balanced salt solution (BSS) into
the ostium of Schlemm's canal. The episcleral venous system
is easily seen, with particular reference to a large scleral collector (black arrows). B. Evidence of enhancement of outflow can be found by simultaneously observing
the episclera vessels and carefully injecting BSS into the canal (white arrow). A rapid blanching of vessels is easily seen. This “white out” phenomena
or laminar flow sign (blue arrows) is a sure sign of correct anatomic location. Observation of this phenomenon
increases awareness of one possible mechanism of postoperative
pressure reduction.
|
Patient selection for NPFS is more complicated than for standard trabeculectomy. Patients
with significant complications from prior trabeculectomy
or at high risk for failure may be candidates for NPFS. The problems
with nonpenetrating procedures are unique and require a completely
different set of preoperative, intraoperative, and postoperative thought
processes and skills compared with trabeculectomy or trabeculotomy. Preoperative Considerations - Patients with severe optic nerve damage may not be optimal candidates for
NPFS because long-term IOP reduction is not as great as trabeculectomy.
- If gonioscopy reveals a preoperative shallow anterior chamber angle, the
iris is more likely to occlude the trabeculodescemet's window. Preoperative
laser iridotomy may be necessary in patients with shallow
anterior chamber angles because peripheral iridectomy is not part of
NPFS.
- If the episcleral venous plexus or scleral collector system is damaged, DSVC
will not be as efficacious. Ocular disease that damages the outflow
system downstream to Schlemm's canal will decrease success with
DSVC. NPFS may be a reasonable procedure in a poorly compliant primary
open-angle glaucoma patient with high pressure and minimal disc damage.
- Patients with high myopia and thin sclera are poor candidates for dual
flap therapy; there is simply not enough tissue to make two flaps. Not
all patients with myopia have inoperable sclera.
- Patients with conjunctival scarring from prior ocular surgery are reasonable
candidates for DSVC because filtration is hopefully mainly internal
and subconjunctival flow is minimal.
- Patients with scleral incisions, especially from cataract surgery, at the
proposed site of NPFS are poor candidates because dual flap therapy
with preservation of the trabeculodescemetic membrane (TDM) is practically
impossible when the iridocorneal angle has been violated.
- Laser trabeculoplasty to the proposed NPFS site should be avoided because
of possible scarring of the outflow structures.110
Patients at high risk for suprachoroidal hemorrhage are still at risk with
NPFS because of the need for substantial intraoperative anterior chamber
decompression. The postoperative risk of delayed suprachoroidal
hemorrhage is minimized by the double guarded technique. DEEP SCLERECTOMY WITH VISCOCANALOSTOMY DSVC is a systematic layer-by-layer dismantling of a defective outflow
system. At the heart of DSVC is the precise fashioning of an intact subscleral
cavern that ultimately functions as a relay station for the passage
of aqueous. Aqueous bypasses the blocked trabecular meshwork by
entering the cavern through an adjacent gossamer quality Descemet's
window111 and exiting the cavern through Schlemm's canal, by uveoscleral channels, or
by transscleral flow. Bleb formation is avoided because aqueous
accumulates inside the sclera instead of underneath the conjunctiva, a
long-term advantage from a dysesthesia and infectious viewpoint. The surgical technique is unforgiving and the learning curve steep. Surgeons
with prior trabeculotomy skills112 will find this procedure easier but still demanding. I would not recommend
topical anesthesia for one's first case. Informed consent should
reflect the investigational nature of these procedures and a 20-point
preoperative checklist113 is helpful in planning filtration surgery. Familiar instruments should
be used for scleral flap dissection, and a special cannula is necessary
to intubate Schlemm's canal. - Fornix-based conjunctival flap (Fig. 24). A fornix-based conjunctival flap significantly aids in limbal exposure. Limbal-based
conjunctival flaps interfere with visibility of limbal
tissues necessary for NPFS. Postoperative wound leaks may be slightly
more common with fornix-based incisions but the leak is not as significant
as in trabeculectomy. Wet-field cautery of episcleral vessels is
used sparingly to prevent coagulation of the collector channels. Thrombin
is helpful if bleeding is excessive (Fig. 25).
- Superficial scleral flap (Figs. 26A and B and 27). Once hemostasis is achieved, a 5 by 5 mm, 300-micron thick scleral flap
is fashioned with either a diamond or razor blade. A specially designed
Grieshaber minilollipop blade is useful to carry out the dissection 1 mm
into clear cornea.
- Outlining the deep scleral flap (Fig. 28).
- Development of the deep sclera flap (Fig. 29). The deep scleral flap technique is useful during any angle procedure
requiring the exposure of Schlemm's canal. This flap should be approximately 600 microns
in thickness and penetrate down to choroid leaving
only a few scleral fibers. The most common mistake is failure to
make the flap deep enough. This leads to eventual loss of landmarks, and
rupture into the anterior chamber is likely. If this occurs and landmarks
are obscure, one should convert to a standard trabeculectomy.
- Unroofing Schlemm's canal (Fig. 30).
- Limbal anatomy during NPFS varies with flap depth (Fig. 31).
- The identification of Schlemm's canal with a suture probe (Fig. 32A to D). Finding Schlemm's canal is one of the most difficult parts of the
procedure. DSVC is totally dependent on finding the ostia of Schlemm's
canal. If the canal is not accurately identified, the procedure
is sure to fail. There are several beneficial suture maneuvers used
to visualize the canal.114 The injection of balanced salt solution into the cut end of the canal
will direct fluid into the episcleral veins, an excellent demonstration
of proper canal location and of how the procedures likely works (see. Fig. 23).
- Identification of Schlemm's canal with a fiberoptic light source (Fig. 33).
- Viscocanalostomy (Fig. 34). The cut ends of Schlemm's canal will close over because of fibrosis. Injecting
viscoelastic into the canal is instrumental to the procedure
and helps prevent closure of the ostium. Care must be taken not
to over fill the canal, which results in rupture into the anterior chamber. After
several days, the viscoelastic is absorbed. If fibrosis is
excessive, the ostia close preventing flow into the canal with subsequent
failure of the procedure. Overfilling the canal may lead to a detachment
of Descemet's membrane.115
- Creation of trabeculodescemetic membrane (TDM) (Fig. 35A and B). After dilating the ostia, the deep scleral flap is carried forward into
clear cornea creating the thin TDM. The key is to preserve the membrane
and visualize the flow of aqueous through the membrane. If no flow
is seen, a thin layer of tissue is removed from the inner wall of Schlemm's
canal. Laceration of the TDM is especially common during
the learning curve for all NPFS procedures. Microperforations do not
seem to hinder results, but macroperforations with iris incarceration
typically require conversion to trabeculectomy. Fashioning of this fine
gossamer membrane is the most demanding portion of the procedure. The
flow through Descemet's membrane seems to be variable, and the
exact size and flow characteristics are poorly delineated.116
- Deep sclerectomy (Fig. 36). Removal of the deep flap creates the potential intrascleral space. This
intrascleral cavern is the collecting station for the exit of aqueous (Fig. 37). It is similar in concept to a bleb but is intrascleral. Keeping aqueous
inside the sclera prevents the many problems associated with the subconjunctival
flow of aqueous.
- Scleral flap and conjunctival closure (Fig. 38A and B). After deep sclerectomy, the superficial flap is tightly closed and the
void filled with viscoelastic. The chamber must not be overfilled. The
conjunctival flap may be hooded or sutured to the limbus.
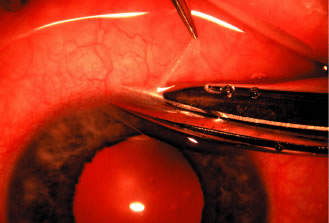 Fig. 24. Deep sclerectomy with viscocanalostomy: fornix-based conjunctival flap. After
obtaining adequate exposure of the superior limbus, a fornix-based
conjunctival flap is dissected. Great care is exercised to avoid trauma
to the episcleral vessels during the dissection. If a corneal traction
suture is used, the pass should be off to the side of the proposed
site to prevent rupture of the limbal tissues. Fig. 24. Deep sclerectomy with viscocanalostomy: fornix-based conjunctival flap. After
obtaining adequate exposure of the superior limbus, a fornix-based
conjunctival flap is dissected. Great care is exercised to avoid trauma
to the episcleral vessels during the dissection. If a corneal traction
suture is used, the pass should be off to the side of the proposed
site to prevent rupture of the limbal tissues.
|
 Fig. 25. Deep sclerectomy with viscocanalostomy: management of episcleral bleeding. A. The management of episcleral bleeding is vastly different than with trabeculectomy
in which the tendency is to eradicate all bleeders. The amount
of cautery used to manage bleeding during trabeculectomy is far
too much for nonpenetrating filtration surgery. Excessive cautery would
obliterate the very episcleral collector system necessary for surgical
success. B. A thrombin soaked sponge (5 mg/cc) applied for 1 minute to the proposed
site is useful. Light cautery to the anterior ciliary artery may be
necessary, one must try to avoid any cautery to the episcleral venous
plexus. Fig. 25. Deep sclerectomy with viscocanalostomy: management of episcleral bleeding. A. The management of episcleral bleeding is vastly different than with trabeculectomy
in which the tendency is to eradicate all bleeders. The amount
of cautery used to manage bleeding during trabeculectomy is far
too much for nonpenetrating filtration surgery. Excessive cautery would
obliterate the very episcleral collector system necessary for surgical
success. B. A thrombin soaked sponge (5 mg/cc) applied for 1 minute to the proposed
site is useful. Light cautery to the anterior ciliary artery may be
necessary, one must try to avoid any cautery to the episcleral venous
plexus.
|
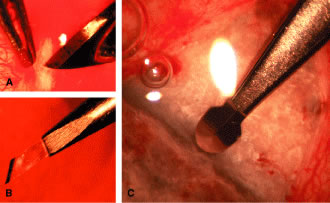 Fig. 26. Deep sclerectomy with viscocanalostomy: development of the superficial
scleral flap. Flap development with NPFS is decidedly more complicated
than trabeculectomy. It is inherently more difficult to make two flaps
from 1 mm of sclera than one flap. The superficial scleral flap should
be approximately 300 microns in thickness, 5 by 5 mm and parabolic
in shape to aid in eventual closure. The most common mistake is making
the flap too thin with dissolution as the limbus is approached. The flap
should look like C, as the limbus is approached. Various free-hand techniques may be used
to dissect the flap including a razor blade (A), diamond blade (B), or a lollipop blade (C) made by Greishaber for dissecting a uniform flap. Fig. 26. Deep sclerectomy with viscocanalostomy: development of the superficial
scleral flap. Flap development with NPFS is decidedly more complicated
than trabeculectomy. It is inherently more difficult to make two flaps
from 1 mm of sclera than one flap. The superficial scleral flap should
be approximately 300 microns in thickness, 5 by 5 mm and parabolic
in shape to aid in eventual closure. The most common mistake is making
the flap too thin with dissolution as the limbus is approached. The flap
should look like C, as the limbus is approached. Various free-hand techniques may be used
to dissect the flap including a razor blade (A), diamond blade (B), or a lollipop blade (C) made by Greishaber for dissecting a uniform flap.
|
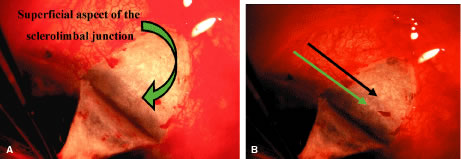 Fig. 27. A. Deep sclerectomy with viscocanalostomy: completion of superficial scleral
flap. The superficial flap should be uniform in thickness, leaving
approximately 700 microns of subscleral tissue for the deep flap dissection. The
flap requires dissection into clear cornea to ensure adequate
exposure of Schlemm's canal and anterior tissues. The subscleral
limbal landmarks start to become visible as the superficial flap is
retracted. It is important to remember a standard trabeculectomy flap
is much thicker and the limbal landmarks are in the deeper sclerolimbal
junction. This is not the case in the Figure 26 in which the flap is only 300 microns. The green arrow indicates the anterior
extent of the superficial sclerolimbal junction and does not contain
the scleral spur, which is much deeper. B. Deep sclerectomy with viscocanalostomy: limbal structures identified with
superficial flap. The anterior limit of the sclerolimbal junction
is easily seen (green arrow) but the posterior limit is more difficult because the flap is very superficial. By
slightly darkening the slide, the more posterior structures
are more easily visible, the black arrow designates the deeper aspect
of the sclerolimbal junction that should be the scleral spur. Fig. 27. A. Deep sclerectomy with viscocanalostomy: completion of superficial scleral
flap. The superficial flap should be uniform in thickness, leaving
approximately 700 microns of subscleral tissue for the deep flap dissection. The
flap requires dissection into clear cornea to ensure adequate
exposure of Schlemm's canal and anterior tissues. The subscleral
limbal landmarks start to become visible as the superficial flap is
retracted. It is important to remember a standard trabeculectomy flap
is much thicker and the limbal landmarks are in the deeper sclerolimbal
junction. This is not the case in the Figure 26 in which the flap is only 300 microns. The green arrow indicates the anterior
extent of the superficial sclerolimbal junction and does not contain
the scleral spur, which is much deeper. B. Deep sclerectomy with viscocanalostomy: limbal structures identified with
superficial flap. The anterior limit of the sclerolimbal junction
is easily seen (green arrow) but the posterior limit is more difficult because the flap is very superficial. By
slightly darkening the slide, the more posterior structures
are more easily visible, the black arrow designates the deeper aspect
of the sclerolimbal junction that should be the scleral spur.
|
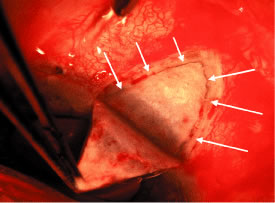 Fig. 28. Deep sclerectomy with viscocanalostomy: outlining the deep scleral flap. There
are several nuances related to outlining the deep flap. The incision
is made in the scleral bed (white arrows), approximately 1 mm from the border of the bed. The depth of the incision
should be down to but not touching choroid. As the limbus is approached, caution
is needed to prevent entering the anterior chamber. However, the
depth of the incision should be kept constant as the limbus
is approached to facilitate a clean dissection of the deep flap up to
and beyond Schlemm's canal. If the incision is made too close to
the border of the bed, postoperative fibrosis is more likely to seal
the ostia of Schlemm's canal. Fig. 28. Deep sclerectomy with viscocanalostomy: outlining the deep scleral flap. There
are several nuances related to outlining the deep flap. The incision
is made in the scleral bed (white arrows), approximately 1 mm from the border of the bed. The depth of the incision
should be down to but not touching choroid. As the limbus is approached, caution
is needed to prevent entering the anterior chamber. However, the
depth of the incision should be kept constant as the limbus
is approached to facilitate a clean dissection of the deep flap up to
and beyond Schlemm's canal. If the incision is made too close to
the border of the bed, postoperative fibrosis is more likely to seal
the ostia of Schlemm's canal.
|
 Fig. 29. Deep sclerectomy with viscocanalostomy: development of the deep scleral
flap. A. Fashioning of the deep scleral flap is a near choroidal experience. The
depth of the dissection is down to choroid, only sparing a few scleral
fibers. Achieving this plane is critical to successfully unroof Schlemm's
canal. If the flap is too superficial, Schlemm's canal
will be passed over and the formation of the trabeculodescemetic membrane
becomes much more difficult. If the plane is deep enough, Schlemm's
canal will unroof as seen in (B), revealing the floor of the canal. Fig. 29. Deep sclerectomy with viscocanalostomy: development of the deep scleral
flap. A. Fashioning of the deep scleral flap is a near choroidal experience. The
depth of the dissection is down to choroid, only sparing a few scleral
fibers. Achieving this plane is critical to successfully unroof Schlemm's
canal. If the flap is too superficial, Schlemm's canal
will be passed over and the formation of the trabeculodescemetic membrane
becomes much more difficult. If the plane is deep enough, Schlemm's
canal will unroof as seen in (B), revealing the floor of the canal.
|
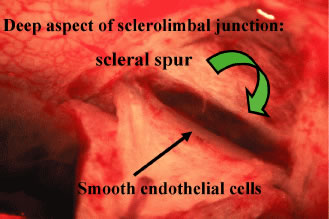 Fig. 30. Deep sclerectomy with viscocanalostomy: unroofing Schlemm's canal. Unroofing
Schlemm's canal is critical to all deep sclerectomy techniques. As
the deep flap scleral dissection is carried towards the limbus, a
transition zone is found. If in the proper plane with the deep
flap dissection, the roof of Schlemm's canal will be found on the
back side of the deep flap (black arrow). It is important to look for this transition zone of the smooth endothelial
cells of Schlemm's canal as they are in contrast to the rough
scleral fibers. Fig. 30. Deep sclerectomy with viscocanalostomy: unroofing Schlemm's canal. Unroofing
Schlemm's canal is critical to all deep sclerectomy techniques. As
the deep flap scleral dissection is carried towards the limbus, a
transition zone is found. If in the proper plane with the deep
flap dissection, the roof of Schlemm's canal will be found on the
back side of the deep flap (black arrow). It is important to look for this transition zone of the smooth endothelial
cells of Schlemm's canal as they are in contrast to the rough
scleral fibers.
|
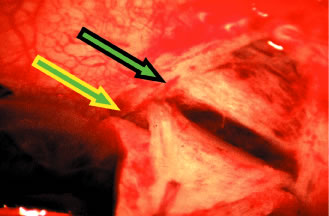 Fig. 31. Deep sclerectomy with viscocanalostomy: limbal landmarks. The green arrows
designate the anterior and posterior limit of the surgeons blue zone. The
anterior limit is designated by the green arrow with the yellow
border and the posterior limit is easiest to see designated by the scleral
spur (green arrow with black border). This zone is usually 1.2 to 1.5 mm in anterior to posterior width but
varies considerably between eyes. Limbal anatomic clues will vary depending
on the depth of the flap. The deep flap dissection verifies the
presumed location of the scleral spur seen in Figure 27. In congenital glaucoma eyes, the scleral spur may be found considerably
more posterior to this figure. Fig. 31. Deep sclerectomy with viscocanalostomy: limbal landmarks. The green arrows
designate the anterior and posterior limit of the surgeons blue zone. The
anterior limit is designated by the green arrow with the yellow
border and the posterior limit is easiest to see designated by the scleral
spur (green arrow with black border). This zone is usually 1.2 to 1.5 mm in anterior to posterior width but
varies considerably between eyes. Limbal anatomic clues will vary depending
on the depth of the flap. The deep flap dissection verifies the
presumed location of the scleral spur seen in Figure 27. In congenital glaucoma eyes, the scleral spur may be found considerably
more posterior to this figure.
|
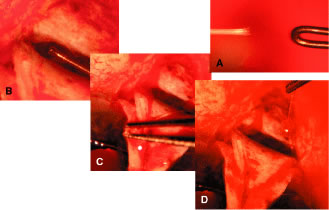 Fig. 32. Deep sclerectomy with viscocanalostomy: identification of Schlemm's
canal with a suture probe. Canal identification is aided by inserting
a thermally blunted (A) 5-0 clear nylon suture into the proposed canal site (B). There are three possible locations the suture may traverse: properly
into the canal, posteriorly into the supraciliary space, or anteriorly
into the anterior chamber. Even though the distal suture end is hidden
from view, verification of canal identity is made by flexing the suture. Flex
the suture anteriorly (C) and release. On release, the suture should bounce back to its original
position if secured properly in the canal. If the suture stays flexed
anteriorly, it is likely in the supraciliary space. If the suture is
mistakenly in the anterior chamber, posterior flexion over the sclera (D) will cause the distal end to migrate into the anterior chamber, an obvious
sign of malposition. Anterior and posterior flexion with immediate
return to the original position is a good sign the probe is in the
canal. Fig. 32. Deep sclerectomy with viscocanalostomy: identification of Schlemm's
canal with a suture probe. Canal identification is aided by inserting
a thermally blunted (A) 5-0 clear nylon suture into the proposed canal site (B). There are three possible locations the suture may traverse: properly
into the canal, posteriorly into the supraciliary space, or anteriorly
into the anterior chamber. Even though the distal suture end is hidden
from view, verification of canal identity is made by flexing the suture. Flex
the suture anteriorly (C) and release. On release, the suture should bounce back to its original
position if secured properly in the canal. If the suture stays flexed
anteriorly, it is likely in the supraciliary space. If the suture is
mistakenly in the anterior chamber, posterior flexion over the sclera (D) will cause the distal end to migrate into the anterior chamber, an obvious
sign of malposition. Anterior and posterior flexion with immediate
return to the original position is a good sign the probe is in the
canal.
|
 Fig. 33. Deep sclerectomy with viscocanalostomy: fiberoptic verification of Schlemm's
canal. Another method of verifying canal identify is to illuminate
the suture probe. Make contact with the fiberoptic light source
against the proximal suture end. The thermally blunted end that is hopefully
in the canal will light up like a search light in the canal lumen (green arrow). Fig. 33. Deep sclerectomy with viscocanalostomy: fiberoptic verification of Schlemm's
canal. Another method of verifying canal identify is to illuminate
the suture probe. Make contact with the fiberoptic light source
against the proximal suture end. The thermally blunted end that is hopefully
in the canal will light up like a search light in the canal lumen (green arrow).
|
 Fig. 34. Deep sclerectomy with viscocanalostomy: viscocanalostomy. After identifying
Schlemm's canal, Healon GV (Pharmacia and Upjohn, Uppsala, Sweden) is
injected carefully into its ostia. This is a very delicate maneuver. A
special cannula with an outside diameter of 150 microns and
inside diameter of 110 microns is inserted 1 mm into the canal. Initially, a
small amount of Healon GV is injected into the canal that refluxes
out. This is irrigated away, and several more applications of viscoelastic
applied, gently sliding the cannula further into the canal to
a maximum of 2 mm. Great care is taken to keep the cannula in the canal. Any
rotation about the axis of the cannula will cause laceration
of the canal. Normally, the height of the canal is 30 microns. Dilation
increases the size to approximately 200 microns. However, postoperatively, the
lumen of the canal gradually decreases. Both sides of the canal
are filled. After removing the deep flap, the canal is filled again
to prevent reflux of blood from Schlemm's canal into the intrascleral
cavern. Fig. 34. Deep sclerectomy with viscocanalostomy: viscocanalostomy. After identifying
Schlemm's canal, Healon GV (Pharmacia and Upjohn, Uppsala, Sweden) is
injected carefully into its ostia. This is a very delicate maneuver. A
special cannula with an outside diameter of 150 microns and
inside diameter of 110 microns is inserted 1 mm into the canal. Initially, a
small amount of Healon GV is injected into the canal that refluxes
out. This is irrigated away, and several more applications of viscoelastic
applied, gently sliding the cannula further into the canal to
a maximum of 2 mm. Great care is taken to keep the cannula in the canal. Any
rotation about the axis of the cannula will cause laceration
of the canal. Normally, the height of the canal is 30 microns. Dilation
increases the size to approximately 200 microns. However, postoperatively, the
lumen of the canal gradually decreases. Both sides of the canal
are filled. After removing the deep flap, the canal is filled again
to prevent reflux of blood from Schlemm's canal into the intrascleral
cavern.
|
 Fig. 35. A and B. Deep sclerectomy with viscocanalostomy: creation of trabeculodescemetic
membrane. Identifying and cannulating Schlemm's canal is difficult, but
creating this thin gossamer membrane is even more difficult. The
deep scleral flap is normally tethered anteriorly by the anterior
boundary of Schlemm's canal. This anterior boundary must be extended
approximately 1.5 mm anteriorly into clear cornea. This is accomplished
by making a radial extension on the sides of the canal into clear
cornea with a diamond knife (A). The tendency is to go to shallow for fear of penetrating into the anterior
chamber. The correct depth down to Descemet's is necessary
to allow the flap to extend anteriorly. A Weck-cell sponge (B) is useful to facilitate blunt dissection of the deep flap from Descemet's, however, excessive force will assuredly cause rupture into
the anterior chamber. The sponge is useful to gauge flow through the thin
membrane. Most investigators recommend removing the thin inner wall
of Schlemm's canal from the floor of the canal to encourage flow. Fig. 35. A and B. Deep sclerectomy with viscocanalostomy: creation of trabeculodescemetic
membrane. Identifying and cannulating Schlemm's canal is difficult, but
creating this thin gossamer membrane is even more difficult. The
deep scleral flap is normally tethered anteriorly by the anterior
boundary of Schlemm's canal. This anterior boundary must be extended
approximately 1.5 mm anteriorly into clear cornea. This is accomplished
by making a radial extension on the sides of the canal into clear
cornea with a diamond knife (A). The tendency is to go to shallow for fear of penetrating into the anterior
chamber. The correct depth down to Descemet's is necessary
to allow the flap to extend anteriorly. A Weck-cell sponge (B) is useful to facilitate blunt dissection of the deep flap from Descemet's, however, excessive force will assuredly cause rupture into
the anterior chamber. The sponge is useful to gauge flow through the thin
membrane. Most investigators recommend removing the thin inner wall
of Schlemm's canal from the floor of the canal to encourage flow.
|
 Fig. 36. Deep sclerectomy with viscocanalostomy: deep sclerectomy. The deep scleral
flap is rotated anteriorly and amputated at its base with Vannas scissors. Avoid
penetrating into the anterior chamber while removing the
deep flap. In this case, the resected flap is placed on the cornea to
view the transition zone of the sclerolimbal tissue. Deep flap removal
creates the potential intrascleral space for the percolation of aqueous. Fig. 36. Deep sclerectomy with viscocanalostomy: deep sclerectomy. The deep scleral
flap is rotated anteriorly and amputated at its base with Vannas scissors. Avoid
penetrating into the anterior chamber while removing the
deep flap. In this case, the resected flap is placed on the cornea to
view the transition zone of the sclerolimbal tissue. Deep flap removal
creates the potential intrascleral space for the percolation of aqueous.
|
 Fig. 37. Deep sclerectomy with viscocanalostomy (DSVC): ultrasound biomicroscopy (UBM). This
UBM demonstrates the intrascleral cavern approximately 2 weeks
after DSVC. The trabeculodescemetic membrane (TDM) is shown as the
window that allows movement of aqueous from the anterior chamber into
the intrascleral cavern. A shallow bleb is not uncommon in the first 2 weeks
after surgery and usually manifested as microcysts. (UBM courtesy of Robert Feldman MD.) Fig. 37. Deep sclerectomy with viscocanalostomy (DSVC): ultrasound biomicroscopy (UBM). This
UBM demonstrates the intrascleral cavern approximately 2 weeks
after DSVC. The trabeculodescemetic membrane (TDM) is shown as the
window that allows movement of aqueous from the anterior chamber into
the intrascleral cavern. A shallow bleb is not uncommon in the first 2 weeks
after surgery and usually manifested as microcysts. (UBM courtesy of Robert Feldman MD.)
|
 Fig. 38. A and B. Deep sclerectomy with viscocanalostomy: closure of scleral and conjunctival
flaps. Both the superficial and scleral flaps are closed in a watertight
fashion. In addition, viscoelastic is reinserted underneath the
scleral flap after its closure to prevent fibrosis in this potential
space. Avoid overfill which could lead to a Descemet's detachment. A
tight closure of the conjunctiva is encouraged in case suture lysis
is necessary along with goniopuncture to encourage subconjunctival
flow if collector system outflow is minimal. Fig. 38. A and B. Deep sclerectomy with viscocanalostomy: closure of scleral and conjunctival
flaps. Both the superficial and scleral flaps are closed in a watertight
fashion. In addition, viscoelastic is reinserted underneath the
scleral flap after its closure to prevent fibrosis in this potential
space. Avoid overfill which could lead to a Descemet's detachment. A
tight closure of the conjunctiva is encouraged in case suture lysis
is necessary along with goniopuncture to encourage subconjunctival
flow if collector system outflow is minimal.
|
Postoperative Care Postoperative care is similar to trabeculectomy, but gonioscopy is even
more critical to visualize the cavern. It is not unusual to see conjunctival
microcysts for the first 3 weeks, but if flap closure is tight, bleb
formation is unusual. Postoperative IOP is usually in the 10 to 20 mm
Hg range during the first week and may increase over a 3-week period. Occasionally, goniopuncture of the TDM is necessary if IOP becomes
unmanageable (Fig. 39). Iris incarceration may require iridoplasty, and Descemet's detachment
has been reported (Fig. 40). It is unusual to see choroidal effusion or shallow anterior chamber. If
optic nerve damage appears imminent, goniopuncture of the TDM along
with suture lysis will usually encourage filtration during the first
two postoperative weeks. Complications include rupture of Descemet's
window, choroidal penetration, hyphema, IOP spike, choroidal detachment, and
cataract formation.  Fig. 39. Deep sclerectomy with viscocanalostomy: goniophotograph of trabeculodescemetic
membrane (TDM). The TDM is visible postoperatively through gonioscopic
means. This goniophotograph reveals pigment in the trabecular
meshwork with the clear membrane located anterior to the pigment. Blood
may reflux into this intrascleral cavern during gonioscopy but rapidly
clears. The red arrow points to a goniopuncture site. Fig. 39. Deep sclerectomy with viscocanalostomy: goniophotograph of trabeculodescemetic
membrane (TDM). The TDM is visible postoperatively through gonioscopic
means. This goniophotograph reveals pigment in the trabecular
meshwork with the clear membrane located anterior to the pigment. Blood
may reflux into this intrascleral cavern during gonioscopy but rapidly
clears. The red arrow points to a goniopuncture site.
|
 Fig. 40. Deep sclerectomy with viscocanalostomy (DSVC): Descemet's detachment. Descemet's
detachment is a complication of any anterior segment
procedure but is more likely to occur with DSVC. The need to inject
viscoelastic multiple times during viscocanalostomy increases the likelihood
of Descemet's detachment. If the spaces are over filled, it
is possible to detach Descemet's as seen in this photo. Of interest
is the clear cornea. Normally Descemet's detachment causes
a cloudy cornea. This cornea remained clear for months because the viscoelastic
prevented aqueous from gaining access to the stroma. This detachment
was not associated with the paracentesis but most likely occurred
from overinjecting viscoelastic into the intrascleral cavern at the
end of the procedure. This is a problem with DSVC because the viscoelastic
is injected underneath the flap and it is difficult to gauge the
amount. Fig. 40. Deep sclerectomy with viscocanalostomy (DSVC): Descemet's detachment. Descemet's
detachment is a complication of any anterior segment
procedure but is more likely to occur with DSVC. The need to inject
viscoelastic multiple times during viscocanalostomy increases the likelihood
of Descemet's detachment. If the spaces are over filled, it
is possible to detach Descemet's as seen in this photo. Of interest
is the clear cornea. Normally Descemet's detachment causes
a cloudy cornea. This cornea remained clear for months because the viscoelastic
prevented aqueous from gaining access to the stroma. This detachment
was not associated with the paracentesis but most likely occurred
from overinjecting viscoelastic into the intrascleral cavern at the
end of the procedure. This is a problem with DSVC because the viscoelastic
is injected underneath the flap and it is difficult to gauge the
amount.
|
DEEP SCLERECTOMY WITH COLLAGEN IMPLANT
The technique for DSCI (Figs.
41, 42, 43,
44, and 45)
is similar to DSVC. Steps 1 through 12 are the same; except step 9 is
eliminated and viscoelastic is not inserted underneath the scleral flap.
DSCI procedures typically produce a bleb. Bleb formation even occurs without
insertion of the collagen implant. One study found raised blebs in 24%
of eyes, diffuse shallow blebs in 34%, and no noticeable bleb in 42% of
eyes.108 Pressure reduction in eyes without
noticeable blebs may be related to flow through Schlemm's canal or uveoscleral
outflow. Complete resorption of the collagen implant typically occurs
between 6 and 9 months.117
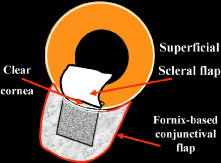 Fig. 41. Deep sclerectomy with collagen implant: superficial scleral flap. After
obtaining adequate limbal exposure, a fornix-based conjunctival flap
is prepared. A 5 × 5 mm superficial limbal based 1/3 thickness scleral
flap is developed in a uniform fashion. Cautery is used judiciously, but
sclerosis of outflow vessels is not as critical as with deep
sclerectomy with viscocanalostomy (DSVC). This superficial flap is dissected 1 mm
into clear cornea to allow space for dissection of canal
area. Fig. 41. Deep sclerectomy with collagen implant: superficial scleral flap. After
obtaining adequate limbal exposure, a fornix-based conjunctival flap
is prepared. A 5 × 5 mm superficial limbal based 1/3 thickness scleral
flap is developed in a uniform fashion. Cautery is used judiciously, but
sclerosis of outflow vessels is not as critical as with deep
sclerectomy with viscocanalostomy (DSVC). This superficial flap is dissected 1 mm
into clear cornea to allow space for dissection of canal
area.
|
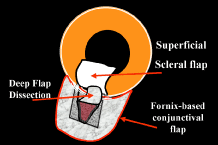 Fig. 42. Deep sclerectomy with collagen implant (DSCI): outlining and dissecting
deep flap. The same principles for deep lamellar dissection apply to
DSCI. The dissection must be carried down to choroid to unroof the canal. Fig. 42. Deep sclerectomy with collagen implant (DSCI): outlining and dissecting
deep flap. The same principles for deep lamellar dissection apply to
DSCI. The dissection must be carried down to choroid to unroof the canal.
|
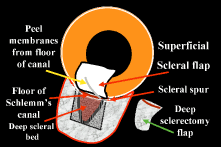 Fig. 43. Deep sclerectomy with collagen implant: deep sclerectomy and juxtacanalicular
membrane peel. The deep flap is dissected anteriorly exposing the
roof of Schlemm's canal. To increase filtration, a fine capsulorhexis
forceps is used to peel off the juxtacanalicular membranes (yellow arrow) associated with the floor of Schlemm's canal. The internal scleral
flap is excised along its base, slightly anterior to Schwalbe's
line (green arrow). The deep sclerectomy specimen contains the roof of Schlemm's canal. Fig. 43. Deep sclerectomy with collagen implant: deep sclerectomy and juxtacanalicular
membrane peel. The deep flap is dissected anteriorly exposing the
roof of Schlemm's canal. To increase filtration, a fine capsulorhexis
forceps is used to peel off the juxtacanalicular membranes (yellow arrow) associated with the floor of Schlemm's canal. The internal scleral
flap is excised along its base, slightly anterior to Schwalbe's
line (green arrow). The deep sclerectomy specimen contains the roof of Schlemm's canal.
|
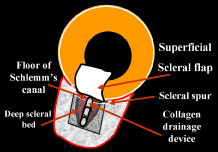 Fig. 44. Deep sclerectomy with collagen implant: placement of collagen implant. The
cylindrical collagen implant drainage device (STAAR Surgical Co., Monrovia, CA) measures 2.5 mm. in length and 1 mm in diameter. The device
is placed radially in the left of the scleral bed and secured with
a 10-0 nylon suture. Fig. 44. Deep sclerectomy with collagen implant: placement of collagen implant. The
cylindrical collagen implant drainage device (STAAR Surgical Co., Monrovia, CA) measures 2.5 mm. in length and 1 mm in diameter. The device
is placed radially in the left of the scleral bed and secured with
a 10-0 nylon suture.
|
 Fig. 45. Deep sclerectomy with collagen implant: closure of scleral flap and conjunctiva. The
scleral flap is sutured loosely back into position using
two 10-0 nylon sutures. The collagen device swells, tenting the scleral
flap upwards. The wound is closed by hooding the conjunctiva and Tenon's
over the flap securing with two 8-0 polyglactin sutures. Fig. 45. Deep sclerectomy with collagen implant: closure of scleral flap and conjunctiva. The
scleral flap is sutured loosely back into position using
two 10-0 nylon sutures. The collagen device swells, tenting the scleral
flap upwards. The wound is closed by hooding the conjunctiva and Tenon's
over the flap securing with two 8-0 polyglactin sutures.
|
The success rates for deep sclerectomy are variable depending on technique
and use of antimetabolite. Mermoud and coworkers104 found comparable pressure reduction between trabeculectomy (53.2%) and
DSCI (48.2%). However, goniopuncture with the Nd:YAG laser was necessary
in 23% of patients with a mean time of 9 months after surgery. Goniopuncture
dropped the IOP from a mean of 21 mm Hg to 12 mm Hg. Another
study found the need for goniopuncture at 41%.118 Welsh and colleagues119 found that approximately 10% of patients required repeat surgery for uncontrolled
IOP, and Dahan and Drusedau108 found that repeat surgery was necessary in 85% of eyes with prior medical
therapy. Complications with a greater than 5% chance of occurrence
after DSCI include: bleb encapsulation, choroidal detachment, wound leak, bleb
fibrosis, hyphema, and cataract progression.120 As long as complications continue to arise related to bleb-forming glaucoma
surgery, efforts to create the perfect bleb-less or minimal-bleb
glaucoma procedure will persist. Only time and controlled clinical and
laboratory studies will reveal the role of NPFS in the long-term care
of the glaucoma patient. Nonpenetrating procedures have created a renaissance
in the anatomy and surgery of the outflow pathways. TUBE SHUNTS FOR GLAUCOMA Introduction When a guarded filtration procedure is unlikely to be successful, tube
shunt surgery may be helpful in improving aqueous outflow.121–123 A tube shunt exits the anterior chamber at the limbus or the vitreous
cavity at the pars plana. It extends posteriorly to a reservoir anchored
behind the insertion of the recti muscles. In this system, the filtration
bleb is displaced from the typical limbal region as seen with a
trabeculectomy to a more posterior location. In this area the blebs are
thick-walled and vascular unlike many limbal blebs that are thin and
avascular. A number of clinical situations with uncontrolled glaucoma
seem to be best served by tube shunt placement (Table 29). Table 29. Candidates for a Tube Shunt
Active neovascular glaucoma
Failed antimetabolite-assisted trabeculectomy
Limbal scarring from previous ocular surgery or trauma
High risk for problem with limbal bleb (e.g., contact lens wearer)
Uncontrolled glaucoma after bleb revision
Epithelial downgrowth
Active uveitis
Co-existing vitreoretinal disease (vitreous hemorrhage)
Current Shunts Shunt devices are either nonvalved (Molteno, Baerveldt) or valved (Ahmed, Krupin) (Fig. 46). The Molteno shunt was the first successful, widely used, commercially
available device.124 They are either a single plate or double plate (Table 30). The latter connects to the first reservoir that the intraocular tube
enters via a connecting tube. A double-plate Molteno requires dissection
and placement of a reservoir in two different quadrants. A comparative
study demonstrated superior IOP control with two plates instead of
only one plate.125 The Baerveldt shunts have an even larger surface area then a double-plated
Molteno shunt.126 However, the largest size 500-mm2 Baerveldt did not yield a more successful outcome then the 350-mm2 version and had a higher complication rate.127 There is a special Baerveldt modification in which a 90-degree angulation
helps to place the tube through the pars plana.128  Fig. 46. Current models of tube shunts: Molteno, Baerveldt, Ahmed, and Krupin. Fig. 46. Current models of tube shunts: Molteno, Baerveldt, Ahmed, and Krupin.
|
Table 30. Shunts
| Shunt | Surface Area (mm2) | Material |
| Nonvalved | | |
| Molteno | — | — |
| Single plate | 135 | Polypropylene |
| Double plate | 270 | — |
| Baerveldt | 350 (250, 425) | Silicone |
| Valved | | |
| Krupin | 184 | Silicone |
| Ahmed | 184 (96) | Polypropylene | To help regulate outflow, the Molteno shunt was modified with V-shaped
ridge in the first reservoir, the dual-chamber version.129 In theory, when the IOP becomes elevated, the conjunctiva and Tenon's
layer are elevated by aqueous off of the reservoir allowing for fluid
to spill over the ridge into the second compartment of the first
reservoir. In clinical practice this V-shaped ridge is often insufficient
to prevent hypotony. There are valved shunts that have been designed
to have opening and closing pressures. This is achieved by slits in
the tube (Krupin shunt)130 or by two apposing membranes (Ahmed shunt).131 Surgical Technique PREOPERATIVE ASSESSMENT. The eye must be examined to decide on the best location for the reservoir
placement (usually supertemporally) and tube entry site (e.g., avoid
high peripheral synechiae). Anticoagulants are discontinued whenever
possible to lower the risk of intraocular bleeding. Intravenous mannitol
may reduce the risk of suprachoroidal hemorrhage and uveal effusion. SHUNT PREPARATION. For the nonvalved shunts, an intraluminal suture (4-0 nylon) and an external
ligature (6-0 Vicryl) are placed to restrict outflow and prevent
postoperative hypotony (Fig. 47).132 The Ahmed shunt valve must be primed by injecting fluid through the tube
tip, which separates the two valve leaflets. This is necessary to “open” the
valve and allow it to become functional (Fig. 48). 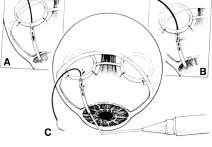 Fig. 47. Tube preparation: an internal 4-0 nylon suture and 6-0 Vicryl external
ligature impedes outflow in nonvalved shunts. Fig. 47. Tube preparation: an internal 4-0 nylon suture and 6-0 Vicryl external
ligature impedes outflow in nonvalved shunts.
|
 Fig. 48. Tube preparation: forceful irrigation through an Ahmed tube “primes” the
valve and opens the pathway for fluid flow with an opening
and closing pressure. Fig. 48. Tube preparation: forceful irrigation through an Ahmed tube “primes” the
valve and opens the pathway for fluid flow with an opening
and closing pressure.
|
RESERVOIR PLACEMENT. The scleral bed is exposed with either a limbal-based or fornix-based conjunctival
flap. Only a single quadrant is prepared except for the double-plate
Molteno that requires exposure of two quadrants. The reservoirs
are secured to the sclera by sutures through the positioning holes
on the reservoir edge (Fig. 49). The sutures are placed with intrascleral passes 8 mm or farther from
the limbus. All the shunts except the Baerveldt have the reservoir sitting
between the recti muscles. The design of the Baerveldt shunt requires
placement of the reservoir wings underneath the recti muscles. Although
some have advocated the use of antimetabolites like an intraoperative
mitomycin C soak in the reservoir site, there has been no study
confirmation of any benefit.133  Fig. 49. Placement of the reservoir: a fornix-based conjunctival flap exposes the
scleral bed for placing and the securing the reservoir about 8 mm or
further from the limbus. Fig. 49. Placement of the reservoir: a fornix-based conjunctival flap exposes the
scleral bed for placing and the securing the reservoir about 8 mm or
further from the limbus.
|
TUBE ENTRY. The tube is trimmed so that there is a bevel facing anteriorly and 2 to 3 mm
will appear within the anterior chamber. After scrutiny for the
ideal tube entry site, a 23-gauge needle is passed through the limbus
to create a tight entry site. The tube tip should not be touching either
the cornea or lens. Alternatively, a pars plana entry could be considered
if a pars plana vitrectomy has been performed.134 After reforming the anterior chamber with balanced salt solution, the
valved shunts should allow for fluid runoff. If the unvalved tube has
been tied tightly so that there is no flow, 1- to 2-mm venting slits are
made with a microblade within the tube proximal to the external ligature. This
will allow for some outflow through the tube in the early
postoperative period. RIPCORD SUTURE PLACEMENT. For the nonvalved shunts, the 4-0 nylon intraluminal suture is passed into
the subconjunctival space of an adjacent quadrant (usually inferotemporally). This
allows for easy removal postoperatively to fully open
the tube for aqueous outflow. WOUND CLOSURE. A donor patch graft is placed over the tube to reduce the risk of tube
erosion through the conjunctiva135 (Fig. 50). Potential donor materials include: sclera, fascia lata, and pericardium. The
conjunctiva is repositioned to carefully cover the tube and overlying
patch graft. For a limbal-based conjunctival flap, a two-layered
closure of Tenon's layer and then conjunctiva is recommended.  Fig. 50. After tube entry into the anterior chamber, a donor tissue patch is placed
over the tube. Fig. 50. After tube entry into the anterior chamber, a donor tissue patch is placed
over the tube.
|
Complications EARLY POSTOPERATIVE PERIOD. Elevated IOP could be due to variety of reasons: valve malfunction, totally
occluded tube by the external ligature, tube tip occlusion, aqueous
misdirection, suprachoroidal hemorrhage, and tube retraction. If there
is an immediate eye pressure elevation with a valved shunt that will
require either repriming the valve by forcible irrigation through the
tube tip or replacing the shunt in an eye with a tight ligature around
the tube without venting slits, medical therapy is initiated to temporize
until removal of the ripcord suture. When the tip of the tube
is occluded with blood, fibrin, or iris it may spontaneously improve. Otherwise, laser
application or sweeping the tissue from the tube may
re-establish patency. Whenever there is a shallow chamber and significant
ocular pain with a high IOP either aqueous misdirection or a suprachoroidal
hemorrhage should be suspected136 (Fig. 51). For aqueous misdirection, medical therapy, cycloplegia, mannitol, and
aqueous suppressants may suffice. If not, then laser disruption of the
anterior hyaloid face or pars plana vitrectomy may be necessary. For
a suprachoroidal hemorrhage, medical management could be attempted before
drainage of the hemorrhage. When the tube tip has retracted and
is no longer visible in the anterior chamber, the tip may be repositioned
if there is extra length available, if not, then an extension piece
of larger caliber tubing can be placed over the tube tip to allow re-entry. It
is also possible to re-enter with the shortened tube through
the pars plana if combined with a pars plana vitrectomy. 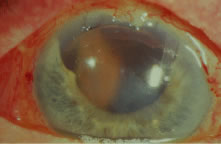 Fig. 51. A shallow anterior chamber resulting from a large suprachoroidal hemorrhage
seen behind the pupil. Fig. 51. A shallow anterior chamber resulting from a large suprachoroidal hemorrhage
seen behind the pupil.
|
Hypotony may be due to a high outflow state and/or choroidal effusion with
hyposecretion. If there is a profound wound leak, it may need to be
repaired. If the anterior chamber has shallowed with a risk of cornea
to lens touch, then reformation of the anterior chamber with a viscoelastic
and possible choroidal effusion drainage may be required. Temporarily
tying off the tube completely in nonvalved shunts may be necessary
in certain cases to help resolve persistent hypotony. LATE POSTOPERATIVE PERIOD. After several weeks if there is an elevation of eye pressure there are
several possible etiologies: bleb encapsulation, steroid response, excessive
subconjunctival fibrosis, or occlusion of the tube tip (Fig. 52). At about 1 month after surgery, there is often an IOP rise despite a
large, thick bleb overlying the reservoir indicating an encapsulation
phase (Fig. 53). This often resolves with conservative management with glaucoma medication. However, needling
of the bleb or surgical revision with partial
excision of the bleb wall may be required in resistant cases.137 Eye pressure elevation secondary to steroid responsiveness138 may be handled by switching to a less potent steroid, tapering off the
topical steroid, or switching to a topical nonsteroidal agent to suppress
inflammation. Failure of the tube shunt because of conjunctival scarring
may require another surgical intervention such as a second tube
shunt in another site or cyclophotocoagulation.139 If the tube tip is enveloped by a fibrous sheet or iris synechiae, it
may be re-opened by YAG laser application.140 Often the tube tip must be repositioned to another entry site. With direct
cornea or lens apposition with the tube, corneal decompensation or
cataract development will ensue. Repositioning of the tube should be
promptly performed at the earliest sign of localized corneal edema. Eyes
with corneal grafts are of greatest concern because of potential danger
to the graft.141 The large posterior blebs seen with shunts may mechanically limit globe
movement and result in diplopia.142 If present in the primary position, prisms may resolve the problem, but
some patients may require strabismus surgery or even removal of the
shunt. Despite use of a patch graft, the tube may erode through the graft
and conjunctiva143 (Fig. 54). Repair must be undertaken because there is a serious risk of intraocular
infection. Insertion of another patch graft and reapproximation of
the overlying conjunctival defect is usually successful. Extrusion of
the reservoir is a more serious and difficult problem to solve satisfactorily (Fig. 55). Closure of the conjunctival defect is hard to maintain, and typically
removal of the shunt is necessary. Placement of another shunt at another
site should be considered.  Fig. 52. Elevated intraocular pressure because of occlusion of the tube tip resulting
from vitreous incarceration. Fig. 52. Elevated intraocular pressure because of occlusion of the tube tip resulting
from vitreous incarceration.
|
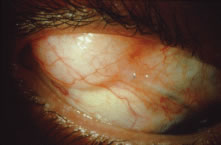 Fig. 53. An encapsulated bleb with a large, thick dome overlying the reservoir that
leads to a transient elevation in the eye pressure. Fig. 53. An encapsulated bleb with a large, thick dome overlying the reservoir that
leads to a transient elevation in the eye pressure.
|
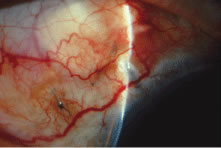 Fig. 54. A conjunctival melt exposing a segment of the tube that is later repaired. Fig. 54. A conjunctival melt exposing a segment of the tube that is later repaired.
|
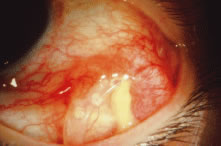 Fig. 55. An extrusion of the reservoir through the conjunctiva that results in explantation
of the shunt device. Fig. 55. An extrusion of the reservoir through the conjunctiva that results in explantation
of the shunt device.
|
Summary Difficult glaucomas are often controlled with tube shunt surgery when other
measures are unsatisfactory. The design of nonvalved and valved shunts
and the techniques for inserting these devices have been modified
to improve the success rate, simplify the operation, and lower the risk
of complications. However, one must still be aware of the potential
complications and appropriately manage them. Many of these postoperative
problems not only threaten to negate the potential eye-pressure lowering
benefit but also may lead to irreversible visual loss. Postoperative Care Topical antibiotics and cycloplegics are routinely used for the first week. Topical
steroids are administered for 2 months or longer. When there
is hypotony and a flat anterior chamber, with imminent lens-cornea
touch, reformation of the anterior chamber with a viscoelastic should
be considered. Increased IOP within the first 3 to 4 weeks should be
managed medically. After that period, there should be a reasonable amount
of fibrosis around the reservoir, which should provide resistance
to outflow and allow for removal of the ripcord intraluminal suture. A
conjunctival incision over the distal end of the 4-0 nylon provides access
to the suture so that it can be pulled out. The timing of the suture
removal will determine the magnitude of the immediate IOP reduction. A
longer waiting period before removal will have a less profound effect
on eye pressure reduction and, correspondingly, less risk of profound
hypotony and its associated complications. |