DIFFERENTIAL DIAGNOSIS
Stage 1 and early stage 2 macular holes can be difficult to diagnose. They have often been confused with epiretinal macular membranes, pseudomacular holes, lamellar macular holes, macular cysts, cystoid macular edema, vitreomacular traction, adult vitelliform degeneration, bull's-eye maculopathy, and idiopathic juxtafoveal telangectasia.
Macular holes usually progress through several stages. A staging method for the development of idiopathic macular holes was originally described by Gass.9 In 1995, he refined his staging classification based on more recent surgical and pathologic data. A modified summary is presented here. Stage 1 occurs when the foveal depression is either decreased or absent, and a yellow ring or spot is present. Stage 2 is marked by early, full-thickness hole formation that is less than 400 μm in diameter. Stage 3 is reached when the hole is fully developed, greater than 400 μm in diameter without a Weiss ring. When the vitreous detaches and a Weiss ring is present, the hole is at stage 4.
Macular holes can resolve spontaneously. This most commonly occurs in stage 1 but has been reported for stage 2 holes as well. The resolution occurs when the posterior hyaloid separates. Traumatic macular holes have also been reported to resolve on their own. Because early holes and traumatic holes may resolve, many surgeons feel it is wise to observe them for a few months. If vision deteriorates or the hole progresses, vitreous surgery is indicated.
The visual acuity of patients with macular holes is variable. Typically, the acuity correlates with the stage of hole development. In stage 1 and early stage 2 holes, the acuity usually falls between 20/25 and 20/60. Late stage 2 and stage three holes fall between 20/70 and 20/200. Stage 4 holes and chronic macular holes (holes greater than 1 year's duration) have visual acuities between 20/400 and counting fingers.
Macular holes are most commonly unilateral.5 However, patients with unilateral macular holes, regardless of stage, should be informed that a hole can develop in their other eye. The incidence of hole formation in the fellow eye in patients with unilateral macular holes is 7%.5 Symptoms of impending holes should be explained to these patients. Symptoms include visual distortion, decreased visual acuity, and changes observed with home Amsler grid testing.
DIAGNOSIS
To diagnose a macular hole accurately, all available methods should be used to rule out other clinically similar conditions. First, the fundus should be examined carefully to determine if there appears to be a clinical posterior vitreous separation, if there is evidence of vitreomacular traction, or if an operculum is present. Next, using a very fine slit beam, the macula should be examined carefully to determine if there is any tissue bridging the apparent macular hole. Also, it should be noted if the edges of the hole are everted or irregular and if there are small, yellowish bodies near the base of the suspected hole. These signs lead to the diagnosis of a true macular hole rather than a pseudohole or other simulating lesion.10
Fundus photography and fluorescein angiography are useful tools in the diagnosis of a macular hole. Stereo color photography helps document the stage, size, and configuration of the hole so that any changes may be detected easily. However, it is important to obtain similar stereo images for serial comparison. Monochromatic photography with use of 490- to 610-nm filters is another useful tool. Monochromatic photos help identify subtle vitreoretinal interface changes.11 Fluorescein angiography, although useful in differentiating a true macular hole from a simulating lesion, can be misleading. The increased transmission of choroidal fluorescence that is associated with macular holes can reverse spontaneously.12 It may also reverse after successful surgical treatment.13
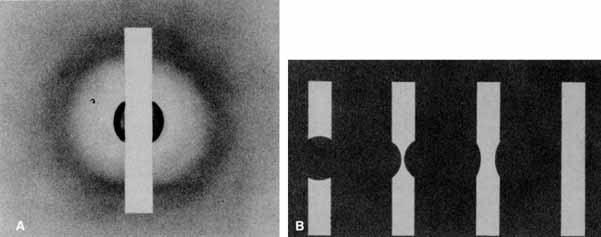
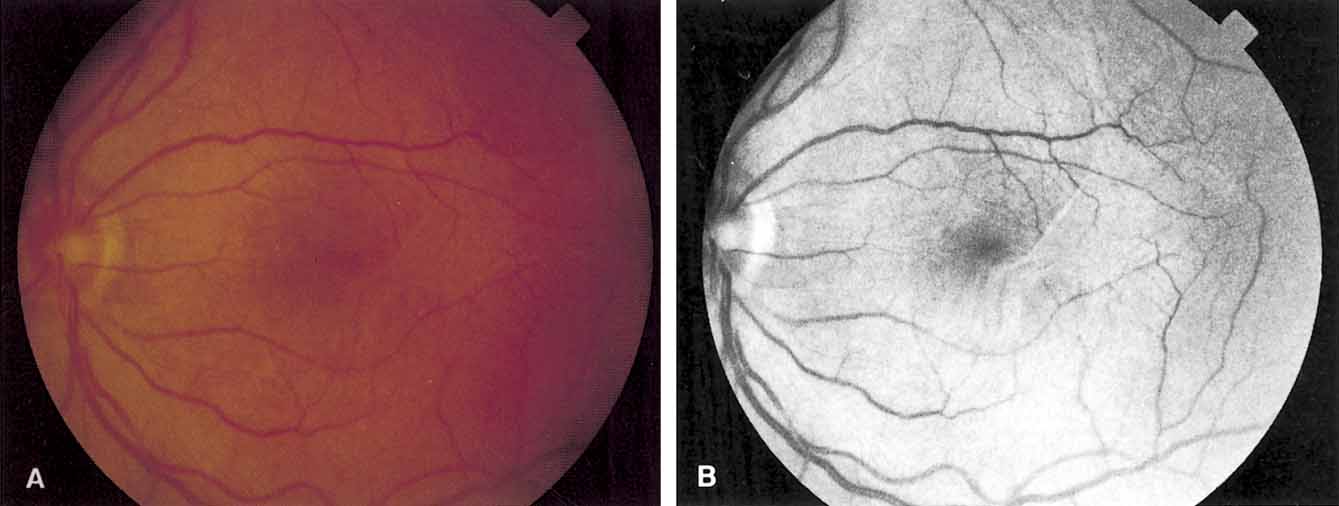
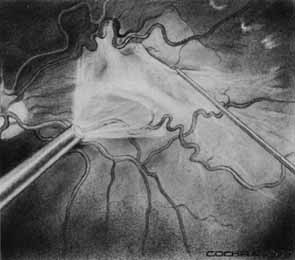
Functional testing has been used to help differentiate pseudoholes or other lesions from true macular holes. The Watzke-Allen sign was one of the first such functional tests.14 This test is performed by presenting a narrow slit beam over the suspected macular hole. The patient is then asked to describe what he or she perceives. If the patient describes a break in the line, a full thickness hole should be suspected (Fig. 1). Another method of performing this test is to pass the slit beam slowly over the macula from varying angles. If the patient perceives a break in the line, at any time, the test is considered to be positive (i.e., there is a full-thickness macular hole).
Another functional test may be performed by aiming the beam of the argon or dye laser. With visualization of the macular region, microperimetry is performed over the macular area. This is accomplished by presenting the 50-μm aiming beam, set at low intensity, to the area of the suspected macular hole and surrounding retina. Cases with full-thickness holes will demonstrate an absolute scotoma in the area of the hole. There will be a relative scotoma at the elevated rim of the neurosensory retina. With other lesions, either no scotoma or only a relative scotoma is seen. In describing the relative scotoma, the patient will usually say that the spot is “present, but different.” The Amsler grid has not been found to be of much value in differentiating true holes from pseudoholes because generally the absolute scotoma in patients with full-thickness macular holes is quite small.
The scanning laser ophthalmoscope (SLO) and the focal electroretinogram have been described as useful for differentiating true holes from other lesions.15–18 Laser biomicroscopy has also been used by some investigators to help demonstrate the fine retinal architecture at the fovea. With this technique, the vitreoretinal interface may be seen with greater detail. This is partly because of the enhanced illumination and the use of monochromatic green light.19,20 Also, some authors believe that a carefully performed B-mode ultrasound can help define the vitreoretinal relationships better than a clinical examination alone.
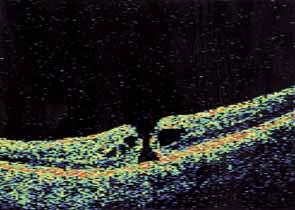
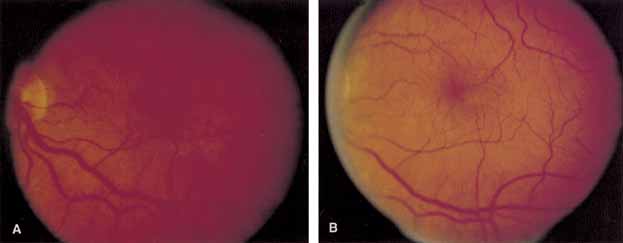
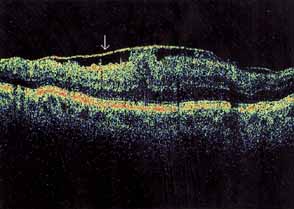
Optical coherence tomography or OCT is a relatively new tool used to diagnose macular lesions. It provides a unique view of the vitreoretinal architecture. OCT is particularly useful in diagnosing lamellar holes. With improvements in resolution and more widespread use, OCT will lead to new advances in the diagnosis and pathogenesis of macular holes (Fig. 2).
|
PATHOGENESIS
The evidence incriminating vitreomacular traction in the pathogenesis of idiopathic macular holes is based on the correlation of clinical and surgical observations with known histopathology and OCT findings. Gass9 proposed that idiopathic macular holes begin from a tractional dehiscence of the umbo with minimal loss of photoreceptors. The tractional forces may be tangential, anteroposterior, or circumferential.21 These forces may resolve with a spontaneous vitreomacular separation22 but in the majority of cases the continuing tractional force will result in a full-thickness macular hole.
Early investigators focused on anteroposterior vitreomacular traction. Schepens,22 in 1955, was the first investigator to relate anteroposterior vitreous traction to the production of macular holes. Subsequently, other authors have made similar observations.6,23,24 Later studies indicated that in most cases, tangential traction plays a major role in the development of idiopathic full-thickness holes.13,25–27
On the basis of histopathologic studies, Foos28 demonstrated the presence of a vitreofoveal attachment that may be involved in the formation of macular holes. The opercula of macular holes obtained during vitrectomy for macular holes have been examined histologically. Macular hole opercula are rarely composed of true retinal tissue.29 The absence of cellular and fibrocellular fragments in the vitreous specimens obtained suggests that mechanisms other than cellular proliferation are important in the generation of the tractional forces required to create a macular hole.
Current theory, based on OCT, biomicroscopy, histology, and surgical experience, suggests that the posterior hyaloid applies traction to the foveola/umbo and causes it to go on stretch. The umbo dehisces because it is the thinnest point in the fovea. Then, according to the hydration theory proposed by Tornambe,30 the middle and inner retina absorbs vitreous fluid at the exposed edges of the hole and begins to swell. The hole enlarges because of a lateral extension of fluid into the outer plexiform layer. There is no mechanical loss of photoreceptors. Moreover, there is no microdetachment beyond the cuff of the hole. Once the inner retina is breached, the macular hole progresses from stage two to three by hydration of the fovea and perifoveal macula. Eventually, the posterior hyaloid separates completely and stage 4 is reached.
Additional support of the macular hole hydration theory may be derived from a report from Chung and Spaide,31 in which emulsified silicone oil migrated (or was absorbed) into the middle layers of the macula, around a successfully closed hole. The authors suggest that internal limiting membrane (ILM) peeling may have allowed the emulsified oil to infiltrate the retina into the macula at the exposed areas of the peel. However, the oil may have entered the middle retina layers through the exposed edges of the macular hole in a fashion similar to vitreous fluid proposed by the hydration theory.
TREATMENT
Stage 1: Impending Macular Holes
The role of vitrectomy for the prevention of full-thickness macular holes is still uncertain. The possible benefits of mechanically removing vitreous traction must be weighed against the known risks. These include retinal detachment, infection, nuclear sclerosis, and the creation of a full-thickness macular hole secondary to surgical manipulation.13,27,32,33 Surgical objectives for stage 1 macular holes consist of removing all tangential and anteroposterior vitreous traction on the foveal region. This must be accomplished without creating a full-thickness hole secondary to forces related to the surgical manipulation.
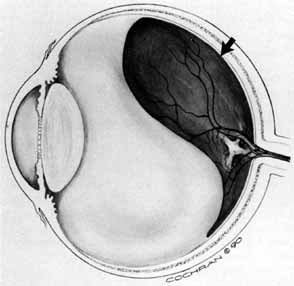
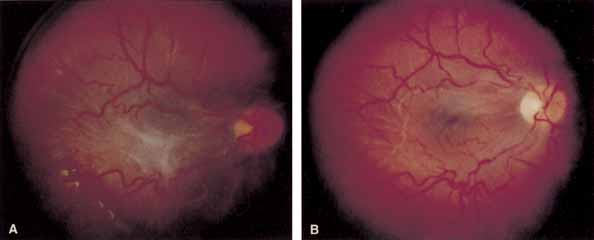
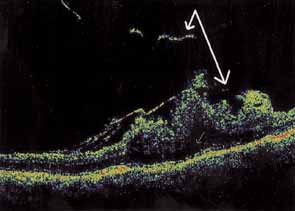
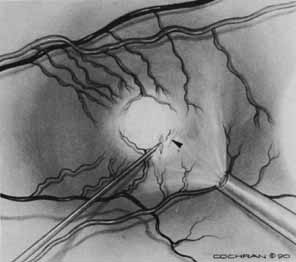
Some authors have commented on the importance of a posterior vitreous detachment in the pathogenesis of a macular hole.34,35 It is difficult to determine the vitreoretinal relationship preoperatively, even with careful slit-lamp evaluation. OCT testing can sometimes be helpful. However, the vitreomacular relationships are more accurately determined intraoperatively with use of oblique intraocular illumination and by noting the effect of gentle tractional forces on the macula during the vitrectomy. In some cases, what was thought to be a posterior vitreous separation preoperatively was actually found to be a large, optically empty space (Fig. 3).
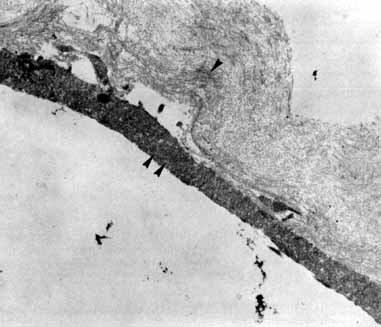
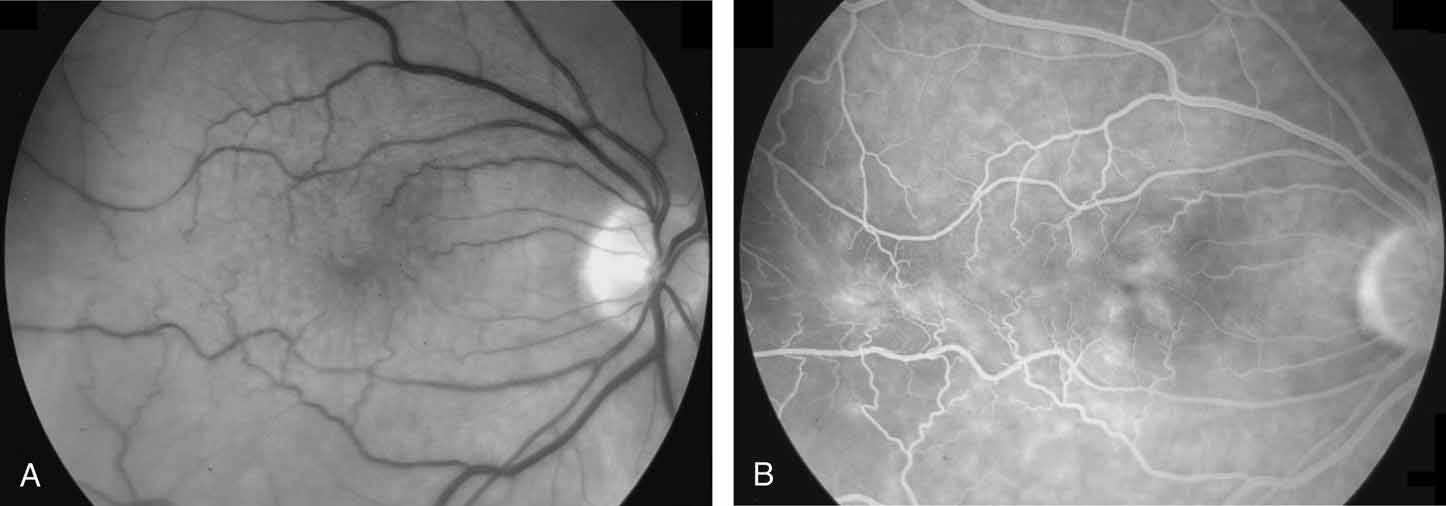
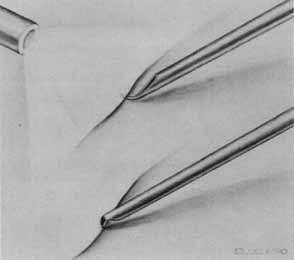
Tissue surgically peeled from the macular region in cases of impending macular hole has been found to be clinically consistent with posterior hyaloid. This finding was supported by electron microscopic examination of the tissue (Fig. 4).13,36 This observation is supported by the work of Kishi and Shimizu.37 They noted a large, optically empty space that appeared to be a complete posterior vitreous detachment in eyes with advanced liquefaction of the vitreous. They termed this area the posterior precortical vitreous pocket (PPVP). They found this pocket in 48 of 84 eyes with either an incomplete or no posterior vitreous detachment, and in 19 of 36 eyes with a posterior vitreous detachment. They noted that in eyes with advanced liquefaction of the vitreous, a large PPVP appeared to be a complete posterior vitreous detachment. In all of their postmortem cases, the posterior layer of the PPVP was found to be a thin layer of cortical vitreous. The presence of this PPVP strengthens the hypothesis that contraction of remaining attached cortical vitreous causes tangential traction on the macula, which gives the clinical appearance of an idiopathic macular cyst or hole.13,25,26,38 These impending holes or cysts' have been noted to resolve with spontaneous or surgical stripping of the membranes.13,25
In the management of eyes considered to be at high risk for the development of full-thickness macular holes, emphasis must be placed on careful follow-up. Patients demonstrating these characteristics should be followed on a monthly or bimonthly basis. Of the patients followed in this manner, approximately one-third will develop a spontaneous vitreomacular separation with an improvement in symptoms. However, if the vision continues to deteriorate to the 20/50 to 20/70 level, surgical intervention should be considered because many of these eyes are likely to progress to full-thickness holes.39 Moreover, better visual acuity results can be achieved with early intervention.
Vitreous Surgery for Full-Thickness Holes
Prior to the landmark paper of Kelly and Wendel,40 full-thickness macular holes were considered to be untreatable. Because there appeared to be an irretrievable loss of foveal tissue, it was assumed that treatment would be of little or no benefit. Once they proved that full-thickness holes could be closed with subsequent improvement in vision, histopathology followed, with proof that photoreceptors were spared in macular hole formation.
Vitreous surgery for macular holes has been refined over the last few years. A standard three-port core vitrectomy is performed. The posterior hyaloid is detached from the retina with the vitrectomy instrument switched to suction mode, at a level of 200 mm Hg. The vitrector port is then placed near the nasal edge of the optic nerve and suction is applied. The posterior hyaloid is engaged and the vitrector tip is pulled anteriorly to promote a posterior hyaloidal separation. A hyaloidal separation is only achieved when a Weiss ring is visualized. After successful detachment of the posterior hyaloid, a more peripheral vitrectomy is performed to provide maximal space for gas tamponade.
An air–fluid exchange is performed with the aid of a soft-tipped cannula. Confirmation of posterior hyaloidal separation can be determined at this time. The soft-tipped cannula is placed near the optic nerve and suction is applied. If the tip engages residual hyaloid, it will bend toward the retina in a fishhook fashion. If residual hyaloid is noted, the vitreous cavity is refilled with fluid and another attempt is made to separate the posterior hyaloid. After the air fluid exchange is completed, 5 to 15 minutes are allowed to elapse for fluid reaccumulation resulting from surface tension. The residual fluid is removed with the soft-tipped cannula and the sclerotomies are trimmed free of vitreous and closed. The air in the vitreous cavity is exchanged with either 16% C3F8 or 20% SF6. The patient is instructed to remain in a face-down position for up to 14 days.
ILM peeling has been reported to be a beneficial adjunct to macular hole surgery. Many surgeons have reported high hole closure rates and excellent visual results.41,42 However, peeling the ILM is the most difficult maneuver in macular hole surgery. New instruments have been designed to aid the surgeon. Vital dyes such as indocyanine green and trypan blue have also been used to help identify the ILM for peeling.43,44
We have found that the ILM does not have to be peeled for surgical success in most cases.45 Moreover, in 2002, Spaide46 reported three cases of successful macular hole surgery with limited vitrectomy over the macular hole without ILM peeling. Similarly, in 2003, Dori et al.47 reported a 100% closure rate without ILM peeling in 50 eyes with stage 2 holes. Ninety-eight percent of these eyes had visual acuity results of 20/50 or better.
Additional complications may occur if ILM peeling is performed. ILM peeling-associated complications include phototoxicity, retinal hemorrhage, RPE defects, and nerve fiber layer defects. Moreover, several recent reports have suggested that indocyanine green, used as an adjunct to ILM peeling, may cause delayed photochemical damage to the RPE.48,49 Indocyanine green has been shown to be present in the fundus and optic disc for up to 12 months after use.50–52 Haritoglou et al.53 found that indocyanine green may alter the cleavage plane in the innermost layers of the retina that may result in less improvement in vision and unexpected visual field defects. Therefore, because of the increased potential for complications, we recommend ILM peeling only for stage 4 macular holes, chronic macular holes, previous surgical failures, and late reopened macular holes.
Laser
Photocoagulation has been used as an adjunct to help repair macular holes. Outpatient laser treatment, combined with fluid gas exchange has been used successfully to close macular holes after initial surgical failures. In 1998, Ohana and Blumenkrantz54 used slit-lamp–based yellow laser to close 13 of 15 holes. Ikuno et al.55 used one spot of argon green to the RPE at the base of the hole to close 12 of 13 holes. Intraoperative endolaser has also played a role in treating macular holes. Woog-Ki et al.56 used one spot of very light endolaser to the RPE at the base of the macular hole and closed 8 of 8 holes. Kwok et al.57 used endophotocoagulation to the base of the hole to help close macular holes in 3 of 4 associated with myopic retinal detachments.
We have reserved the use of endolaser to stage 4 holes, chronic holes, initial surgical failures, and late reopened holes in which the ILM is not peeled or is only partially peeled. We know from experience that these cases are likely to fail if the ILM is not peeled or an endolaser is not used. We use an argon green endolaser at very low power and short duration. A test spot is placed in the macula in a safe zone near an arcade vessel. Laser power is slowly increased until a faint grey blanching of the RPE is achieved. The probe is then placed over the hole and very close to the RPE to minimize the spot size. One spot is then applied to the RPE through the opening in the hole. We have had excellent success with this technique. The application of laser to the RPE at the base of the hole may stimulate the RPE to pump out the intraretinal fluid proposed by Tornambe's macular hole hydration theory.30
Complications
Complications associated with macular hole surgery, in addition to those that may occur with any intraocular procedure, include elevated intraocular pressure, peripheral retinal tears, retinal detachment, lens opacities, visual field defects, vitreous hemorrhage, and late reopenings.58 Some of these conditions will resolve spontaneously or with treatment; others may result in permanent loss of vision. Most of these eyes will develop visually significant nuclear sclerotic lens opacities within 1 year.58–61
CHRONIC MACULAR HOLES
Chronic macular holes are generally considered to be stage 3 or stage 4 holes that have been present for more than 1 year. These holes, possibly due to long-term intraretinal fluid accumulation, epiretinal membrane formation, and RPE atrophy are more difficult to close compared to acute macular holes. Fortunately, because of increased awareness of macular holes by the general ophthalmic community, chronic holes are becoming far more rare. Visual improvement may occur with successful hole closure. However, these improvements are generally not as pronounced as those seen with acute macular holes. Roth et al.62 were able to close 9 of 11 chronic holes in their series, with a mean postoperative vision of 20/100. ILM peeling, adjuvants, and endolaser may assist in macular hole closure in this challenging subgroup.
TRAUMATIC MACULAR HOLES
Traumatic macular holes are much less common than idiopathic macular holes. Most develop from significant blunt trauma to the eye. These holes are commonly preceded by Berlin's edema. Traumatic macular holes may close spontaneously,63,64 therefore, these holes should be observed for 3 to 6 months before surgery is contemplated. Surgical closure rates for traumatic holes are similar to idiopathic macular holes. Autologous plasmin enzyme has been used as an adjuvant to help close pediatric and adult cases.65,66 Adult traumatic holes have also been successfully closed without the use of adjuvants.67