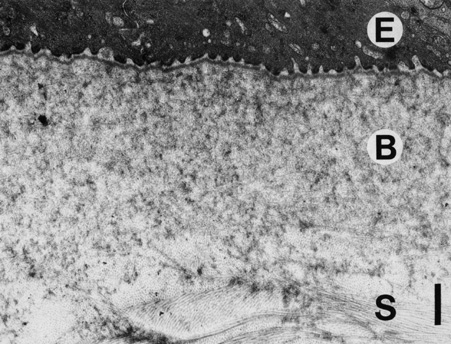
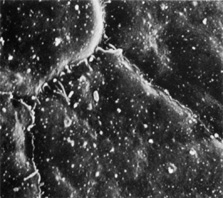
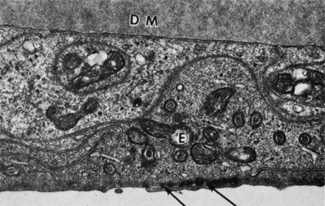
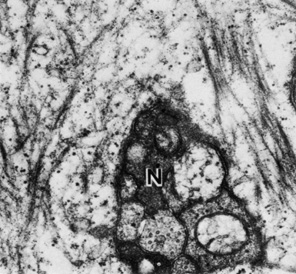
1. Tripathi RC, Tripathi BJ: Anatomy, orbit, and adnexa of the human eye. In Davson H (ed): The Eye. 3rd ed. New York: Academic Press, 1984:1–268 2. Maurice DM: The cornea and sclera. In Davson H (ed): The Eye. 3rd ed. New York, Academic Press, 1984: 1–158 3. Smolek MK, Klyce SD: Is Keratoconus a true ectasia? An evaluation of corneal surface area. Arch Ophthalmol 118:1179, 2000 4. Mishima S, Gassett A, Klyce SD: Determination of tear volume and tear flow. Invest Ophthalmol 5:264, 1966 5. Rolando M, Refojo MF: Tear evaporimeter for measuring water evaporation rate from the tear film
under controlled conditions in humans. Exp Eye Res 36:25, 1983 6. Van Haeringen NJ: Clinical biochemistry of tears. Surv Ophthalmol 26:84, 1981 7. Gipson IK, Yankauchas M, Spurr-Michaud SJ, et al: Characteristics of a glycoprotein in the ocular surface glycocalyx. Invest Ophthalmol Vis Sci 33:218, 1992 8. Nichols BA, Chiappino ML, Dawson CB: Demonstration of the mucous layer of the tear film by electron microscopy. Invest Ophthalmol Vis Sci 26:464, 1985 9. Hazlett LD, Wells P, Spann B, et al: Epithelial desquamation in the adult mouse cornea: a correlative TEM-SEM
study. Ophthalmic Res 12:315, 1980 10. Wolosin JM: Regeneration of resistance and ion transport in rabbit corneal epithelium
after induced surface cell exfoliation. J Membr Biol 104:45, 1988 11. Hanna C, Bicknell DS, O'Brien JE: Cell turnover in the adult human eye. Arch Ophthalmol 65:695, 1961 12. Davenger M, Evenson A: Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature 229:560, 1971 13. Alldredge OC, Krachmer JH: Clinical types of corneal transplant rejection. Their manifestations, frequency
preoperative correlates, and treatment. Arch Ophthalmol 99:599, 1981 14. Thoft RA, Friend J: The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci 24:1442, 1983 15. Wiley L, SunderRaj N, Sun TT, et al: Regional heterogeneity in human corneal and limbal epithelia: an immunohistochemical
evaluation. Invest Ophthalmol Vis Sci 32:594, 1991 16. Dua HS, Azuara-Blanco A: Limbal stem cells of the corneal epithelium. Surv Ophthalmol 44:415, 2000 17. Wilson SE, Liu JJ, Mohan RR: Stromal-epithelial interactions in the cornea. Prog Retin Eye Res 18:293, 1999 18. Doughty MJ: Morphometric analysis of the surface cells of rabbit corneal epithelium
by scanning electron microscopy. Am J Anat 189:316, 1990 19. Nichols B, Dawson CR, Tongi B: Surface features of the conjunctiva and cornea. Invest Ophthalmol Vis Sci 24:570, 1983 20. McLaughlin BJ, Cadwell RB, Sasaki Y, et al: Freeze-fracture quantitative comparison of rabbit corneal epithelial
and endothelial membranes. Curr Eye Res 4:951, 1985 21. Hogan MJ, Alvarado JA, Weddell E: Histology of the Human Eye. Philadelphia: WB Saunders, 1971 22. Buschke W, Friedenwald JS, Fleischmann W: Studies on the mitotic activity of the corneal epithelium: methods- The
effects of colchicine, ether, cocaine, and ephedrin. Bull Johns Hopkins Hosp 73:143, 1943 23. Gipson IK, Spurr-Machaud S, Tisdale A, et al: Redistribution of the hemidesmosome components alpha 6 beta 4 integrin
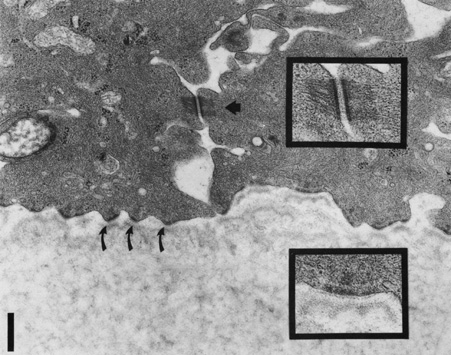
and bullous pemphigoid antigens during epithelial wound healing. Exp Cell Res 207:86, 1993 24. Shams NB, Hanninen LA, Chaves HV, et al: Effect of vitamin A deficiency on the adhesion of rat corneal epithelium
and the basement membrane complex. Invest Ophthalmol Vis Sci 34:2646, 1993 25. Kurpakus MA, Jones JC: A novel hemidesmosomal plaque component tissue distribution and incorporation
into assembling hemidesmosomes in an in vitro model. Exp Cell Res 194:139, 1991 26. Owaribe K, Nishizawa Y, Franke WW: Isolation and characterization of hemidesmosomes from bovine corneal epithelial
cells. Exp Cell Res 192:622, 1991 27. Stepp MA, Spurr-Michaud S, Gipson IK: Integrins in the wounded and unwounded stratified squamous epithelium of
the cornea. Invest Ophthalmol Vis Sci 34:1829, 1993 28. Kurpakus MA, Stock EL, Jones JCR: Analysis of wound healing in an in vitro model: Early appearance of laminin
and a 125 × 103 Mr polypeptide during adhesion complex formation. J Cell Sci 96:651, 1990 29. Trinkhaus-Randall V, Tong M, Thomas P, et al: Confocal imaging of the alpha 6 and beta 4 integrin subunits in the human
cornea with aging. Invest Ophthalmol Vis Sci 34:3103, 1993 30. Fine JD, Horiguchi Y, Jester J, et al: Detection and partial characterization of a midlamina lucida-hemidesmosome-associated antigen (19-DEJ-1) present
within human skin. J Invest Dermatol 92:825, 1989 31. Gipson IK, Spurr-Michaud SJ, Tisdale AS: Anchoring fibrils form a complex network in human and rabbit cornea. Invest Ophthalmol Vis Sci 28:212, 1987 32. Keene DR, Sakai L Y, Lunstrum GP et al: Type VII collagen forms an extended network of anchoring fibrils. J Cell BioI 104:611, 1987 33. Burgeson RE: Type VII collagen. In Mayne R, Burgeson R (eds): Structure and Function of Collagen Types. New York: Academic Press, 1987:145–172 34. Marshall GE, Konstas AG, Lee WR: Immunogold fine structural localization of extracellular matrix compounds
in aged human cornea: I. Types I–IV collagen and laminin. Graefes Arch Clin Exp Ophthalmol 229:157, 1991 35. Konomi H, Hayashi T, Nakayasu K, et al: Localization of type V collagen and type IV collagen in human cornea, lung, and
skin. Am J PathoI 116:417, 1984 36. Newsome DA, Foidart JM, Hassell JH, et al: Detection of specific collagen types for normal and keratoconus corneas. Invest Ophthalmol Vis Sci 20:738, 1981 37. Nakayasu K, Tanaka M, Konomi H, et al: Distribution of types I, II, III, IV, and V collagen in normal and keratoconus
corneas. Ophthalmic Res 18:1, 1986 38. Kolega J, Manabe M, Sun T-T: Basement membrane heterogeneity and variation in corneal epithelial differentiation. Differentiation 42:54, 1989 39. Cleutjens JP, Havenith MG, Kasper M, et al: Absence of type IV collagen in the centre of the corneal epithelial basement
membrane. Histochem J 22:688, 1990 40. Fujikawa LS, Foster CS, Gipson IK, et al: Basement membrane components in healing rabbit corneal epithelial wounds: Immunofluorescence
and ultrastructural studies. J Cell Biol 98:128, 1984 41. Scheinman JI, Tsai C: Monoclonal antibody to type IV collagen with selective basement membrane
localization. Lab Invest 50:101, 1984 42. Linsenmayer TF, Fitch JM, Mayne R: Basement membrane structure and assembly: Inferences from immunological
studies with monoclonal antibodies. In Trelstad R (ed): The Role of Extracellular Matrix in Development. New York: Alan R Liss, 1984:145–172 43. Fitch JM, Linsenmayer TF: Monoclonal antibody analysis of ocular basement membranes during development. Dev Biol 95:137, 1983 44. Yurchenco PD, Schittny JC: Molecular architecture of basement membranes. FASEB J 4:1577, 1990 45. Cleutjens JP, Havenith MG, Vallinga M, et al: Monoclonal antibodies to native basement membranes reveal heterogeneous
immunoreactivity patterns. Histochemistry 92:407, 1989 46. Komai Y, Ushiki T: The three dimensional organization of collagen fibrils in the human cornea
and sclera. Invest Ophthalmol Vis Sci 32:2244, 1991 47. Hanna KD, Jouve FE, Waring GO: Preliminary computer simulation of the effects of radial keratotomy. Arch OphthalmoI 107:911, 1986 48. Marshall GE, Konstas AG, Lee WR: Immunogold fine structural localization of extracellular matrix compounds
in aged human cornea: II. Collagen types V–VI. Graefes Arch Clin Exp Ophthalmol 229:164, 1991 49. Von der Mark K, Von der Mark H, Timpl R, et al: Immunofluorescent localization of collagen types I, II, and III in the
embryonic chick eye. Dev Biol 59:75, 1977 50. Birk DE, Fitch JM, Linsenmayer TF: Organization of collagen types I and V in the embryonic chicken cornea. Invest Ophthalmol Vis Sci 27:1470, 1986 51. Fitch JM, Birk DE, Mentzer A, et al: Corneal collagen fibrils: dissection with specific collagenases and monoclonal
antibodies. Invest Ophthalmol Vis Sci 29:1125, 1988 52. Linsenmayer TF, Bruns RR, Mentzer A, et al: Type VI collagen: immunohistochemical identification as a filamentous component
of the extracellular matrix of the developing avian corneal stroma. Dev Biol 118:425, 1986 53. Tripathi RC, Bron AJ: Secondary anterior crocodile shagreen of Vogt. Br J Ophthalmol 59:59, 1975 54. Linsenmayer TF, Gibney E, Igoe F, et al: Type V collagen: molecular structure and fibrillar organization of the
chicken alpha 1 (V) NH2 -terminal domain, a putative
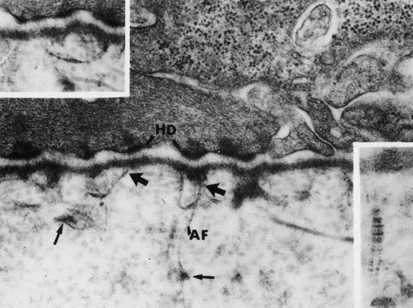
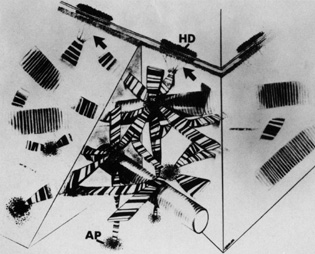
regulator of corneal fibrillogenesis. J Cell Biol 121:1181, 1993 55. Yamamato S, Hashizume H, Hitome J, et al: The subfibrillar arrangement of corneal and scleral collagen fibrils as
revealed by scanning electron and atomic force microscopy. Arch Histol Cytol 63:127, 2000 56. Meller D, Peters K, Meller K: Human cornea and sclera studied by atomic force microscopy. Cell Tissue Res 288:111, 1977 57. Holmes DF, Gilpin CJ, Baldock C, et al: Corneal collagen fibril structure in three-dimensions: structural
insights in fibril assembly, mechanical properties, and tissue organization. Proc Natl Acad Sci USA 98:7307, 2001 58. Ploetz C, Zycband EI, Birk DE: Collagen fibril assembly and deposition in the developing dermis: segmental
deposition in extracellular compartments. J Struct Biol 106:73, 1991 59. Trelstad RL, Coulombre AJ: Morphogenesis of the collagenous stroma in the chick cornea. J Cell Biol 50:840, 1971 60. Meek KM, Blamires T, Elliott GF, et al: The organisation of collagen fibrils in the human corneal stroma: a synchrotron
x-ray diffraction study. Curr Eye Res 6:841, 1987 61. Meek KM, Newton RH: Organization of collagen fibrils in the corneal stroma in relation to mechanical
properties and surgical practice. J Refract Surg 15:695, 1999 62. Maurice DM: Mechanics of the cornea. In Cavanagh HD (ed): The Cornea: Transactions of the World Congress on the Cornea III. New York: Raven Press, 1988:1987–1993 63. Smolek MK, McCarey BE: Interlamellar adhesive strength. Invest Ophthalmol Vis Sci 31:1087, 1990 64. Scott JE: Proteoglycan-fibrillar collagen interactions. Biochem J 252:313, 1988 65. Scott JE, Bosworth TR: A comparative biochemical and ultrastructural study of proteoglycan-collagen
interactions in corneal stroma. Biochem J 270:491, 1990 66. Rada JA, Cornuet PK, Hassell JR: Regulation of corneal collagen fibrillogenesis in vitro by corneal proteoglycan (lumican
and decorin) core proteins. Exp Eye Res 56:635, 1993 67. Cintron C, Covington HI: Proteoglycan distribution in developing rabbit cornea. J Histochem Cytochem 38:675, 1990 68. Castoro JA, Bettelheim AA, Bettelheim FA: Water concentration gradients across bovine cornea. Invest Ophthalmol Vis Sci 29:963, 1988 69. Klyce SD, Russell SR: Numerical solution of coupled transport equations applied to corneal hydration
dynamics. J Physiol 292:107, 1979 70. Borcherding MS, Blacik LJ, Sittig RA, et al: Proteoglycans and collagen fibre organization in human corneoscleral tissue. Exp Eye Res 21:59, 1975 71. Muller LJ, Pels E, Schurmans LRHM, et al: A new three-dimensional model of the organization of proteoglycans
and collagen fibrils in the human corneal stroma. Exp Eye Res 78:493, 2004 72. Maurice DM: The structure and transparency of the cornea. J Physiol 136:263, 1957 73. Hart RW, Farrell RA: Light scattering in the cornea. J Opt Soc Am 59:766, 1969 74. Benedek GB: The theory of transparency of the eye. Appl Optics 10:459, 1971 75. Farrell RA, McCally RL, Tatham PER: Wavelength dependencies of light scattering in normal and cold swollen
rabbit corneas and their structural implications. J Physiol 233:589, 1973 76. Watsky MA: Keratocyte gap junctional communication in normal and wounded rabbit corneas
and human corneas. Invest Ophthalmol Vis Sci 36:2568, 1995 77. Moller-Pedersen T: A comparative study of human corneal keratocyte and endothelial cell density
during aging. Cornea 16:333, 1997 78. Muller LJ, Pels L, Vrensen GF: Novel aspects of the ultrastructural organization of human corneal keratocytes. Invest Ophthal Vis Sci 36:2557, 1995 79. Andresen JL, Ehlers N: Chemotaxis of human keratocytes is increased by platelet-derived
growth factor-BB, epidermal growth factor, transforming growth
factor-alpha, acidic fibroblast growth factor, insulin-like
growth factor-I, and transforming growth factor-beta. Curr Eye Res 17:79, 1998 80. Jester JV, Barry PA, Lind GJ, et al: Corneal keratocytes: in situ and in vitro organization of cytoskeletal
contractile proteins. Invest Ophthalmol Vis Sci 35:730. 1994 81. Andresen JL, Ledet T, Hager H, et al: The influence of corneal stromal matrix proteins on the migration of human
corneal fibroblasts. Exp Eye Res 71:33, 2000 82. Crosson CE: Cellular changes following epithelial abrasion. In Beuerman RW, Crosson CE, Kaufman HE (eds): Healing Processes in the Cornea, Vol 1. Advances in Applied Biotechnology
Series. Houston, TX: Gulf Publishing, 1989: 3–14 83. Moller-Pedersen T: Keratocyte reflectivity and corneal haze. Exp Eye Res 78:553, 2004 84. Zhao J, Nagasaki T: Lacrimal gland as the major source of mouse tear factors that are cytotoxic
to corneal keratocytes. Exp Eye Res 77:297, 2003 85. Birk DE, Trelstad RL: Extracellular compartments in matrix morphogenesis: collagen fibril, bundle, and
lamellar formation by corneal fibroblasts. J Cell Bioi 99:2024, 1984 86. Johnson DH, Bourne WM, Campbell RJ: The ultrastructure of Descemet's membrane. I. Changes with age in
normal cornea. Arch Ophthalmol 100:1942, 1982 87. Bourne WM, Johnson DH, Campbell RJ: The ultrastructure of Descemet's membrane. III. Fuchs' dystrophy. Arch Ophthalmol 100:1952, 1982 88. Binder PS, Rock ME, Coalwell Schmidt K, et al: High-voltage electron microscopy of normal human cornea. Invest Ophthalmol Vis Sci 32:2234, 1991 89. Tamura Y, Konomi H, Sawada H, et al: Tissue distribution of type VIII collagen in human adult and fetal eyes. Invest Ophthalmol Vis Sci 32:2636, 1991 90. Klyce SD, Beuerman RW: Structure and function of the cornea. In Kaufman HE, Barron BA, McDonald MB, Waltman SR (eds): The Cornea. New York: Churchill Livingstone, 1988:3–54. 91. Williams K, Watsky M: Gap junctional communication in the human corneal endothelium and epithelium. Curr Eye Res 25:29, 2002 92. Waring GO, Bourne WM, Edelhauser HF, et al: The corneal endothelium: Normal and pathological structure and function. Ophthalmology 89:531, 1982 93. Kreutziger GO: Lateral membrane morphology and gap junction structure in rabbit corneal
endothelium. Exp Eye Res 23:285, 1976 94. Amann J, Holley GP, Lee SB, et al: Increased endothelial cell density in the paracentral and peripheral regions
of the human cornea. Am J Ophthalmol 135:584, 2003 95. Bourne WM, Nelson LR, Hodge DO: Central corneal endothelial cell changes over a ten-year period. Invest Ophthalmol Vis Sci 38:779, 1997 96. Gallagher B: Primary cilia of the corneal endothelium. Am J Anat 159:475, 1980 97. Senoo T, Obara Y, Joyce N: EDTA. a promoter of proliferation in human corneal endothelium. Invest Ophthal Vis Sci 41:2930, 2000 98. Senoo T, Joyce NC: Cell cycle kinetics in corneal endothelium from old and young donors. Invest Ophthal Vis Sci 41:660, 2000 99. Joyce N: Proliferative capacity of the corneal endothelium. Prog Retin Eye Res 22:359, 2003 100. Sherrard ES, Ng YL: The other side of the corneal endothelium. Cornea 9:48, 1990 101. Rae JL, Watsky MA: Ionic channels in corneal endothelium. Am J Physiology 270:975, 1996 102. Rozsa AJ, Beuerman RW: Density and organization of free nerve endings in the corneal epithelium
of the rabbit. Pain 14:105, 1982 103. Zander E, Weddell G: Observations on the innervation of the cornea. J Anat 85:68, 1951 104. Mawas MJ: L'innervation de la cornee humaine. Bull Soc Ophtalmol Paris 2:162, 1951 105. Muller LJ, Vrensen GF, Pels L, et al: Architecture of human corneal nerves. Invest Ophthalmol Vis Sci 38:985, 1997 106. Klyce SD, Beuerman RW, Crosson CE: Alteration of corneal epithelial ion transport by sympathectomy. Invest Ophthalmol Vis Sci 26:434, 1985 107. Klyce SD, Jenison GL, Crosson CE, et al: Distribution of sympathetic nerves in the rabbit cornea. ARVO Abstract. Invest Ophthalmol Vis Sci 27:354, 1986 |