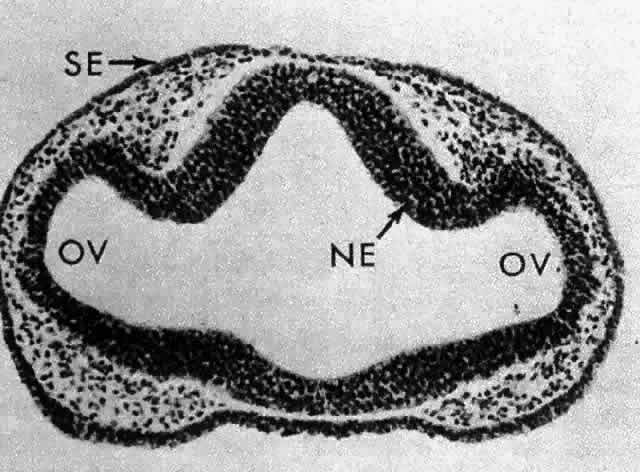
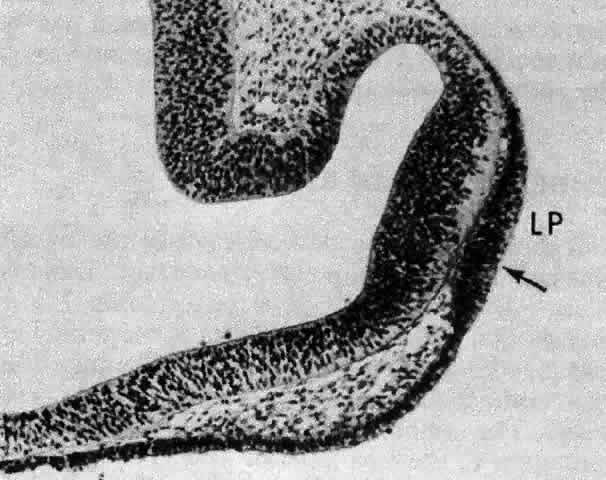
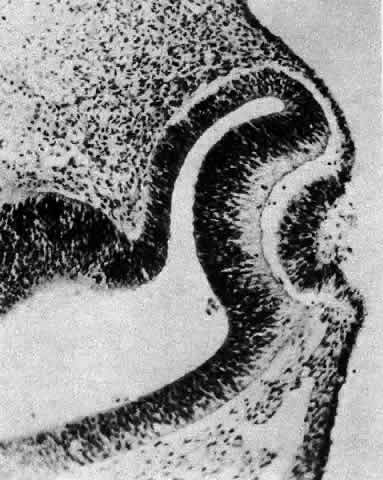
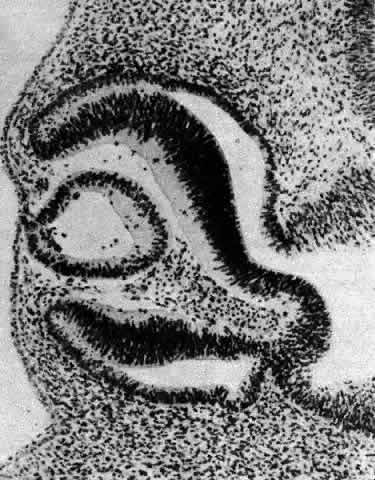
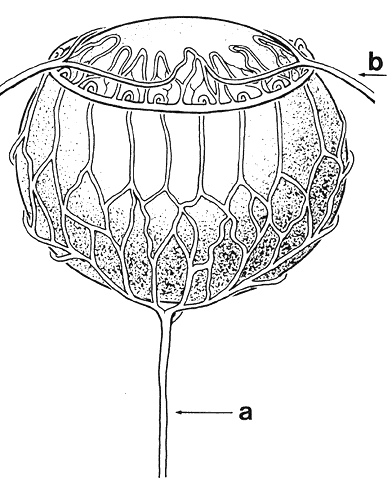
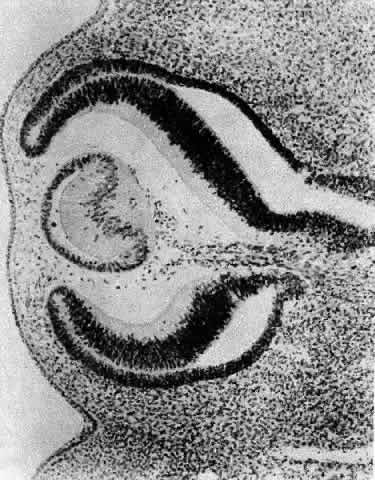
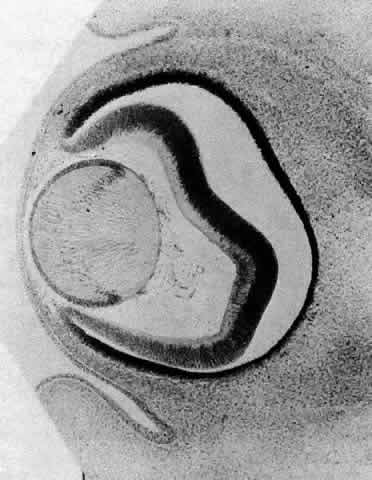
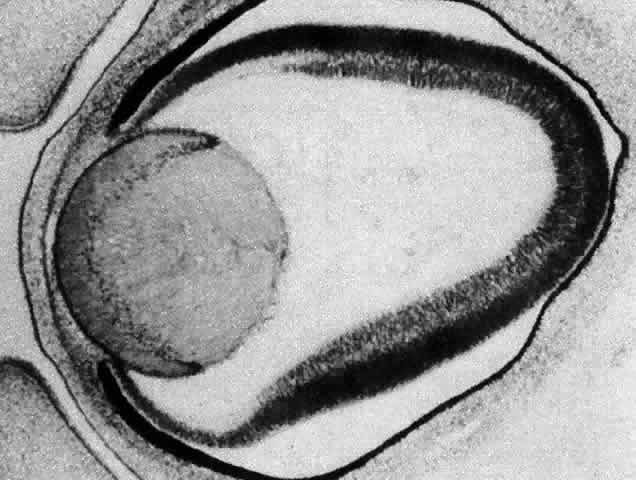
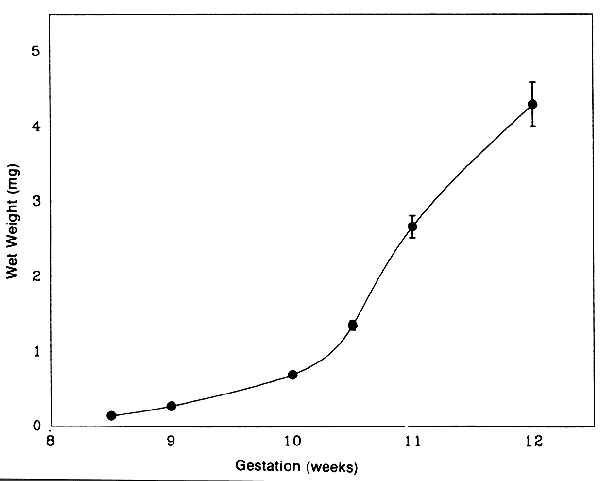
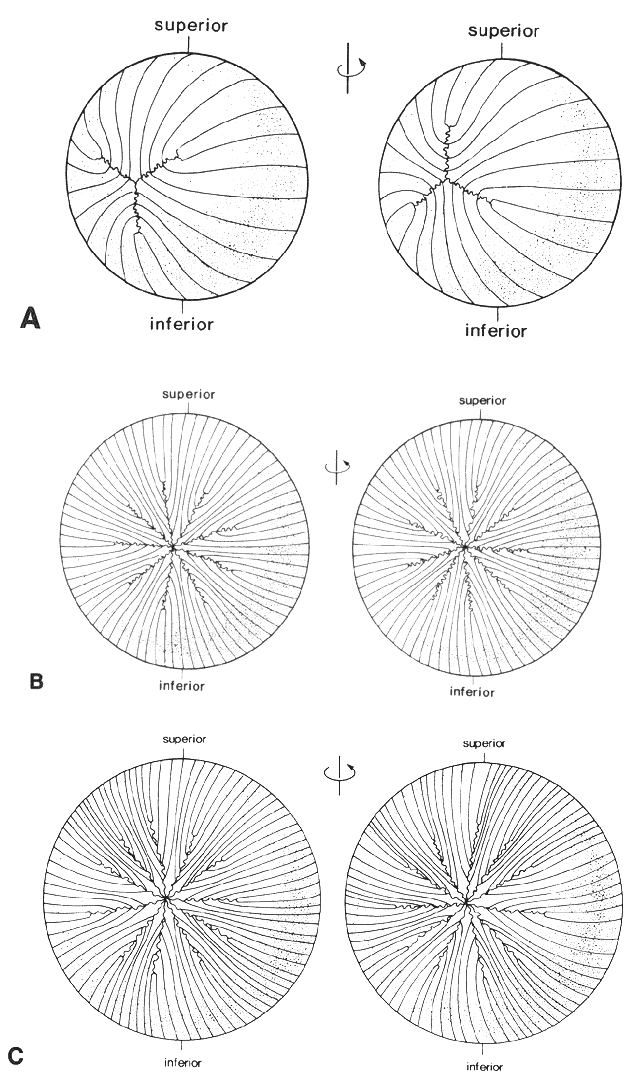
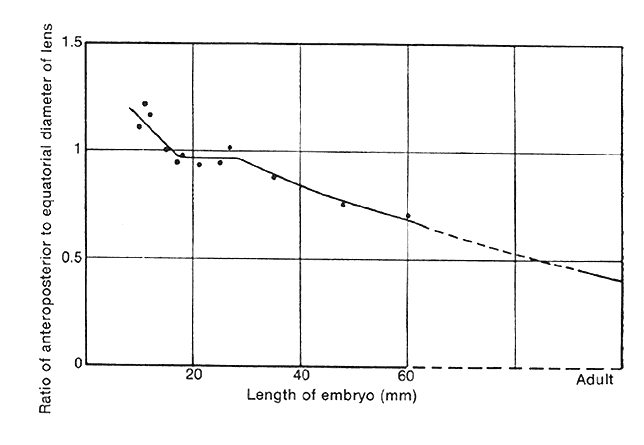
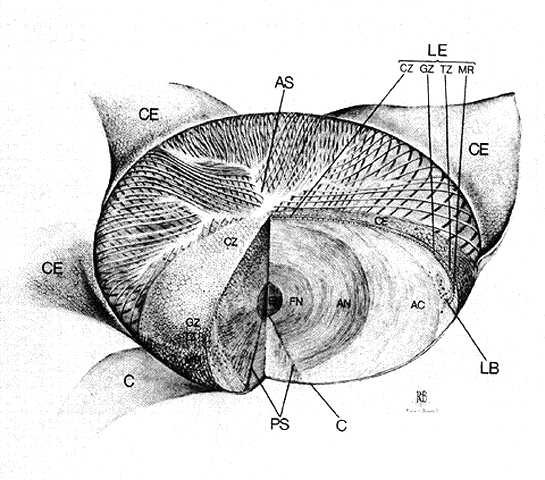
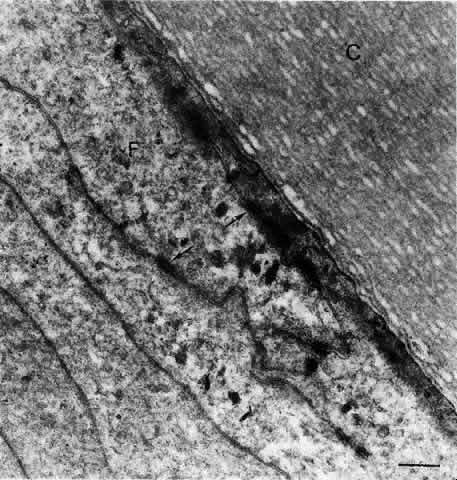
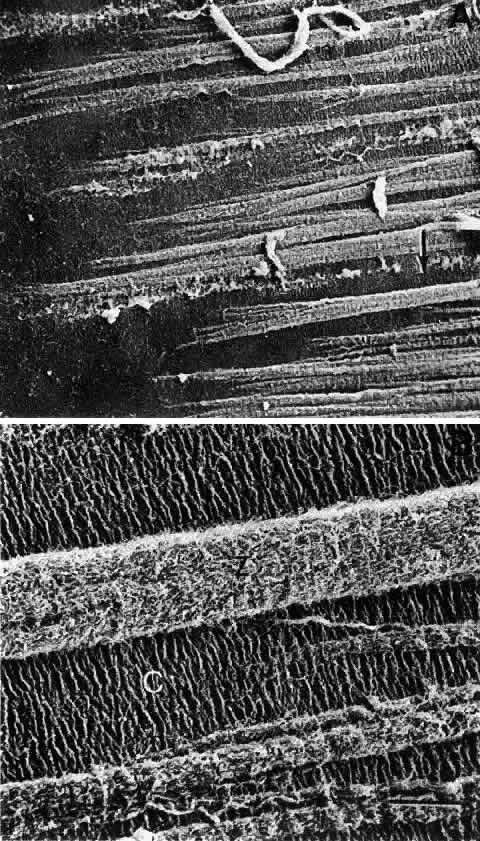
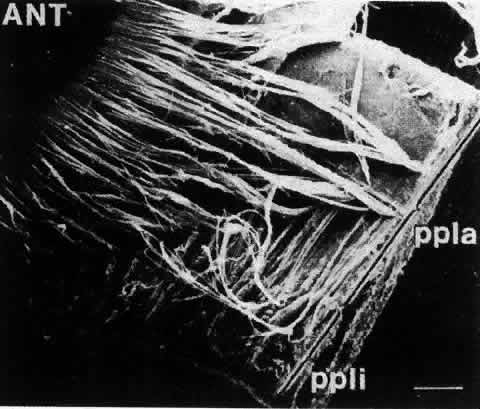
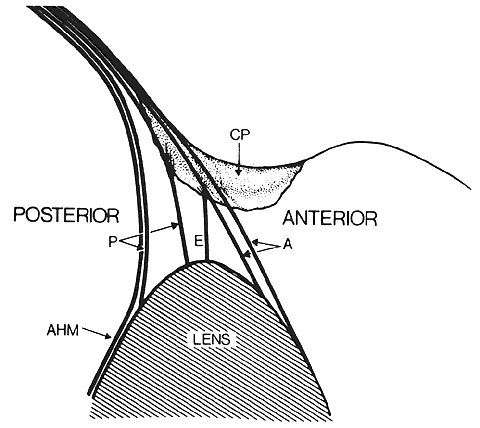
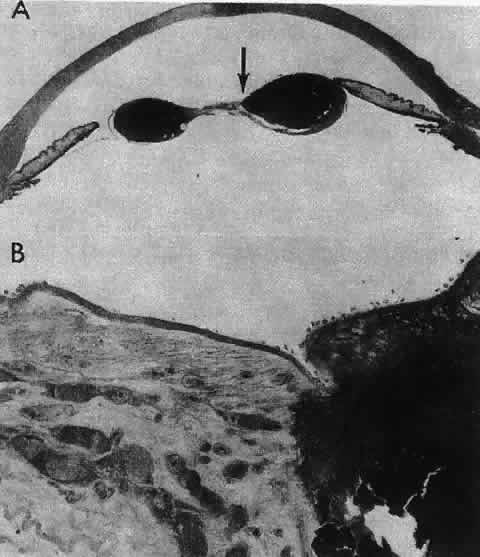
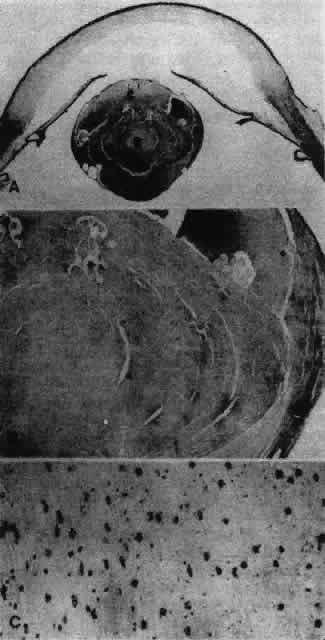
1. van Leeuwenhoek A: Letter no. 11, September 7th, 1674. The Collected Letters
of Antoni van Leeuwenhoek, Part I, pp 137–167 (English). Amsterdam, Swets
Zeitlinger, 1939 2. van Leeuwenhoek A: Letter no. 80, April 14th, 1684. The Collected Letters
of Antoni van Leeuwenhoek, Vol IV, pp 209–251 (English). Amsterdam, Swets
Zeitlinger, 1952 3. van Leeuwenhoek A: Letter no. 81, July 25, 1684. The Collected Letters
of Antoni van Leeuwenhoek, Vol IV, pp 253–299 (English). Amsterdam, Swets
Zeitlinger, 1952 4. van Leeuwenhoek A: Letter no. 82, January 5, 1685. The Collected Letters
of Antoni van Leeuwenhoek, Vol V, pp 3–67 (English). Amsterdam, Swets, Zeitlinger, 1957 5. Stricker L: The Crystalline Lens System. Cincinnati, L Stricker, 1899 6. McKeehan MS: Cytological aspects of embryonic lens induction in the chick. J Exp Zool 117:31, 1951 7. Coulombre J, Coulombre AJ: Lens development: IV. Size, shape and orientation. Invest Ophthalmol 8:251, 1969 8. Coulombre AJ: Experimental embryology of the vertebrate eye. Invest Ophthalmol 4:411, 1965 9. McKeehan M: Induction of portions of the chick lens without contact with the optic
cup. Anat Rec 132:297, 1958 10. O'Rahilly R, Meyer DB: The early development of the eye in the chick: Gallus domesticus. Acta Anat 36:20, 1959 11. Cohen AI: Electron microscopic observations of the developing mouse eye: I. Basement
membranes during early development and lens formation. Dev Biol 3:297, 1961 12. Weiss P, Fitton-Jackson S: Fine structural changes associated with lens determination in the avian
embryo. Dev Biol 3:532, 1961 13. Smelser GK: Embryology and morphology of the lens. Invest Ophthalmol 4:398, 1965 14. Saha MS, Spann CL, Grainger RM: Embryonic lens induction: More than meets the optic vesicle. Cell Differ 28:153, 1989 15. Tripathi BJ, Tripathi RC, Livingston AM, Borisuth NS: The role of growth factors in the embryogenesis and differentiation of
the eye. Am J Anat 192:442, 1991 16. McAvoy JW, Chamberlain CG, deLongh RU et al: The role of fibroblast growth factor in eye lens development. Ann NY Acad Sci 638:256, 1991 17. Henry JJ, Grainger RM: Early tissue interactions leading to embryonic lens formation in Xenopus laevis. Dev Biol 141:149, 1990 18. Grainger RM, Henry JJ, Saha MS, Servetnick M: Recent progress on the mechanics of embryonic lens formation. Eye 6:117, 1992 19. Grainger RM: Embryonic lens induction: Shedding light on vertebrate tissue determination. Trends Genet 8:349, 1992 20. Zwaan J, Bryan PR, Pearce TL: Interkinetic nuclear migration during the early stages of lens formation
in the chicken embryo. J Embryol Exp Morphol 21:71, 1969 21. Cuadros MA, Martin C, Rios A et al: Macrophages of hemangioblastic lineage invade the lens vesicle-ectoderm
interspace during closure and detachment of the avian embryonic lens. Cell Tissue Res 266:117, 1991 22. Lerche W, Wulle KG: Electron microscopic studies on the development of the human lens. Ophthalmologica 158:296, 1969 23. Silver PHS, Wakely J: The initial stage in the development of the lens capsule in chick and mouse
embryos. Exp Eye Res 19:73, 1974 24. Wang JL, Toida K, Uehara Y: The tunica vasculosa lentis; an expedient system for studying vascular
formation and regression. J Electron Microsc (Tokyo) 39:46, 1990 25. Wistow GJ, Piatigorsky J: Lens crystallins: The evolution and expression of proteins for a highly
specialized tissue. Annu Rev Biochem 57:479, 1988 26. van de Kamp M, Zwaan J: Intracellular localization of lens antigens in the developing eye of the
mouse embryo. J Exp Zool 186:23, 1973 27. Piatigorsky J: Lens differentiation in vertebrates. A review of cellular and molecular
features. Differentiation 19:134, 1981 28. McAvoy JW: Cell division, cell elongation and the coordination of crystallin gene
expression during lens morphogenesis in the rat. J Embryol Exp Morphol 45:271, 1978 29. Treton JA, Jacquemin E, Courtois Y, Jeanny JC: Differential localization by in situ hybridization of specific crystallin
transcripts during mouse lens development. Differentiation 47:143, 1991 30. McAvoy JW: Cell division, cell elongation and distribution of α-, ß- and Γ-crystallins
in the rat lens. J Embryol Exp Morphol 44:149, 1978 31. Coulombre JL, Coulombre AJ: Lens development: Fiber elongation and lens orientation. Science 142:1489, 1963 32. Jacobson AG: Inductive processes in embryonic development. Science 152:25, 1966 33. Yamamoto Y: Growth of lens and ocular environment: Role of neural retina in the growth
of mouse lens as revealed by an implantation experiment. Dev Growth Differ 18:273, 1976 34. Beebe DC, Silver MH, Belcher KS et al: Lentropin, a protein that controls lens fiber formation, is related functionally
and immunologically to the insulin-like growth factors. Proc Natl Acad Sci USA 84:2327, 1987 35. McAvoy JW: Beta- and gamma-crystallin synthesis in rat lens epithelium explanted with
neural retina. Differentiation 17:85, 1980 36. Zelenka PS, Pallansch L, Vatal M, Nath P: Proto-oncogene expression during
in vitro differentiation of embryonic chicken lens epithelial explants. In
Piatigorsky J, Shinohara T, Zelenka P (eds): Molecular Biology
of the Eye, Genes, Vision and Ocular Disease, pp 249–258. New
York, Alan R. Liss, 1988 37. Rinaudo JA, Zelenka PS: Expression of c-fos and c-jun mRNA in the developing chicken lens: Relationship
to cell proliferation, quiescence and differentiation. Exp Cell Res 199:147, 1992 38. Kuszak JR, Bertram BA, Macsai MS, Rae JL: Sutures of the crystallin lens: A review. Scanning Electron Microsc 3:1369, 1984 39. Kuszak, Sivak JG, Weerheim JA: Lens optical function is a direct function of lens sutural architecture. Invest Ophthalmol Vis Sci 32:2119, 1991 40. Kuszak JR: Ultrastructure of mammalian lens fiber cells and epithelium. Int
Rev Cytol Surv Cell Biol (in press, 1994) 41. Kuszak JR, Herbert KL, Sivak JG: The interrelationship of lens anatomy
and optical quality: Primate lenses. Exp Eye Res (in press, 1994) 42. Harding JJ, Rixon KC, Marriott FHC: Men have heavier lenses than women of the same age. Exp Eye Res 25:651, 1977 43. Weekers R, Delmarcelle Y, Luyckx-Bacus J, Collignon J: Morphological changes
of the lens with age and cataract. Ciba Foundation Symposium 19, The
Human Lens in Relation to Cataract. Amsterdam, Associated Scientific, 1973 44. Brown N: The change in lens curvature with age. Exp Eye Res 19:175, 1974 45. Charles MW, Brown N: Dimensions of the human eye relevant to radiation protection. Phys Med Biol 20:202, 1975 46. Farnsworth PN, Shyne SE: Anterior zonular shifts with age. Exp Eye Res 28:291, 1979 47. Lowe RF, Clark BAJ: Posterior corneal curvature: Correlations in normal eyes and in eyes involved
with primary angle-closure glaucoma. Br J Ophthalmol 57:464, 1973 48. Lowe RF, Clark BAJ: Radius of curvature of the anterior lens surface. Correlations in normal
eyes and in eyes involved with primary angle-closure glaucoma. Br J Ophthalmol 57:471, 1973 49. Duke-Elder S, Wybar K: System of Ophthalmology, Vol 2, The Anatomy of the
Visual System, p205. London, Henry Kimpton, 1961 50. Wolff E, Warwick R: Anatomy of the Eye and Orbit. Philadelphia, WB Saunders, 1976 51. Karim AKA, Jacob TJC, Thompson GM: The human anterior lens capsule: Cell density, morphology and mitotic index
in normal and cataractous lenses. Exp Eye Res 45:865, 1987 52. Kuszak JR, Brown HG: Embryology and anatomy of the crystallin lens. In
Albert DM, Jakoviec FA (eds): Principles and Practice of Ophthalmology, pp 82–96. Philadelphia, WB Saunders, 1994 53. Howard A: Whole-mounts of rabbit lens epithelium for cytological study. Stain Technol 27:313, 1952 54. Rothstein H: Experimental techniques for the investigation of the amphibian
lens epithelium. In Prescott DM (ed): Methods in Cell Physiology, Vol
III, pp 45–74. New York, Academic Press, 1968 55. Harding CV, Reddan JR, Unakar NJ, Bagchi M: The control of cell division in the ocular lens. Int Rev Cytol 31:215, 1971 56. Brown NAP, Bron AJ: An estimate of the human lens epithelial size in vivo. Exp Eye Res 44:899, 1987 57. Guggenmoos-Holtzman I, Engel B, Henke V, Naumann GOH: Cell density of human lens epithelium of human cataracts. Ophthalmology 94:875, 1987 58. Vasavada AR, Cherian M, Yadav S, Rawal UM: Lens epithelial cell density and histomorphological study in cataractous
lenses. J Cataract Refract Surg 17:798, 1991 59. Konofsky K, Naumann GOH: Cell density and sex chromatin in lens epithelium of human cataracts. Ophthalmology 94:875, 1987 60. Rafferty NS: Lens morphology. In Maisel H (ed): The Ocular Lens, Structure
Function and Pathology, pp 1–60. New York, Marcel Dekker, 1985 61. Brown HG, Pappas GD, Ireland ME, Kuszak JR: Ultrastructural, biochemical and immunologic evidence of receptor mediated
endocytosis in the crystalline lens. Invest Ophthalmol Vis Sci 31:2579, 1990 62. Gorthy WC, Snavely MR, Berrong ND: Some aspects of transport and digestion in the lens of the normal young
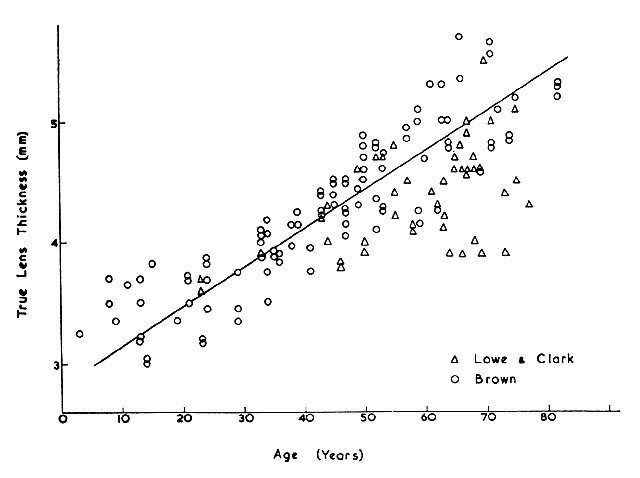
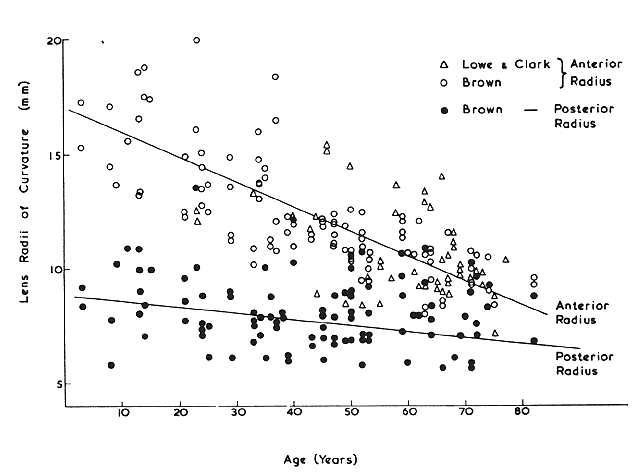
adult rat. Exp Eye Res 12:112, 1971 63. Rae JL, Stacey T: Lanthanum and procion yellow are extracellular markers in the crystalline
lens of the rat. Exp Eye Res 28:1, 1979 64. Worgul BV, David J, Odrich S et al: Evidence of genotoxic damage in human cataractous lenses. Mutagenesis 6:495, 1991 65. Harding CV, Donn A, Srinivasan BD: Incorporation of thymidine by injured lens epithelium. Exp Cell Res 18:582, 1959 66. Rafferty NS: Studies of an injury induced growth in the frog lens. Anat Rec 146:299, 1963 67. Rafferty NS: Proliferative response in experimentally injured frog lens epithelium: Autoradiographic
evidence for movement of DNA synthesis toward injury. J Morphol 121:295, 1967 68. Rafferty NS: The cytoarchitecture of normal mouse lens epithelium. Anat Rec 173:225, 1972 69. Rafferty NS: Mechanism of repair of lenticular wounds in Rana pipiens: I. Role of cell migration. J Morphol 133:409, 1972 70. Rothstein H, Weinsieder A, Blaiklock R: Response to injury in the lens epithelium of the bullfrog, Rana catesbeina. Exp Cell Res 35:548, 1964 71. Rothstein H, Reddan JR, Weinsieder A: Response to injury in the lens epithelium of the bullfrog: II. Spatio-temporal
patterns of DNA synthesis and mitosis. Exp Cell Res 37:440, 1965 72. Weinsieder A, Rothstein H, Drebert D: Lenticular wound healing: Evidence for genomic activation. Cytobiologie 7:406, 1973 73. Gierthy JF, Rothstein H: Cell disorganization and the stimulation of mitosis in the cultured leopard
frog lens. Exp Cell Res 64:170, 1971 74. Rothstein H, Worgul B: Mitotic variations in the lens epithelium of the frog: II. Possible role
of temperature and hormones. Ophthalmol Res 5:151, 1973 75. van Buskirk R, Worgul BV, Rothstein H, Wainwright N: Mitotic variations in the lens epithelium of the frog: III. Somatotropin. Gen Comp Endocrinol 25:52, 1975 76. Worgul BV, Rothstein H: On the mechanism of thyroid mediated mitogenesis in adult anura: I. Preliminary
analyses of growth kinetics and macromolecular syntheses, in
lens epithelium, under the influence of exogenous triiodothyronine. Cell Tissue Kinet 7:415, 1974 77. Weinsieder A, Briggs R, Reddan J et al: Induction of mitosis in ocular tissue by chemotoxic agents. Exp Eye Res 20:33, 1975 78. Reddan J, Weinsieder A, Wilson D: Aqueous humor from traumatized eyes triggers cell division in the epithelia
of cultured lenses. Exp Eye Res 28:267, 1979 79. Worgul BV, Merriam GR Jr: The effect of endotoxin induced intraocular inflammation on the rat lens
epithelium. Invest Ophthalmol Vis Sci 18:401, 1979 80. Muggleton-Harris AL: Cellular changes occurring with age in the lens cells of the frog (Rana pipiens) in reference to the developmental capacity of the transplanted nuclei. Exp Gerontol 5:227, 1970 81. Alcala J, Maisel H: Biochemistry of lens plasma membranes and cytoskeleton. In
Maisel H (ed): The Ocular Lens, pp 169–222. New York, Marcel
Dekker, 1985 82. Rafferty NS, Scholz DL, Goldberg M, Lewyckyj M: Immunocytochemical evidence for an actin-myosin system in lens epithelial
cells. Exp Eye Res 51:591, 1990 83. Rafferty NS, Scholz DL: Polygonal arrays of microfilaments in epithelial cell of the intact lens. Curr Eye Res 3:1141, 1984 84. Wanko T, Gavin MA: The fine structure of the lens epithelium, an electron microscopic study. Arch Ophthalmol 60:868, 1958 85. Hertl M: Kernvolumen und Nukleolarapparat wachsender Linsenzellen. Zellforsch Micro Anat Abt Histochem 43:228, 1955 86. Srinivasan BD, Iwamoto T: Electron microscopy of rabbit lens nucleoli. Exp Eye Res 16:9, 1973 87. Kuwabara T: The maturation of the lens cell: A morphologic study. Exp Eye Res 20:427, 1975 88. Eguchi G: Electron microscopic studies on lens regeneration: II. Formation and growth
of lens vesicle and differentiation of lens fibers. Embryologia 8:247, 1964 89. Karasaki S: An electron microscopic study of wolffian lens regeneration in the adult
newt. J Ultrastruct Res 11:246, 1964 90. Papaconstantinou J: Molecular aspects of lens cell differentiation. Science 156:338, 1967 91. Kuwabara T: Microtubules in the lens. Arch Ophthalmol 79:189, 1968 92. Rafferty NS, Goossens W: Ultrastructure of traumatic cataractogenesis in the frog: A comparison
with mouse and human lens. Am J Anat 148:385, 1977 93. Maisel H: Filaments of the vertebrate lens. Experientia 33:525, 1977 94. Maisel H, Lieska N, Bradley R: Isolation of filaments of the chick lens. Experientia 34:352, 1978 95. Piatigorsky J, Webster H, Wolberg H: Cell elongation in the cultured embryonic chick lens epithelium with and
without protein synthesis. J Cell Biol 55:82, 1972 96. Mousa GY, Trevithick JR: Differentiation of rat lens epithelial cells in tissue culture: II. Effects
of cytochalasins B and D on actin organization and differentiation. Dev Biol 60:14, 1977 97. Worgul BV, Rothstein H: On the mechanism of radiocataractogenesis. Medikon 6:5, 1977 98. Worgul BV, Rothstein H: Congenital cataracts associated with disorganized meridional rows in a
new laboratory animal: The degu (Octodon degus). Biomed Exp 23:1, 1975 99. Streeten BW, Eshaghian J: Human posterior subcapsular cataract. Arch Ophthalmol 96:1653, 1978 100. Messier B, Leblond C: Cell proliferation and migration as revealed by radioautography after injection
of thymidine-H3 into male rats and mice. Am J Anat 106:247, 1960 101. Brolin SE, Diderholm H, Hammar H: An autoradiographic study on cell migration in the eye lens epithelium. Acta Soc Med Ups 66:43, 1961 102. Mikulicich A, Young R: Cell proliferation and displacement in the lens epithelium of young rats
injected with tritiated thymidine. Invest Ophthalmol 2:344, 1963 103. Hammar H: An autoradiographic study on cell migration in the eye lens epithelium
from normal and alloxan diabetic rats. Acta Ophthalmol 43:442, 1965 104. Worgul BV, Merriam GR Jr, Szechter A, Srinivasan BD: Lens epithelium and radiation cataract: I. Preliminary studies. Arch Ophthalmol 94:996, 1976 105. Hayden J, Rothstein H: A correlation between mitosis and cell migration in the Rana pipiens lens. J Cell Biol 79:19a, 1978 106. Moduk SP, Bollum FJ: Detection and measurement of single-strand breaks in nuclear DNA in fixed
lens sections. Exp Cell Res 75:307, 1972 107. Kuwabara T, Imaizumi M: Denucleation process of the lens. Invest Ophthalmol 13:973, 1974 108. Vrensen GF, Graw J, DeWolf A: Nuclear breakdown during terminal differentiation of primary lens fibers
in mice: A transmission electron microscopic study. Exp Eye Res 52:647, 1991 109. Jurand A, Yamada T: Elimination of mitochondria during wolffian lens degeneration. Exp Cell Res 46:636, 1967 110. Brown NAP: Lens change with age and cataract; slit-image photography. Ciba
Foundation Symposium 19, The Human Lens in Relation to Cataract, pp 65–78. New
York, Associated Scientific, 1973 111. Vinciguerra MJ, Bettelheim FA: Packing and orientation of fiber cells. Exp Eye Res 11:214, 1971 112. Tardieu A, Delaye M: Eye lens proteins and transparency: From light transmission theory to solution
x-ray structural analysis. Annu Rev Biophys Chem 17:47, 1988 113. Thoft RA, Kinoshita JH: The extracellular space of the lens. Exp Eye Res 4:287, 1965 114. Paterson CA: The extracellular space of the crystallin lens. Am J Physiol 218:797, 1970 115. Yorio T, Bentley PJ: Distribution of the extracellular space of the amphibian lens. Exp Eye Res 23:601, 1976 116. Kuszak JR, Ennesser CA, Bertram BA et al: The contribution of cell-to-cell fusion to the ordered structure of the
crystalline lens. Lens Eye Toxic Res 6:639, 1989 117. Dickson DH, Crock GW: Interlocking patterns on primate lens fibers. Invest Ophthalmol 11:809, 1972 118. Harding CV, Susan S, Murphy H: Scanning electron microscopy of the adult rabbit lens. Ophthalmic Res 8:443, 1976 119. Farnsworth PN, Shyne SE, Burke PA et al: Maturation of the human lens fiber. In
Srivastava S (ed): The Red Blood Cell and the Lens, pp 45–48. New
York, Elsevier, 1980 120. Cohen AI: The electron microscopy of the normal human lens. Invest Ophthalmol 4:433, 1965 121. Worgul BV, Iwamoto T, Merriam GR Jr: RNA-containing cytoplasmic inclusions at the termini of maturing fibers
in the rat lens. Ophthalmic Res 9:388, 1977 122. Lo WK: Adherens junctions in the ocular lens of various species; ultrastructural
analysis with an improved fixation. Cell Tissue Res 254:31, 1988 123. Kuszak J, Maisel H: Morphology of differentiating lens fiber cells. Invest Ophthalmol Vis Sci 17(suppl):281, 1978 124. Kuszak JR, Sheh YH, Carney KC, Rae JL: A correlate freeze-etch and electrophysiological study of communicating
junctions in crystalline lenses. Curr Eye Res 4:1145, 1985 125. Nicholson BJ, Takemoto LJ, Hunkapiller MW: Differences between liver gap junction protein and lens MIP26 from rat: Implications
for tissue specificity of gap junctions. Cell 32:967, 1983 126. Gorin MB, Yancy SB, Cline J et al: The major intrinsic protein (MIP) of the bovine fiber membrane: Characterization
and structure based on cDNA cloning. Cell 39:49, 1984 127. Philipson BT, Hanninen L, Balazs EA: Cell contacts in human and bovine lenses. Exp Eye Res 21:205, 1975 128. Mathias RT, Rae JL: Transport properties of the lens. Am J Physiol 249:C181, 1985 129. Duncan G: Relative permeabilities of the lens membrane to sodium and potassium. Exp Eye Res 8:315, 1969 130. Rae JL: Potential profiles in the crystalline lens of the frog. Exp Eye Res 19:227, 1974 131. Young RW, Ocumpaugh DE: Autoradiographic studies on the growth and development of the lens capsule
in the rat. Invest Ophthalmol 5:583, 1966 132. Wislocki GB: The anterior segment of the eye of the rhesus monkey investigated by histochemic
means. Anat Rec 113:579, 1952 133. Wulle KG, Lerche W: Zur Feinstrukur der embryonalen menschlichen Linsenblase. Albrecht von Graefes Arch Klin Exp Ophthalmol 173:141, 1969 134. Farnsworth PN, Mauriello JA, Burke-Gadomski P et al: Surface ultrastructure of the human lens capsule and zonule attachments. Invest Ophthalmol 15:36, 1976 135. Hettlich HJ, Wenzel M, Janssen M, Miltermayer C: Immunohistochemische untersuchungen der menschlichen Linsenkapsel. Fortschr Ophthalmol 87:147, 1990 136. Marshall GE, Konstas AG, Bechrakis NE, Lee WR: An immunoelectron microscope study of the aged human lens capsule. Exp Eye Res 54:393, 1992 137. Kohno T, Sorgente N, Ishibashi T et al: Immunofluorescent studies of fibronectin and laminin in the human eye. Invest Ophthalmol Vis Sci 28:506, 1987 138. Parmigiani CM, McAvoy JW: The role of laminin and fibronectin in the development of the lens capsule. Curr Eye Res 10:501, 1991 139. Wolff E: Anatomy of the Eye and Orbit, 6th ed, pp 207–224. Philadelphia, WB
Saunders, 1968 140. Fisher RF: The elastic constant of the human lens. J Physiol 212:147, 1971 141. Hogan MJ, Alvarado JA, Weddell JE: Histology of the Human Eye. Philadelphia, WB
Saunders, 1971 142. Davanger M: The suspensory apparatus of the lens: The surface of the ciliary body. Acta Ophthalmol 53:19, 1975 143. Streeten B: The zonular insertion: A scanning electron microscopic study. Invest Ophthalmol 16:364, 1977 144. Streeten BN, Swann DA, Licari PA et al: The protein composition of the ocular zonules. Invest Ophthalmol Vis Sci 24:119, 1983 145. Streeten BN, Licari PA, Marucci AA, Dougherty RM: Immunohistochemical comparison of ocular zonules and the microfibrils of
elastic tissue. Invest Ophthalmol Vis Sci 21:130, 1981 146. Farnsworth PN, Burke P: Three-dimensional architecture of the suspensory apparatus of the lens
of the rhesus monkey. Exp Eye Res 25:563, 1977 147. Marshall J, Beaconsfield M, Rothery S: The anatomy and development of the human lens and zonules. Trans Ophthalmol Soc UK 102:423, 1982 148. Mann I: Developmental Abnormalities of the Eye, 1st ed. Philadelphia, JB
Lippincott, 1937 149. Mann I: Developmental Abnormalities of the Eye, 2nd ed. Philadelphia, JB
Lippincott, 1957 150. Baker WH: Congenital aphakia. N Y Med J 46:595, 1887 151. Mann I: Congenital absence of the lens, with special reference to an aphakic human
embryo. Br J Ophthalmol 5:301, 1921 152. Duke-Elder S: System of Ophthalmology, Vol 3, Normal and Abnormal Development: Part 2. Congenital
Deformities. St. Louis, CV Mosby, 1963 153. Pesme P, Montoux P: Staphylome congenital de la cornée (étude
anatomique). Bull Soc Ophtalmol Fr 935, 1956 154. Farnsworth PN, Burke PA, Blanco J, Maltzman B: Ultrastructural abnormalities in a microspherical ectopic lens. Exp Eye Res 27:399, 1973 155. Dayal MB, Mehra KS: Spherophakia. Am J Ophthalmol 50:333, 1960 156. Lisch K: Uber kongenitale formanomalien der linse. Albrecht von Graefes Arch Klin Exp Ophthalmol 157:287, 1956 157. Coulombre AJ, Herrmann H: Lens development: III. Relationship between the growth of the lens and
the growth of the outer eye coat. Exp Eye Res 4:302, 1965 158. Nordmann J: Biologie du Cristallin. Paris, Masson et Cie, 1954 159. Pergens E: Buphthalmus mit lenticonus posterior. Arch Augenheilk 35:1, 1897 160. Sachs E, Larsen RL: Cancer and the lens. Am J Ophthalmol 31:561, 1948 161. Franceschetti A, Rickli H: Posterior (eccentric) lenticonus. Report of first case with clinical and
histological findings. Arch Ophthalmol 51:499, 1954 162. Makley TA: Posterior lenticonus. Report of a case with histologic findings. Am J Ophthalmol 39:308, 1955 163. Sand BJ, Abraham SV: Anterior lenticonus. An operated case and a review of the literature. Am J Ophthalmol 53:636, 1962 164. Krusius FF: Ueber zwei seltene anomalien des linsen systemes. Arch Augenheilk 65:233, 1910 165. Feigenbaum A: The origin of lenticonus anterior. Folia Ophthalmol Orient 1:103, 1932 166. Petronio G: Su di un caso tipico e bilaterale “di lenticono anteriore.” Arch Ottalnologia 48:153, 1941 167. Hiles DA, Kitty LA: Disorders of the lens. In Isenberg SJ (ed): The Eye
in Infancy, pp 336–373. St. Louis, CV Mosby, 1994 168. Cross HE: Differential diagnosis and treatment of dislocated lenses. In
Bergsma D, Bron AJ, Cotlier E (eds): The Eye and Inborn Errors of Metabolism, pp 335–346. New York, Alan R Liss, 1976 169. Burke PA, Shyne SE, Caputo AR, Farnsworth PN: Ultrastructural abnormalities in Weill-Marchesani syndrome lens. Invest Ophthalmol Vis Sci 17(suppl):279, 1978 170. Cross HE: Ectopia lentis in systemic heritable disorders. Birth Defects 10:113, 1974 171. Farnsworth PN, Burke P, Dotto ME, Cinotti AA: Ultrastructural abnormalities in a Marfan's syndrome lens. Arch Ophthalmol 95:1601, 1977 172. Lloyd IC, Goss-Sampson M, Jeffrey BG et al: Neonatal cataract: Aetiology, pathogenesis and management. Eye 6:184, 1992 173. Donaldson DD: The Crystalline Lens. St. Louis, CV Mosby, 1976 174. Phelps CD: Examination and functional evaluation of crystalline lens. In
Duane TD (ed): Clinical Ophthalmology, Vol I, pp 1–23. New York, Harper & Row, 1978 175. Yanoff M, Fine BS: Ocular Pathology. New York, Harper & Row, 1975 176. Lowenstein A: Ueber die genese angeborener linsentrubungen. Klin Monatsbl Augenheilkd 87:382, 1931 177. Clapp CA: Alterations in the capsular epithelium in immature cataract. Trans Am Ophthalmol Soc 39:73, 1941 178. Samuels B: Lesions in the lens caused by purulent corneal ulcers. Trans Am Ophthalmol Soc 39:66, 1941 179. Samuels B: Lesions in the lens caused by purulent corneal ulcers. Arch Ophthalmol 27:345, 1942 180. Samuels B: Cataract complicating corneal scars after perforating ulcers. Arch Ophthalmol 29:583, 1943 181. Riedl A: Ueber Beiziehung von angeborenen Linsentrubungen zur Pupillarmembran. Klin Monatsbl Augenheilkd 69:482, 1922 182. Seefelder R: Stammbaum einer Familie mit angeborenem Star. Ber Dtsch Ophthalmol Ges 52:414, 1938 183. Waardenburg PJ: Gross remnants of the pupillary membrane, anterior polar cataract and microcornea
in a mother and her children. Ophthalmologica 118:828, 1949 184. Ogata T, Matsui M: Electron microscopic studies on the capsule and epithelial cells of anterior
polar cataract of human lens. Acta Soc Ophthalmol Jpn 76:1286, 1972 185. Font RI, Brownstein S: A light and electron microscopic study of anterior subcapsular cataracts. Am J Ophthalmol 78:972, 1974 186. Pellaton R: Die physiologischen linsentrubungen im kindesalter nach spaltlampenunterschung
an 164 normalen kinderaugen. Albrecht von Graefes Arch Klin Exp Ophthalmol 111:341, 1923 187. Cordes FC: Types of congenital cataract. Am J Ophthalmol 30:397, 1947 188. Nettleship E, Ogilvie FM: Peculiar form of hereditary congenital cataract. Trans Ophthalmol Soc UK 26:191, 1906 189. Smith P: A pedigree of Doyne's discoid cataract. Trans Ophthalmol Soc UK 30:37, 1910 190. Vogt A: Weitere Ergebnisse der Spaltlampen-microscopic des vordern Bulbasabschnittes
III. Albrecht von Graefes Arch Klin Exp Ophthalmol 107:196, 1922 191. VonPoos F: Ueber eine familiar aufgetretene besondere Schichtstarform “Cataracta
Zonularis Pulverulenta.” Klin Monatsbl Augenheilkd 76:502, 1926 192. von Szily A: The contribution of pathological examinations to the elucidation of the
problems of cataract. Trans Ophthalmol Soc UK 58:595, 1938 193. Francois J: Heredity in Ophthalmology, pp 364–368. St. Louis, CV
Mosby, 1961 194. Falls HF: Developmental cataracts (results of surgical treatment in one hundred and
thirty-one cases). Arch Ophthalmol 29:210, 1943 195. Marner E: A family with eight generations of hereditary cataract. Acta Ophthalmol 27:537, 1949 196. Nettleship E: On heredity in the various forms of cataract. R Lond Ophthalmol Hosp Rep 16:179, 1905 197. Rosenstern I: Zur wirkung des lebertrans auf rachitis und spasmophie diathese. Berl Klin Wochenschr 47:822, 1910 198. Huggert A: The connection between the position of zonular opacities in the lens and
the time of their formation estimated from simultaneously occurring
enamel hypoplasia. Acta Ophthalmol 26:7, 1948 199. von Szily A, Eckstein A: Vitaminmangel und Schichtstargenese Karakte als eine erscheinungsform der
Avitaminose mit Storung des Kalkstuffwechsels bei saugenden Ratten, hervorgerufen
durch qualitative unterernahrung der muttertiere. Klin Monatsbl Augenheilkd 71:545, 1923 200. von Goldschmidt M: Zur frage der kataraktbildung bei vitaminmangel. Klin Wochenschr 6:635, 1927 201. Adams PH: 1) A family with congenital displacement of lenses. 2) A family with congenital
opacities of lenses. Trans Ophthalmol Soc UK 29:274, 1909 202. Marty F: Les couzbes de frequence en fonction de l'age, de quelques alterations
congenitales ou seniles frequentes du segment anterieur. Arch Ophtal (Paris) 17:5, 1957 203. Muller O: Uber Haufigkeit und form der vorderen axialen nahtpunktierung und der vorderen
axialen embryonalkatarakt. Albrecht von Graefes Arch Klin Exp Ophthalmol 124:444, 1930 204. Mann IC: The correlation of the embryology of the lens with slit-lamp appearances. Trans Ophthalmol Soc UK 45:696, 1925 205. Stocklin P: Normvarianten der morphologie der kindlichen linse. Albrecht von Graefes Arch Klin Exp Ophthalmol 158:346, 1957 206. Treacher-Collins E: Developmental deformities of the crystalline lens. Ophthalmoscope 6:577, 1908 207. Koby FE: Cataracte familiale d'un type particulier se transmettant apparement suivant
le mode dominant. Arch Ophthalmol (Paris) 40:492, 1923 208. Sautter H: Die Trubungsformen der menschlichen Linse. Stuttgart, Thieme, 1951 209. Renard G, Laporte P: Un cas de cataracte congénitale a cristaux associée a des
malformations craniofacialis. Bull Mem Soc Fr Ophtalmol 64:176, 1951 210. Offret G, Durand R: Cataracte en cristaux polychromes chez deux consanguins. Bull
Soc Ophtalmol Fr, p 491, 1955 211. Tadros MA: Coralliform cataract. Bull Ophthalmol Soc Egypt 49:177, 1956 212. Rieger H, Stark M, von Zeynek: Zur frage der cataracta coralliformis. Albrecht von Graefes Arch Klin Exp Ophthalmol 147:426, 1944 213. Verhoeff FH: Microscopic findings in a case of coralliform cataract, with remarks on
congenital cataracts in general. Arch Ophthalmol 47:558, 1918 214. Gifford SR, Puntenney I: Coralliform cataract and a new form of congenital cataract with crystals
in the lens. Arch Ophthalmol 17:885, 1937 215. Parker CO: Spear cataract. Arch Ophthalmol 55:23, 1956 216. Cordes FC: Types of congenital and juvenile cataracts. In Haik GM (ed): Symposium
on Diseases and Surgery of the Lens. St. Louis, CV Mosby, 1957 217. Cordes FC: Types of congenital cataract. Am J Ophthalmol 30:397, 1947 218. Lisch K: Zur Genese des vorderen Polstars. Albrecht von Graefes Arch Klin Exp Ophthalmol 145:393, 1942 219. Franceschetti A, Dieterle P: Cataracte fusiforme congénitale, (en pagode) et ses relations avec
la cataracte pulvérulente centrale. Bull Mem Soc Fr Ophtalmol 72:341, 1959 220. von Szily A: Ueber angeborene familiare “Ringstarlinse” nebst Hinweisen
auf ihre Entstehung. Klin Monatsbl Augenheilkd 81:145, 1928 221. Franceschetti A, Forni S, Rizzini V: Cataracte annulaire ou en bouée de sauvetage. Ophthalmologica 125:342, 1953 222. Avanza C, Balavoine C: Un nouveau biotype de la cataracte en bouée de sauvetage (cataracta
umbilicata) Associée a une brachymorphie une ophtalmoplégie
externe et une hyperaminoacidurie. Bull Mem Soc Fr Ophtalmol 72:331, 1959 223. Haro ES: Hereditary disk-shaped (ring) cataract. Arch Ophthalmol 36:82, 1946 224. Francois J: Total and regressive cataracts. In Francois J (ed): Congenital
Cataracts, pp 175–193. Assen, Netherlands, Royal Van Gorcum, 1959 225. Jaensch PA: Anatomische Untersuchungen angeborenen Totalstars. Albrecht von Graefes Arch Klin Exp Ophthalmol 115:81, 1924 226. Gregg MM: Congenital cataracts following German measles in the mother. Trans Ophthalmol Soc NZ 3:35, 1941 227. Zimmerman LE, Font RL: Congenital malformations of the eye. Some recent advances in knowledge
of the pathogenesis and histopathological characteristics. JAMA 196:684, 1966 228. Yanoff M: Pathology of cataract. In Bellows JG (ed): Cataract and Abnormalities
of the Lens, pp 155–189. New York, Grune & Stratton, 1975 229. Swan C: Study of three infants dying from congenital defects following maternal
rubella in early stages of pregnancy. J Pathol 56:289, 1944 230. Alford CA, Neva FA, Weller TH: Virologic and serologic studies on human products of conception after maternal
rubella. N Engl J Med 271:1275, 1964 231. Bellanti JA, Artenstein MS, Olson LC et al: Congenital rubella: Clinicopathologic, virologic and immunologic studies. Am J Dis Child 110:464, 1965 232. Cooper LZ, Green RH, Krugman S et al: Rubella in contacts of infants with rubella-associated anomalies. MMWR Morb Mortal Wkly Rep 14:44, 1965 233. Karkinen-Jaaskelainen M, Saxen L, Vaheri A, Leinikki P: Rubella cataract in vitro: Sensitive period of the developing human lens. J Exp Med 141:1238, 1975 234. Menser MA, Harley JD, Hertzberg R et al: Persistence of virus in lens for three years after prenatal rubella. Lancet 3:327, 1967 235. World Health Organization. Epidemiological Vital Statistics Report 19:433, 1966 236. Jay M: Linkage and chromosomal studies in congenital cataract. Trans Ophthalmol Soc UK 102:350, 1982 237. Lubsen NH, Renwick JH, Schoenmakers JGG: Hereditary cataract: Perspective for prenatal screening. Ophthalmic Paediatr Genet 7:195, 1986 238. Lewis RA: Mapping the gene for X-linked cataracts and microcornea with facial, dental
and skeletal features to Xp22: An appraisal of the Nance-Horan syndrome. Trans Am Ophthalmol Soc 87:658, 1990 239. Zhu D, Alcorn DM, Antonarakis SE et al: Assignment of the Nance-Horan syndrome to the distal short arm of the X
chromosome. Hum Genet 86:54, 1990 240. Stambolin D, Lewis RA, Buetow K et al: Nance-Horan syndrome; localization within the region Xp21.1–Xp22.3 by
linkage analysis. Am J Hum Genet 47:13, 1990 241. Sparkes RS, Mohandes T, Heinzmann C et al: Assignment of a human beta-crystallin gene to 17 cen-q23. Hum Genet 74:133, 1986 242. Shiloh Y, Donlon T, Bruns G: Assignment of human Γ-crystallin gene cluster (CRYG) to the long
arm of chromosome 2, region q33–36. Hum Genet 73:17, 1986 243. den Dunnen JT, Jongbloed RJE, Geurts van Kessel AHM et al: Human lens Γ-crystallin sequences are located in the p12-qter region
of chromosome 2. Hum Genet 70:217, 1985 244. Sparkes RS, Mohandes T, Heinzmann C et al: The gene for the major intrinsic protein (MIP) of the ocular lens is assigned
to human chromosome 12 cen-q14. Invest Ophthalmol Vis Sci 27:1351, 1986 245. Barrett DJ, Sparkes RS, Gorin MB et al: Genetic linkage analysis of autosomal dominant congenital cataracts with
lens specific DNA probes and polymorphic phenotypic markers. Ophthalmology 95:538, 1988 246. Eiberg H, Marner E, Rosenberg T, Mohr J: Marner's cataract (CAM) assigned to chromosome 16: Linkage to haptoglobin. Clin Genet 34:272, 1988 247. Lubsen NH, Renwick JH, Tsui L-C: A locus for a human hereditary cataract is closely linked to the Γ-crystallin
gene family. Proc Natl Acad Sci USA 84:489, 1987 248. Reese PD: Autosomal dominant congenital cataract associated with chromosomal translocation [t(3;4) (p26,2;p15)]. Arch Ophthalmol 105:1382, 1987 249. Conneally PM, Wilson AF, Merritt AD et al: Confirmation of genetic heterogeneity in autosomal dominant forms of congenital
cataracts from linkage studies. Cytogenet Cell Genet 22:295, 1978 250. Marner E, Rosenberg T, Eiberg H: Autosomal dominant congenital cataract, morphology and genetic mapping. Acta Ophthalmol 67:151, 1989 251. Westat: Summary and critique of available data on the prevalence and economic
and social costs of visual disorders and disabilities. Quoted in
Vision Research: A National Plan, Vol 2, p456. The 1977 Report of the
National Advisory Eye Council, DHEW Publication No (NIH) 78–1259, February 16, 1976 |