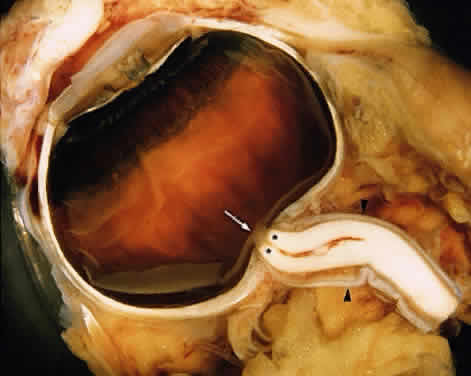
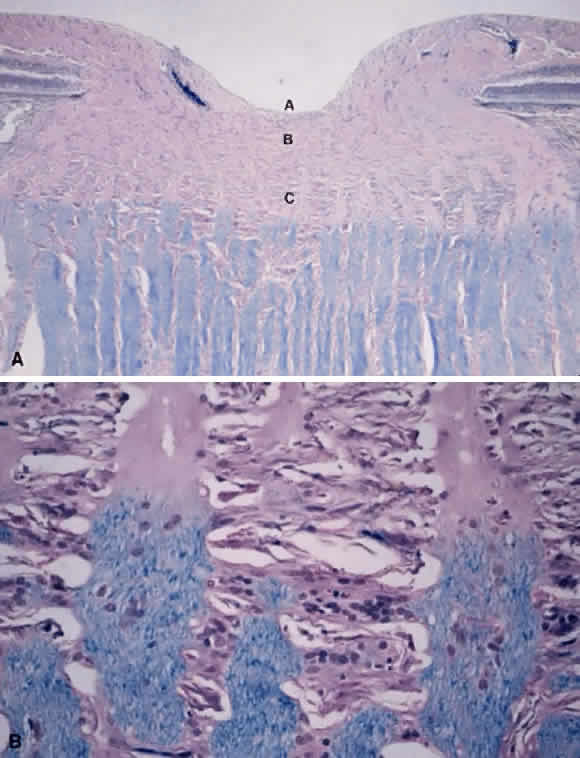
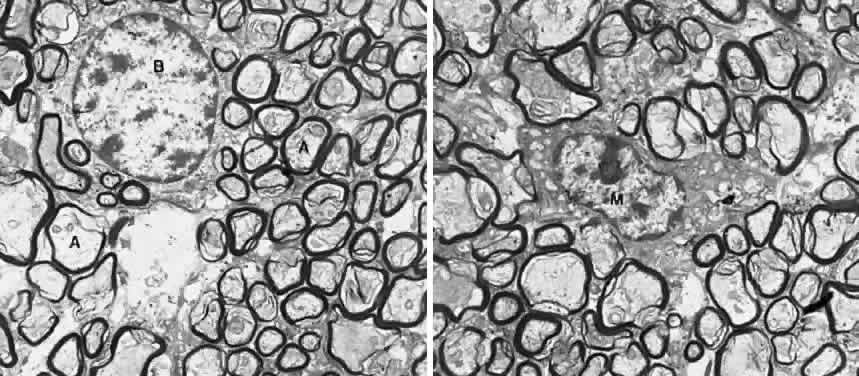
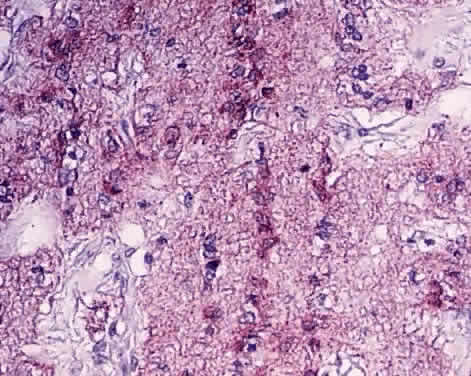
1. Fine B, Yanoff M: The optic nerve. In Fine B, Yanoff M. Ocular Histology: A
Text and Atlas, 2d ed., pp 272–287. Hagerstown, MD, Harper & Row, 1979 2. Hayreh SS: Anatomy and physiology of the optic nerve head. Trans Am Acad Ophthalmol Otolaryngol 78:240, 1974 3. Hernandez MR, Igoe F, Neufeld AH: Extracellular matrix of the human optic nerve head. Am J Ophthalmol 102:139, 1986 4. Hernandez MR, Luo XX, Igoe F, Neufeld AH: Extracellular matrix of the human lamina cribrosa. Am J Ophthalmol 104:567, 1987 5. Ye H, Hernandez MR: Heterogeneity of astrocytes in human optic nerve head. J Comp Neurol 362:441, 1995 6. Raff MC, Mirsky R, Fields KL et al: Galactocerebroside is a specific cell-surface antigenic marker for oligodendrocytes
in culture. Nature 274:813, 1978 7. Eisenbarth GS, Walsh FS, Nirenberg M: Monoclonal antibody to a plasma membrane antigen of neurons. Proc Natl Acad Sci USA 76:4913, 1979 8. Schuller-Petrovic S, Gebhart W, Lassmann H, Rumpold H, Kraft D: A shared antigenic determinant between natural killer cells and nervous
tissue. Nature 306:179, 1983 9. Arimatsu Y, Naegele JR, Barnstable CJ: Molecular markers of neuronal subpopulations in layers 4, 5, and 6 of cat
primary visual cortex. J Neurosci 7:1250, 1987 10. Naegele JR, Barnstable CJ: A carbohydrate epitope defined by monoclonal antibody VC1.1 is found on
N-CAM and other cell adhesion molecules. Brain Res 559:118, 1991 11. Hoyt WF, Luis O: Visual fiber anatomy in the infrageniculate pathway of the primate. Arch Ophthalmol 68:94, 1962 12. Cowey A, Stoerig P: The neurobiology of blindsight. Trends Neurosci 14:140, 1991 13. Morin LP: The circadian visual system. Brain Res Rev 19:102, 1994 14. Wolter JR: The centrifugal nerves in the human optic tract, chiasm, optic nerve and
retina. Trans Am Ophthalmol Soc 63:678, 1965 15. Sturrock RR: A light and electron microscopic study of proliferation and maturation
of fibrous astrocytes in the optic nerve of the human embryo. J Anat 119:223, 1975 16. Sturrock RR: Microglia in the human embryonic optic nerve. J Anat 139:81, 1984 17. Ling EA, Wong WC: The origin and nature of ramified and amoeboid microglia: a historical
review and current concepts. Glia 7:9, 1993 18. Raff MC: Glial cell diversification in the rat optic nerve. Science 243:1450, 1989 19. Miller RH, Raff MC: Fibrous and protoplasmic astrocytes are biochemically and developmentally
distinct. J Neurosci 4:585, 1984 20. Skoff RP, Knapp PE, Bartlett WP: Astrocytic diversity in the optic nerve: a
cytoarchitectural study. In Fedoroff S, Vernadakis A (eds): Astrocytes: Development, Morphology, and Regional Specialization of Astrocytes, Vol. 1., p 269. Orlando, Academic Press, 1986 21. Lampert PW, Vogel MH, Zimmerman LE: Pathology of the optic nerve in experimental acute glaucoma. Electron microscopic
studies. Invest Ophthalmol 7:199, 1968 22. Penfold PL, Madigan MC, Provis JM: Antibodies to human leucocyte antigens indicate subpopulations of microglia
in human retina. Vis Neurosci 7:383, 1991 23. Penfold PL, Provis JM, Liew SCK: Human retinal microglia express phenotypic characteristics in common with
dendritic antigen-presenting cells. J Neuroimmunol 45:183, 1993 24. Orgul S, Cioffi GA: Embryology, anatomy, and histology of the optic nerve vasculature. J Glaucoma 5:285, 1996 25. Onda E, Cioffi GA, Bacon DR, Van Buskirk EM: Microvasculature of the human optic nerve. Am J Ophthalmol 120:92, 1995 26. Lieberman MF, Maumenee AE, Green WR: Histologic studies of the vasculature of the anterior optic nerve. Am J Ophthalmol 82:405, 1976 27. Anderson DR, Braverman S: Reevaluation of the optic disc vasculature. Am J Ophthalmol 82:165, 1976 28. Anderson DR: Vascular supply to the optic nerve of primates. Am J Ophthalmol 70:341, 1970 29. Zhao Y, Li F: Microangioarchitecture of optic papilla. Jpn J Ophthalmol 31:147, 1987 30. Henkind P, Levitzky M: Angioarchitecture of the optic nerve. I. The papilla. Am J Ophthalmol 68:979, 1969 31. Levitzky M, Henkind P: Angioarchitecture of the optic nerve. II. Lamina cribrosa. Am J Ophthalmol 68:986, 1969 32. Ernest JT, Archer D: Fluorescein angiography of the optic disk. Am J Ophthalmol 75:973, 1973 33. Hayreh SS: The central artery of the retina: its role in the blood supply of the optic
nerve. Br J Ophthalmol 47:651, 1963 34. Goder G: The capillaries of the optic nerve. Am J Ophthalmol 77:684, 1974 35. Olver JM, Spalton DJ, McCartney ACE: Microvascular study of the retrolaminar optic nerve in man: the possible
significance in anterior ischaemic optic neuropathy. Eye 4:7, 1990 36. Olver JM, Spalton DJ, McCartney ACE: Quantitative morphology of human retrolaminar optic nerve vasculature. Invest Ophthalmol Vis Sci 35:3858, 1994 37. Torres M, Gómez-Pardo E, Gruss P: Pax2 contributes to inner ear patterning and optic nerve trajectory. Development 122:3381, 1996 38. Deiner MS, Kennedy TE, Fazeli A, Serafini T, Tessier-Lavigne M, Sretavan DW: Netrin-1 and DCC mediate axon guidance locally at the optic disc: loss
of function leads to optic nerve hypoplasia. Neuron 19:575, 1997 39. Brittis PA, Silver J: Multiple factors govern intraretinal axon guidance: a time-lapse study. Mol Cell Neurosci 6:413, 1995 40. Takayama S, Yamamoto M, Hashimoto K, Itoh H: Immunohistochemical study on the developing optic nerves in human embryos
and fetuses. Brain Dev 13:307, 1991 41. Rhodes RH: Development of the optic nerve. In Jakobiec F (ed): Ocular Anatomy, Embryology
and Teratology, p 601. Philadelphia, Harper & Row, 1982 42. Duke-Elder ST, Cook C: Normal and abnormal development. Part I. Embryology. In: Duke-Elder
ST (ed). System of Ophthalmology, Vol.III, p 109. London, Henry
Kimpton, 1963 |