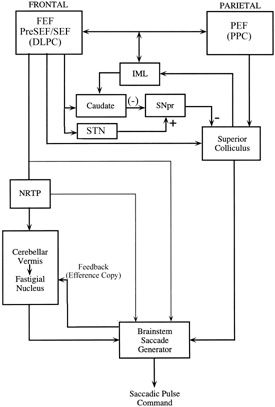
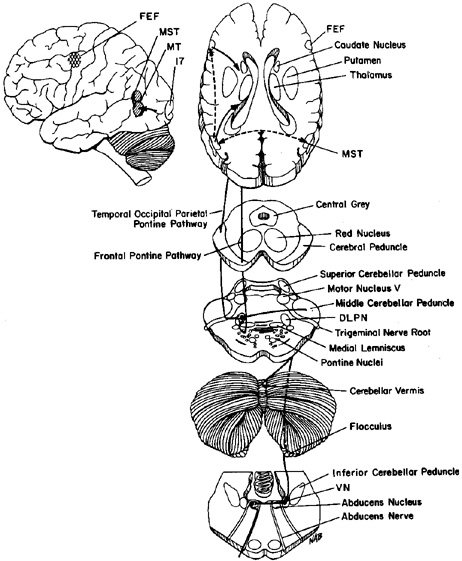
ANATOMY OF THE ORBITAL CONTENTS AND ROTATIONS OF THE EYES
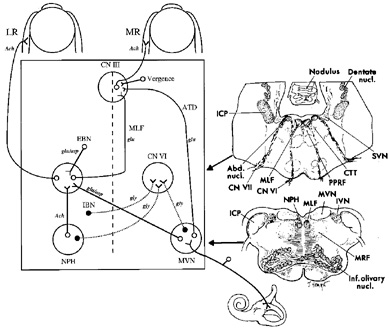
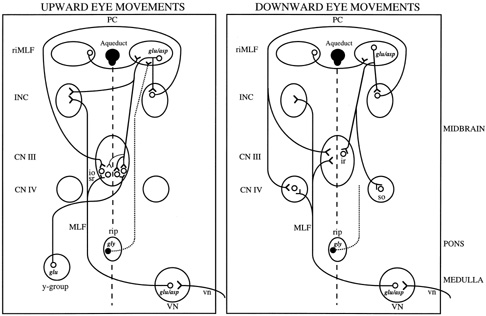
The eyeball is suspended in the orbit by fascia, the main component of which is Tenon's capsule. Each eye is rotated by six muscles: four rectus muscles and two oblique muscles. The directions of pulling action of the extraocular muscles depend on the starting position of the eye. The primary action of the muscle refers to the axis around which the eye principally rotates when that muscle contracts; the secondary and tertiary actions refer to the axes around which there are lesser rotations. The pulling actions of each muscle are summarized in Table 2. Concepts of the way that the extraocular muscles attach to the eye and move it have undergone a revolutionary change in the past decade, based on magnetic resonance imaging (MRI) in volunteers and dissection of cadaver orbits.11,12 These studies have demonstrated fibroelastic sleeves for the extraocular muscles that act as pulleys and, for example, stop slide-slip of the horizontal rectus muscles during vertical eye movements. It is well known that the extraocular muscles have two layers, but it now appears that the outer orbital layer inserts into the pulley of the muscle while the inner global layer passes through the pulley to insert onto the globe. Different populations of ocular motoneurons may supply the global and orbital layers, and their proprioception may differ.13 At first sight, such an arrangement raises many complications about the control of eye movements, because it suggests that separate populations of ocular motoneurons will be needed to contract the orbital layer to position the pulley (which is the functional origin of the muscle) and contract the global layer to move the eye. However, this active pulley hypothesis may simplify certain aspects of movements.12 For example, the pulleys may govern rotations of the eyes during saccades and pursuit so that they obey Listing's law.14 Listing's law states that when the eye rotates to a tertiary position, this is achieved by rotation around a single axis perpendicular to the primary position of gaze and to the new position of gaze. Together these axes form Listing's plane, which is approximately frontoparallel. Donder's law states that every tertiary eye position is associated with a specific torsional rotation. The noncommutative properties of rotations pose a computational challenge to the brain (the order of rotations must be specified), but the pulleys may simplify that problem mechanically in the orbit. It also seems possible that certain forms of congenital strabismus may be caused by misplacement of pulleys in the coronal plane.12 Precise measurements of eye rotations in all three directions,15 and high-definition imaging of the extraocular muscles are likely to clarify the role played by the extraocular muscle pulleys further.
TABLE 2. Actions Of The Extraocular Muscles With The Eye In Primary Position*
| Muscle | Primary action | Secondary action | Tertiary action |
| Medial rectus | Adduction | — | — |
| Lateral rectus | Abduction | — | — |
| Superior rectus | Elevation | Intorsion | Adduction |
| Inferior rectus | Depression | Extorsion | Adduction |
| Superior oblique | Intorsion | Depression | Abduction |
| Inferior oblique | Extorsion | Elevation | Abduction |
*Adapted from Leigh RJ, Zee DS: The Neurology of Eye Movements, 3rd ed. New York: Oxford University Press, 1999.
PROPERTIES OF THE EXTRAOCULAR MUSCLES
Extraocular muscles differ anatomically and physiologically from limb muscles.16,17 The former have fibers that are smaller and more richly innervated; some extraocular muscle fibers are among the fastest contracting and yet are relatively fatigue-resistant. The extraocular muscles contain twitch fibers that have a single endplate per fiber and generate action potentials. In addition, there are nontwitch fibers that cannot generate action potentials but show graded contractions to trains of electrical pulse stimuli. These tonic fibers are capable of a smoothly modulated muscle contraction, which may be important for maintaining steady gaze. The extraocular muscles are selectively vulnerable to some disorders (e.g., myasthenia gravis) but resistant to others (e.g., Duchenne's dystrophy). Furthermore, the appearances of diseases that affect the muscle are different when they involve the extraocular muscles. Both central global and peripheral orbital muscle layers contain fibers more suited for either sustained contraction or brief rapid contraction. However, the orbital layer contains many fatigue-resistant twitch fibers and the global zone contains twitch fibers with variable degrees of fatigue resistance; the different muscle types receive different innervation.13
Six types of fibers have been defined in the extraocular muscles.16,17 (i) In the orbital layer, approximately 80% of fibers are singly-innervated, have fast-type myofibrillar adenosine triphosphatase (ATPase), and high oxidative activity (with numerous mitochondria in dense clusters). They are very fatigue-resistant. (ii) The remaining orbital fibers are multiply innervated fibers, with multiple nerve terminals. They have twitch capacity near the center of the fiber, and nontwitch activity proximal and distal to end plate band. (iii) In the global layer, approximately 33% of fibers are red, singly innervated, fast-twitch, and fatigue-resistant. (iv) The global layer contains intermediate, singly innervated fibers (approximately 25%) with fast-twitch properties, numerous mitochondria, and intermediate level of fatigue resistance. (v) Global, pale singly innervated fibers (approximately 33%) have fast-twitch properties but contribute only sporadically because of their low fatigue resistance. (vi) Global multiply innervated fibers (approximately 10%) have slow-twitch, slow tonic properties that exhibit slow, graded, nonpropagated responses to neural or pharmacologic activation; this fiber type is unparalleled in any other human skeletal muscle. It should be noted that levator palpebrae lacks multiply innervated fibers; this may reflect the importance of this fiber type for fixation and smooth eye movements. Unlike other skeletal muscles, in which embryonic myosin is transformed to adult isoforms, extraocular muscles preserve their embryonic myosin in the proximal and distal portions of both types of orbital layer fibers.16 This may underlie the remarkable capacity of extraocular muscles to adapt to changes in innervation and disease states.
Ocular motoneurons and muscle fibers are the final common pathway for all ocular motor systems. The contribution that different fiber types make to different types of eye movements was clarified in classic experiments by Scott and Collins,18 who used miniature electrode needles with multiple recording sites. They reported that fibers are differentiated functionally by the amount of work performed and that the electromyographic (EMG) activity of a given unit correlates with an eye position, irrespectively of the type of movement. (It should be noted, however, that fibers that do not generate action potentials will not be evident on EMG). Orbital layer fibers are recruited before the global, but both types of fibers participate in every movement. Scott and Collins proposed a division of labor such that orbital fibers are active throughout nearly the entire range of movement but during fixation, global fibers are recruited only as the eye is called into the field of action of that muscle. The classic scheme of Scott and Collins is being reinterpreted in the light of the discovery of pulleys for the extraocular muscles and proprioceptive innervation of the extraocular muscles (discussed below).12,13
The different structure of the extraocular muscles apparently determines their differential involvement in neuropathic and myopathic pathologic processes. One example is myasthenia gravis (MG), an autoimmune condition with antibodies against acetylcholine receptors, in which extraocular muscles are preferentially affected.16,19 This may be accounted for by the difference in structure of the acetylcholine receptor in the extraocular muscles, in which the embryonic 2β type is preserved at the neuromuscular junction of multiply innervated fibers (in contrast to the adult 2β type of the receptor in the skeletal muscles).16,20 However, because multiply innervated fibers are absent from the levators, this explanation cannot account for frequent ptosis in myasthenic patients.21 Generally normal saccadic metrics in the presence of the affected ductions again argue for the preferential involvement of the multiply-innervated fibers, with preservation of the fast-twitch singly innervated fibers. Recently, it has been shown that injection of a standard dose of edrophonium (Tensilon) increased peak velocity-amplitude relationship in patients with MG while decreasing it in normal controls or patients with ocular palsies of other causes.22 Because clinical improvement in the amplitude is not specific for MG, the difference in the velocity response may prove to be of higher diagnostic value. The effect in normal controls may be the result of subclinical cholinergic excess (depolarizing blockade-like) and suggests that 10 mg is too high a dose.
Duchenne's Muscular Dystrophy
In this systemic myopathy, as in most others, eye movements are spared.23 This correlates with the absence of necrosis in the extraocular muscles despite the deficiency of a subsarcolemmal protein, dystrophin.24 In other muscles, the initial pathology is increase in intracellular free calcium because of disruption of sarcolemmal integrity.16 It has been suggested that higher capacity of extraocular muscles to scavenge free radicals caused by higher levels of superoxide dismutase might account for this selective preservation of function.25
Although it has long been known that the extraocular muscles contain end-organs necessary for proprioception, their role in the control of eye movements has remained moot until recently.13 In part this has been because vision exerts much more powerful and immediate feedback control on eye movements than any non-visual signal. In addition, experimental studies in monkey have supported a role for the other extraretinal signal - efference copy or corollary discharge of eye movement commands;26 this internal neural signal is consistent with Helmholtz's idea that the brain monitors its own effort of will. One conceptual reason to doubt that proprioception has any role in the control of eye movements is that no external loads are applied to the extraocular muscles, and evidence exists to refute the presence of a stretch reflex in the extraocular muscles.27
In extraocular muscles, the main sources of proprioceptive input are muscle spindles, which lie mainly in the orbital layer; myotendinous cylinders (palisade endings), which are associated with the global layer; and Golgi tendon organs that lie in the peripheral layer.28 It has been hypothesized that only twitch fibers play a substantial role in eye movement, whereas the global layer nontwitch muscle fibers adjust the tension on the palisade endings and modulate the afferent proprioceptive signal.28 Extraocular proprioceptors project to the brain via the ophthalmic branch of the trigeminal nerve and the Gasserian ganglion, to the spinal trigeminal nucleus (pars interpolaris and pars caudalis).29 From the trigeminal nucleus, proprioceptive information is distributed widely to structures involved in oculomotor control: superior colliculus, vestibular nuclei, nucleus prepositus hypoglossi, cerebellum, frontal eye fields, and also structures involved in visual processing: lateral geniculate body, pulvinar, visual areas 17 and 18.30 It has been shown that extraocular proprioception contributes to the development of visual binocularity, aspects of pattern recognition, and formation of visuospatial maps.30
Studies have suggested that ocular proprioception is important for adaptive recalibration, such as occurs after ocular motor palsies. Thus, after experimentally induced, unilateral extraocular palsies, it was shown that proprioceptive deafferentation of the paretic eye produced gradual worsening of both static alignment and saccadic conjugacy.31 Taken together, this evidence argues that proprioception contributes to long-term adaptive mechanisms responsible for eye alignment during fixation and saccades.
ANATOMY OF THE CRANIAL NERVES
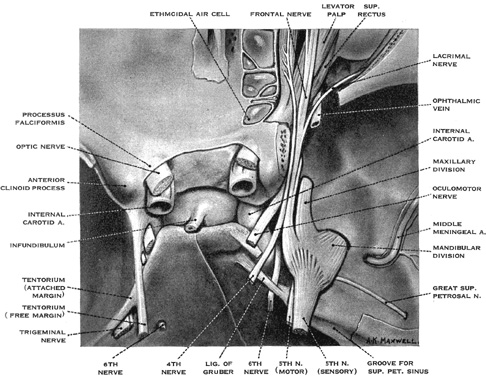
The ocular motor nuclei are located in the brainstem, close to the midline. The intracranial courses of the ocular motor nerves are summarized in Figure 1.
The abducens nucleus lies in the floor of the fourth ventricle, in the lower pons; it is capped by the genu of the facial nerve. As discussed below, the abducens nucleus contains two distinct populations of cells: motor neurons that innervate the lateral rectus muscle and internuclear neurons that innervate, via the medial longitudinal fasciculus, contralateral medial rectus motoneurons. From the medial aspect of the nucleus, fibers destined for the ipsilateral, lateral rectus muscle pass ventrally, laterally and caudally through the pontine tegmentum and medial lemniscus, and lateral to the corticospinal tract, to emerge from the pons at its caudal border. The abducens nerve then courses nearly vertically along the clivus, through the prepontine cistern, and rises to the petrous crest, where it bends forward to penetrate the dura. Here it lies medial to the trigeminal nerve, and passes under the petroclinoid ligament in Dorello's canal. Because the nerve is relatively tethered in the last two locations, it is vulnerable to shear forces; thus, sixth nerve palsy is a nonlocalizing sign in the context of elevated intracranial pressure or trauma. In the cavernous sinus, it lies lateral to the internal carotid artery and medial to the ophthalmic division of the trigeminal nerve. For a short portion, pupillosympathetic fibers run with the sixth nerve as they leave the carotid artery to reach the first division of the trigeminal nerve. Therefore, the combination of the sixth-nerve palsy with Horner's syndrome is suggestive of a cavernous sinus process. The sixth nerve then enters the orbit through the superior orbital fissure, and passes through the annulus of Zinn to innervate the lateral rectus on its inner surface. The most common causes of the sixth-nerve palsy are (peripheral) vascular disease caused by diabetes mellitus or hypertension in an elderly population, and tumors in younger patients.
The trochlear nucleus lies at the ventral border of the periaqueductal gray matter at the level of the inferior colliculus. It innervates the contralateral superior oblique muscle. The trochlear nerve is the longest and the thinnest of cranial nerves; therefore, it is susceptible to even minor head trauma. Its fibers pass dorsolaterally and caudally, around the central gray matter, and decussate completely in the roof of the aqueduct, within the superior medullary velum. Lesions there can produce bilateral fourth nerve palsies. The trochlear nerve emerges from the dorsal aspect of the brainstem, caudal to the inferior colliculus, and close to the tentorium cerebelli, and passes laterally around the upper pons to reach the prepontine cistern. It then runs forward on the free edge of the tentorium before entering the cavernous sinus. Within the lateral wall of the sinus the fourth nerve lies below the third nerve and above the ophthalmic division of the fifth nerve. It crosses over the oculomotor nerve to enter the superior orbital fissure and passes to the medial aspect of the orbit to supply the superior oblique muscle.
The oculomotor nucleus is a paramedian structure that lies at the ventral border of the periaqueductal gray matter. It extends rostrally to the level of the posterior commissure and caudally to the trochlear nucleus. It sends fibers to the medial rectus, superior rectus, inferior rectus and inferior oblique muscles, and to the levator palpebrae superioris. Warwick's anatomic scheme for the oculomotor nucleus32 has been revised with the demonstration that the neurons supplying the medial rectus muscle are distributed into three areas of the oculomotor nucleus.33 The neurons innervating each superior rectus muscle lie next to each other, and their axons decussate in this part of the nucleus. The central caudal nucleus, which supplies both levator palpebrae superioris muscles, is a single structure. All projections from the oculomotor nucleus are ipsilateral except for those to the superior rectus, which are totally crossed, and those to the levator palpebrae superioris, which are bilateral.
The fascicles of the oculomotor nerve pass ventrally through the red nucleus, the substantia nigra, and the medial part of the cerebral peduncle. A topographic organization has been proposed on the basis of the effects of clinical lesions; from lateral to medial, the order is inferior oblique, superior rectus, medial rectus and levator palpebrae, inferior rectus, and parasympathetic pupillary fibers from Edinger-Westphal nucleus.34 The rootlets of the third nerve emerge from the interpeduncular fossa and then fuse and pass between the posterior cerebral artery and superior cerebellar artery into the basal cistern. The third nerve passes lateral to the posterior communicating artery and below the temporal lobe uncus, where it runs over the petroclinoid ligament just lateral to the posterior clinoid process. During its subarachnoid course, parasympathetic pupillary fibers lie in the peripheral, dorsomedial part of the nerve. Their peripheral location, however, is not the only reason for the pupillary involvement with structural lesions of the third nerve; early pupillary involvement also reflects pressure-sensitive nature of these fibers. The oculomotor nerve pierces the dura close to the free edge of the tentorium cerebelli. Within the cavernous sinus, the third nerve lies initially above the trochlear nerve, where it receives sympathetic fibers from the carotid artery. As it leaves the cavernous sinus, it divides into superior and inferior branches; these pass through the superior orbital fissure. The superior branch runs laterally to the optic nerve and ophthalmic artery to supply the superior rectus and levator palpebrae muscles. The inferior branch supplies the medial rectus, inferior rectus and inferior oblique muscles, and the ciliary ganglion. The most common cause of isolated third nerve palsy is vascular disease (in association with diabetes or hypertension); in such cases the pupil is usually spared. The second most common cause are aneurysms, typically of the supraclinoid portion of the internal carotid or posterior communicating artery; such cases characteristically have pupillary involvement; exceptions to this rule, however, have been reported.