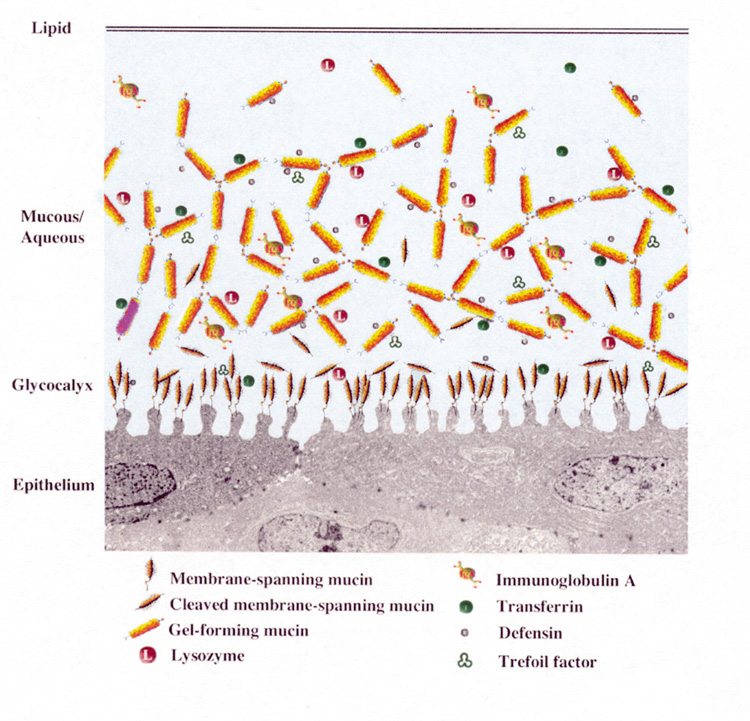
MUCIN LAYER
Ocular mucus is composed of mucin, immunoglobulins, urea, salts, glucose, leukocytes, cellular debris, and enzymes.1 Mucins are high molecular weight glycoproteins that are heavily glycosylated: 50% to 80% of their mass can be attributed to their carbohydrate side chains.2 Tandem repeats of amino acids rich in serine and threonine found in their protein backbone serve as sites for O-type glycosylation.3,4 Heavy glycosylation imparts an overall negative charge to the glycoprotein, making the mucins highly hydrophilic and able to admix with the aqueous layer and maintain water on the surface of the eye.
Mucins are classified as either membrane-associated or secretory. Secretory mucins are further divided into two groups: large gel-forming mucins or small soluble mucins.
Membrane-associated mucins form the glycocalyx, a dense barrier to pathogen penetrance, at the epithelial cell–tear film interface.2 The membrane-associated mucins contain a hydrophobic transmembrane domain that anchors the mucin on the apical surface of the epithelial cells, a short cytoplasmic tail that extends into the cytoplasm, and an extracellular domain that reaches into the tear film.5
Estimated to extend 200 to 500 nm from the cell surface, the extracellular, highly glycosylated tandem repeat domains, also called ectodomains, function as a disadhesive preventing cell–cell and cell–matrix interactions.2,6 This property provides a lubricating surface that allows lid epithelia to glide over the corneal and conjunctival epithelia without adherence.7
The cytoplasmic tail of the membrane-associated mucin is thought to affect epithelial activity by interacting with cytoplasmic proteins and facilitating signal transduction.2 The short cytoplasmic domains are also reported to be associated with the actin cytoskeleton,8,9 which helps support the microplicae structure.2 The presence of epidermal growth factor (EGF)-like domains on the membrane associated mucins suggests a potential role in the regulation of epithelial cell growth.9–14
Soluble forms of the membrane-associated mucins have also been identified in the tear film, although the exact mechanisms for this occurrence are still unknown.2,13,15 Possible mechanisms include: cleavage and release of the extracellular domain into the tear film in a process termed ectodomain shedding; posttranslational processing of the mucin into two subunits where one subunit remains anchored in the plasma membrane, and the other soluble subunit is packaged in secretory granules and released into the tear film; or as some data suggest, mucin shedding from the cell surface over time leaves the oldest cells without microplicae and membrane associated mucins. The oldest cells lose their disadhesive character with the loss of the mucin, which results in the adherence of the old cells to the mucus of the tear film and their removal via the nasolacrimal duct.
Secreted mucins move easily over the mucins composing the glycocalyx because of the repulsive forces between them, which result from their anionic character.7 Secretory mucins act as a “cleaning crew,” moving through the tear fluid and collecting debris that can then be removed via the nasolacrimal duct during blinking.7 The secreted mucins are classified as either gel-forming or soluble. The large gel-forming mucins are probably the largest glycoproteins known based on their high molecular weight and are considered gel forming because they are responsible for the rheological properties of mucus.2,16 The small soluble mucins lack cysteine-rich D domains and are present as monomeric species.2
The majority of ocular mucins are secreted by the conjunctival goblet cells;1 however, ocular mucins are also produced by the stratified squamous epithelium of the cornea and conjunctiva, and new evidence suggests that the lacrimal gland also contributes to mucin production.17–19 Corneal and conjunctival stratified squamous cells contain the membrane-associated mucins MUC1, MUC4, and MUC16.20–22 MUC1 is a likely candidate for the glycocalyx as it is present in the apical cell membranes of the superficial ocular surface cells.23,24 Soluble forms of MUC1, MUC4, and MUC16 have also been detected in the tears2 and MUC4 has been detected in the lacrimal gland.18 MUC2, a gel-forming secretory mucin, and MUC7, a soluble secretory mucin, have been identified in tears and are both present in the conjunctiva, although the exact cellular source in the conjunctiva is unknown.17,19 MUC7 is also secreted by the lacrimal gland.19 MUC5AC, a large gel-forming mucin, is expressed by the goblet cells of the conjunctival epithelium and has been identified in tears in some individuals.25 MUC5AC is the major mucin present at the ocular surface providing the scaffolding of the mucus layer.2,17,20 Mucin secretion by the corneal and conjunctival stratified squamous epithelial cells is not as well studied as mucin secretion by conjunctival goblet cells, which is discussed later in the section on the lacrimal functional unit.
AQUEOUS LAYER
The middle aqueous layer of the tear film consists of water, electrolytes, proteins, peptide growth factors, immunoglobulins, cytokines, vitamins, antimicrobials, and hormones secreted by the lacrimal glands (Table 1).26,27
TABLE 1. The normal tear film has numerous constituents
| Tear Constituents |
| Water |
| Electrolytes |
| Na+ |
| K+ |
| Mg2+ |
| Ca2+ |
| Cl- |
| HCO3- |
| PO43- |
| Proteins |
| Albumin |
| β-Lysin |
| Ceruloplasmin |
| Complement |
| Defensins |
| Group II phospholipase A2 |
| Histamine |
| Interferon |
| Lactoferrin |
| Lipocalin |
| Lysozyme |
| Matrix metalloproteinases |
| Plasminogen activator |
| Prostaglandins |
| Proteases |
| Transferrin |
| Peptide growth factors |
| Epidermal growth factor (EGF) |
| Hepatocyte growth factor (HGF) |
| Transforming growth factor-β (TGF-β) |
| Immunoglobulins |
| IgA |
| IgG |
| IgD |
| IgE |
| IgM |
| sIgA (2 molecules of IgA joined by SC) |
| Cytokines |
| IL-1α |
| IL-1β |
| IL-6 |
| Vitamins |
| Lipids |
| Mucins |
Electrolytes present in the tear film include sodium, potassium, magnesium, calcium, chloride, bicarbonate, and phosphate ions.27 The electrolytes are responsible for the osmolarity of tears, acting as a buffer to maintain a constant pH and contribute to maintaining epithelial integrity of the ocular surface.28,29 An increase in osmolarity of the aqueous layer is a global feature of dry eye syndrome and damages the ocular surface directly and indirectly by triggering inflammation.27,30
Proteins found in human tears are species-specific. More than 60 proteins have been identified in human tears including albumin, immunoglobulins, metal-carrying proteins, complement, histamine, plasminogen activator, prostaglandins, proteases, and antimicrobials.31 Considering that the thin nonkeratinzed epithelium and abundant blood supply of the conjunctiva make the conjunctiva an ideal entrance for infectious agents, it is imperative that the ocular surface have a strong defense system to protect against invading microorganisms. The primary defense system of the ocular surface is composed of the nonspecific immunity conferred by lysozyme, lactoferrin, β-lysin, complement, defensins, and group II phospholipase A2 and the specific immunity of antibodies, such as secretory immunoglobulin A (sIgA).27 In aqueous-deficient dry eye syndrome, the concentration of lysozyme, lactoferrin, lipocalin, and sIgA are reduced, compromising the integrity of the defense system, which may make the ocular surface more susceptible to infection, in addition to the symptoms of dry eye.32,33
Lysozyme, lactoferrin, and lipocalin are regulated antimicrobial proteins, secreted in response to an intracellular stimulus with a rate of secretion that approximately matches the rate of tear flow. Therefore, their concentration remains relatively constant with various flow rates.34–36 Lysozyme, an antimicrobial enzyme, lyses bacterial cell walls in the same manner as penicillin. It is one of the most important protein components in the tear film and acts synergistically with β-lysin, an enzyme that attacks bacterial cell membranes. Lactoferrin, a metal-binding protein, also has antimicrobial properties and may enhance antibody activity against certain microorganisms. Lipocalin, a lipid-binding protein, scavenges potentially harmful hydrophobic molecules and has recently been shown to inhibit bacterial and fungal infections through sequestering microbial siderophores.37 Further evidence suggests that lipocalin may contribute to a stable tear film by interacting with meibomian lipids and delivering them to the aqueous layer, and when complexed with other tear components, may be responsible for the high viscosity and low surface tension of tears.38–40Immunoglobulins are constitutively produced and transported to the tear film from the conjunctiva. Thus, reflex tearing reduces the concentration of immunoglobulins and a reduction in tear flow increases their concentration.36 An example of one such immunoglobulin is sIgA. sIgA is formed by two molecules of immunoglobulin A (IgA) produced by plasma cells in the adenoid layer of the conjunctiva being joined by secretory component (SC), a protein molecule produced by the conjunctival epithelium. sIgA is produced at a rate dependent on its rate of synthesis, which is regulated by its local endocrine environment.34,35,41
In nonstimulated tears, a small proportion of proteins are derived through the leakage of plasma components through ocular surface capillaries.27 These components include albumin; IgG; ceruloplasmin, a copper-carrying protein with oxidizing potential; transferrin, an iron-carrying protein; and monomeric IgA.27 Their concentrations increase in dry eye syndrome resulting from decreased tear volume and leakage from chronically inflamed surface capillaries.27 Peptide growth factors (such as EGF, transforming growth factor-β (TGF-β) and hepatocyte growth factor [HGF]), and vitamin A act via autocrine and paracrine mechanisms to regulate epithelial proliferation, motility, and differentiation. Peptide growth factors are also involved in corneal wound healing and immune modulation.42,43 Evidence suggests that the concentration of EGF is decreased in dry eye syndrome, and it seems reasonable to suppose that the other growth factors secreted by the lacrimal glands are similarly affected.27,44
LIPID LAYER
The anterior layer of the tear film is composed of meibomian oil secreted by the meibomian glands and is the major barrier to evaporation from the ocular surface.45,46 The lipid layer is also responsible for providing stability to the tear film through interaction with the aqueous-mucin phase, providing a smooth optical surface for the cornea, and acting as a barrier against foreign particles.38 McCulley and Shine47 have proposed that the anterior lipid layer is composed of two phases: a polar surfactant phase overlaid by a nonpolar phase. The polar lipid phase, primarily composed of phospholipids and glycolipids, is multifunctional. The highly structured polar lipid layer acts as a surfactant between the hydrophilic aqueous mucin layer and the thick, nonpolar lipid layer; facilitates interaction with the aqueous–mucin layer; provides a barrier; and offers a structural component on which the nonpolar phase depends.47,48 The nonpolar phase (mainly composed of wax, cholesterol esters, and triglycerides) provides the air-tear film interface and is responsible for retarding evaporation.47
A normal tear film lipid layer is able to reduce evaporation by approximately 90% to 95%.49–51 The rate of evaporation is affected by the thickness of the lipid layer, and it has been postulated that a decrease in thickness may cause evaporative dry eye.45 In fact, mild to moderate dry eye states exhibit a lack of confluence in the tear film lipid layer.52 Lipid composition may also affect evaporation because it has been reported that the phospholipid content of meibomian oil is decreased in patients with dry eye and meibomian lipid composition is altered in anterior and posterior blepharitis, although the exact mechanisms have yet to be elucidated.48,53–58 The melting range of meibomian oil is dependent on lipid composition and may be lowered by the presence of branched and unsaturated fatty acids and alcohols.38 The low melting range of meibomian oil (19.5°C to 32.0°C), attributed to the variety of lipids present in the meibomian oil, facilitates meibomian delivery and contributes to tear film stability as the surface corneal temperature (32°C) is close to the upper limit of the melting range, allowing the lipid to exist in a relatively solid state.45
Meibomian oil secretion is a continuous process, occurring 24 hours per day during waking and sleeping hours, and is aided by blink action.45 The rate of secretion is controlled by neural, hormonal, and vascular influences.38 The lid margin reservoir of oil in an adult male has been estimated to contain approximately 300 μg of meibomian oil, which is more than adequate to refresh the lipid layer after each blink considering that the preocular tear film holds 9 μg of lipid.59,60 The casual oil level (or resting level) in the marginal lid reservoir is highest just after waking and lower late morning and at the end of the day, suggesting that oil retained during sleep is discharged when blinking is resumed after waking.59,61 Forced blinking, as well as deliberate expression of meibomian oil, has been shown to increase the thickness of the tear film lipid layer;62,63 however, evidence also shows that oil delivery can be maintained in the absence of blinking, implying that blinking is not essential for delivery. Studies show a rise in the casual level of lipid on the lid margin with age in both genders; however, lower rises in levels in women before menopause suggests a hormonal influence on meibomian secretion.23