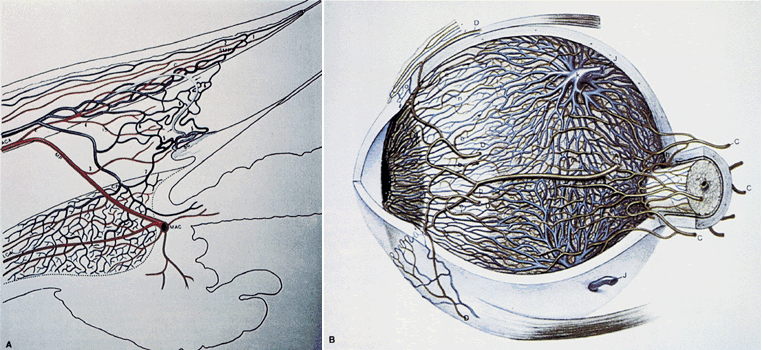
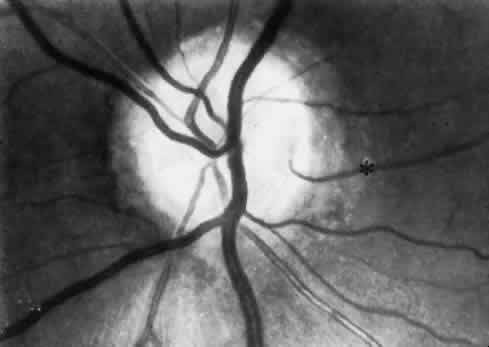
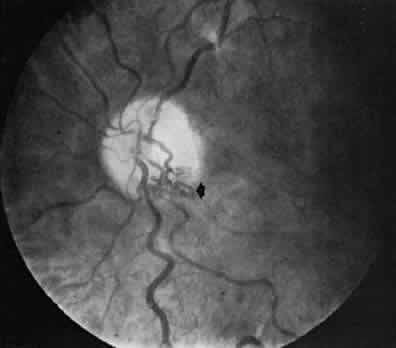
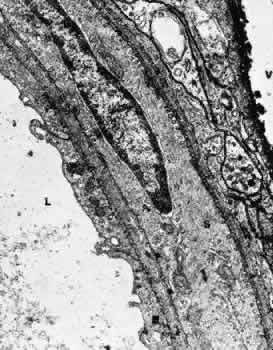
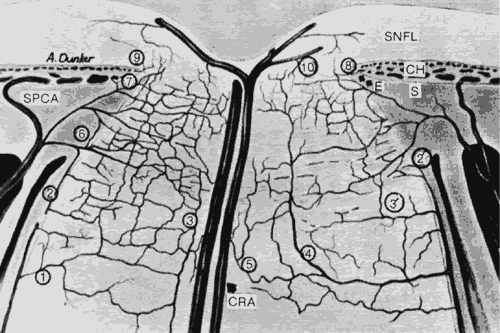
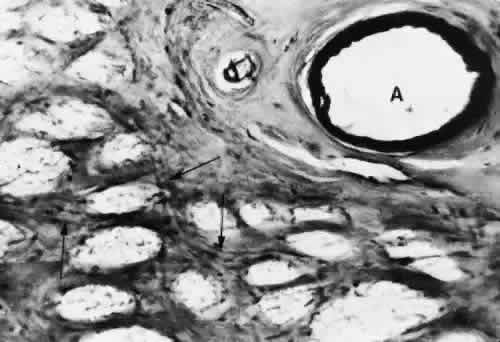
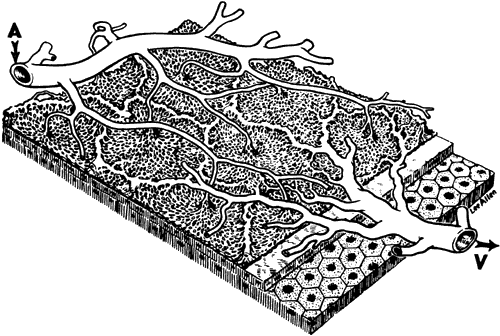
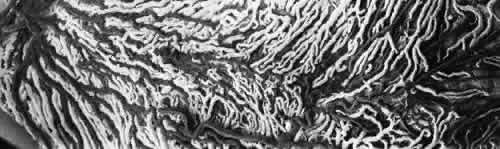
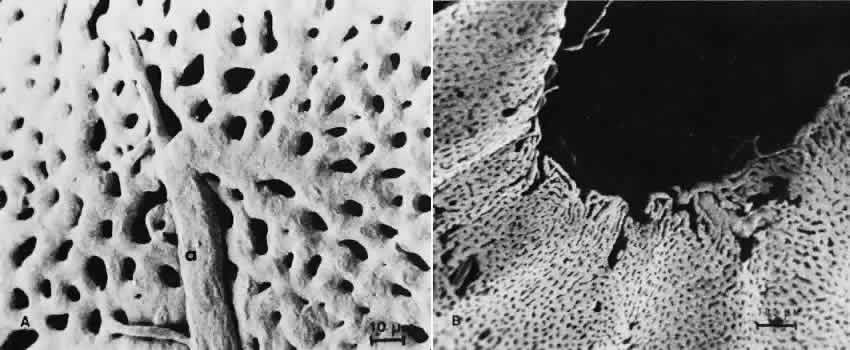
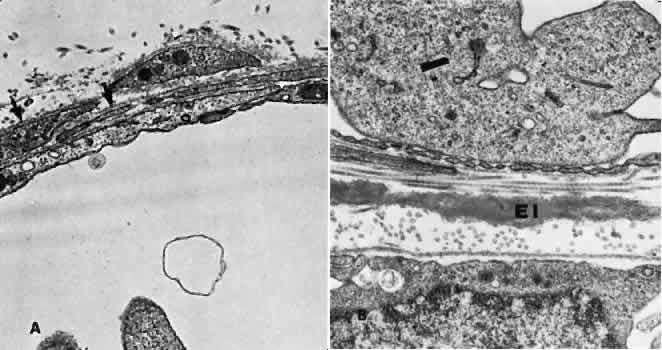
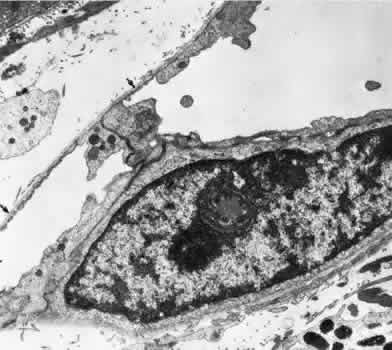
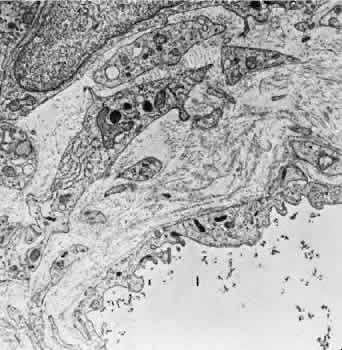
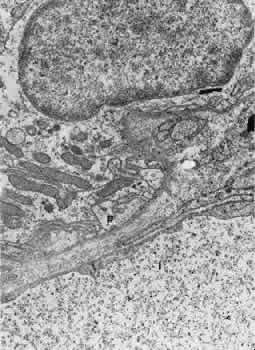
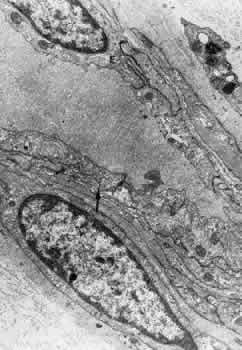
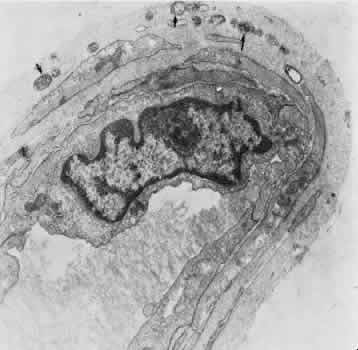
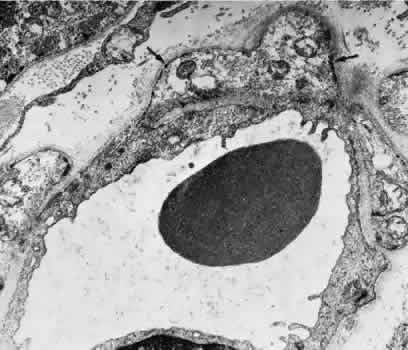
1. Hayreh SS, Dass R: The ophthalmic artery, part II. Intraorbital course. Br J Ophthalmol 46:165, 1962 2. Hayreh SS: The ophthalmic artery, part III. Branches. Br J Ophthalmol 46:212, 1962 3. Ashton N: The mode of development of the retinal vessels in man. In Cant
JS, ed: The Ocular Circulation in Health and Disease, p 7. St. Louis, CV
Mosby, 1969 4. Olver JM: Functional anatomy of the choroidal circulation. Eye 4:262–272, 1990 5. Krause R: New technique to demonstrate the network of blood capillaries of the human
retina in their three-dimensional arrangement. Graefes Arch Clin Exp Ophthalmol 228:187, 1990 6. Williamson JR, Tilton RG, Kilo CK et al: Immunofluorescent imaging of capillaries and pericytes in human skeletal
muscle and retina. Microvasc Res 20:233, 1980 7. Kuwabara T, Cogan DG: Studies of retinal vascular patterns, part 1. Normal architecture. Arch Ophthalmol 64:904, 1960 8. Cuthbertson RA, Mandel TE: Anatomy of the mouse retina: Endothelial cell-pericyte ratio and capillary
distribution. Invest Ophthalmol Vis Sci 27:1659, 1986 9. Hogan MJ, Feeney L: Ultrastructure of the retinal vessels, part 1. The larger vessels. J Ultrastruct Res 9:10, 1963 10. Yamada E, Shikano S: Electron microscopy. In Atlas of Ophthalmology. Tokyo, Igaku
Shoin, 1972 11. Justice J, Lehmann RP: Cilioretinal arteries. Arch Ophthalmol 94:1355, 1976 12. Das A, Frank RN, Zhang NL et al: Ultrastructural localization of extracellular matrix components in human
retinal vessels and Bruch's membrane. Arch Ophthalmol 108:421, 1990 13. Laties A: Central retinal artery innervation: Absence of adrenergic innervation to
the intraocular branches. Arch Ophthalmol 77:405, 1967 14. Furukawa H: Autonomic innervation of preretinal blood vessels of the rabbit. Invest Ophthalmol 28:1752, 1987 15. Hogan MJ, Feeney L: Ultrastructure of the retinal vessels, part II: te small vessels. J Ultrastruct Res 9:29, 1963 16. Duker JS, Brown GC: Anterior location of the crossing artery in branch retinal vein obstruction. Arch Ophthalmol 107:998, 1989 17. Seitz R: The Retinal Vessels. St. Louis, CV Mosby, 1964 18. Iwasaki M, Inomata H: Relation between superficial capillaries and foveal structures in the human
retina. Invest Ophthalmol Vis Sci 27:1698, 1986 19. Henkind P, Bellhorn RW, Poll D: Radial peripapillary capillaries, part III. Their development in the cat. Br J Ophthalmol 57:595, 1973 20. Henkind P: New observations on the radial peripapillary capillaries. Invest Ophthalmol 6:103, 1967 21. Henkind P: Radial peripapillary capillaries: Past, present, future. In
Shimizu K, ed: Fluorescein Angiography, p 91. Tokyo, Igaku Shoin, 1974 22. Kornzweig AL, Eliasoph I, Feldstein M: Selective atrophy of the radial peripapillary capillaries in chronic glaucoma. Arch Ophthalmol 80:696, 1968 23. Ishikawa T: Fine structure of retinal vessels in man and the macaque monkey. Invest Ophthalmol 2:1, 1963 24. Raviola G, Butler JM: Unidirectional vesicular transport mechanism in retinal vessels. Invest Ophthalmol Vis Sci 24:1465, 1983 25. Frank RN, Turczyn TJ, Das A: Pericyte coverage of retinal and cerebral capillaries. Invest Ophthalmol Vis Sci 31:999, 1990 26. Yamashita T, Becker B: The basement membrane in the human diabetic eye. Diabetes 10:881, 1961 27. Cogan DG, Toussaint D, Kuwabara T: Retinal vascular patterns, part IV. Diabetic retinopathy. Arch Ophthalmol 66:366, 1961 28. Hohman TC, Nishimura C, Robison WG: Aldose reductase and polyol in cultured pericytes of human retinal capillaries. Exp Eye Res 48:55, 1989 29. Leuenberger P: Ultrastructure of the aging retinal vascular system with special reference
to quantitative and qualitative changes of capillary basement membranes. Gerontologia 19:1, 1973 30. Wise GN, Dollery CT, Henkind P: Retinal Circulation. New York, Harper & ow, 1971 31. Nyborg NCB, Nielsen PJ: The level of spontaneous myogenic tone in isolated human posterior ciliary
arteries decreases with age. Exp Eye Res 51:711, 1990 32. Francois J, Neetens A: Vascularization of the optic pathway, part 1. Br J Ophthalmol 38:472, 1954 33. Francois J, Neetens A: Vascularization of the optic pathway, part 11. Br J Ophthalmol 40:45, 1954 34. Lieberman MF, Maumenee AK, Green WR: Histologic studies of the vasculature of the anterior optic nerve. Am J Ophthalmol 82:405, 1976 35. Hayreh SS: Blood supply of the optic nerve head and its role in optic atrophy, glaucoma, and
oedema of the optic disc. Br J Ophthalmol 53:721, 1969 36. Henkind P, Levitzky M: Angioarchitecture of the optic nerve, part 1. The papilla; part II. Lamina
cribrosa. Am J Ophthalmol 68:979, 1969 37. Hayreh SS: Blood supply and vascular disorders of the optic nerve. Ann Inst Barraquer 4:7, 1963 38. Ernest JT: Autoregulation of optic disc oxygen tension. Invest Ophthalmol Vis Sci 13:101, 1974 39. Weinstein JM, Duckrow RB, Beard D et al: Regional optic nerve blood flow and its autoregulation. Invest Ophthalmol Vis Sci 24:1559, 1983 40. Geijer C, Bill A: Effects of raised intraocular pressure on retinal, prelaminar, laminar, and
retrolaminar optic nerve blood flow in monkeys. Invest Ophthalmol Vis Sci 18:1030, 1979 41. Quigley HA, Hohman RM, Addicks EM et al: Blood vessels of the glaucomatous optic disc in experimental primate and
human eyes. Invest Ophthalmol Vis Sci 25:918, 1984 42. Jonas JB, Nguyen XN, Naumann GOH: Parapapillary retinal vessel diameter in normal and glaucoma eyes, part 1. Morphometric
data. Invest Ophthalmol Vis Sci 30:1599, 1989 43. Sebag J, Delori FC, Feke GT et al: Effects of optic atrophy on RBF and oxygen saturation in humans. Arch Ophthalmol 107:222, 1989 44. Alm A, Bill A: Ocular circulation. In Moses RA, Hart WM, eds: Adler's
Physiology of the Eye. St Louis, Mosby, 1987 45. Yannzzi LA, Flower RW, Slakter JS: Indocyanine Green Angiography. St. Louis, Mosby, 1997 46. Lieb W, Shields JA, Cohen SM et al: Color Doppler imaging of intraocular tumors. Ophthalmology 97:1660, 1990 47. Parver LM, Auker C, Carpenter DO: Choroidal blood flow as a heat dissipating mechanism in the macula. Am J Ophthalmol 89:641, 1980 48. Wagenmann A: Experimentelle Untersuchungen Uber den Einfluss der Circulation inden Netzhautund
Aderhautgenfassen auf die Ernahrung des Auges, insbes der Retina
und uber die Folgen der Sehnervendurchschneidung. Graefes Arch Clin
Exp Ophthalmol 36:1, 1890. Quoted by Ernest T et al: Am J Ophthalmol 81:574, 1976 49. Amalric P: Veines choroidiennes etat normal et pathologique. Ann Ocul (Paris) 207:161, 1974 50. Ring HO, Fujino T: Observations on the anatomy and pathology of the choroidal vasculature. Arch Ophthalmol 78:431, 1967 51. Dollery CT, Henkind P, Kohner EM et al: Effect of raised intraocular pressure on the retinal and choroidal circulation. Invest Ophthalmol 7:191, 1968 52. Hayreh SS: Segmental nature of the choroidal vasculature. Br J Ophthalmol 59:631, 1975 53. Torczynski E, Tso MO: The architecture of the choriocapillaris at the posterior pole. Am J Ophthalmol 81:428, 1976 54. Weiter JJ, Ernest JT: Anatomy of the choroidal vasculature. Am J Ophthalmol 78:583, 1974 55. Ernest JT, Stern WH, Archer DB: Submacular choroidal circulation. Am J Ophthalmol 81:574, 1976 56. Fryczykowski AW, Sherman MD, Walker J: Observations on the lobular organization of the human choriocapillaris. Int Ophthalmol 15:109–120, 1991 57. Weiter J, Schacher RA, Ernest T: Control of intraocular blood flow, part II. Effects of sympathetic tone. Invest Ophthalmol 12:332, 1973 58. Ruskell GL: Facial parasympathetic innervation of the choroidal blood vessels in monkeys. Exp Eye Res 12: 166, 1971 59. Hogan MJ, Feeney L: Electron microscopy of the human choroid, part III. The blood vessels. Am J Ophhalmol 1:1084, 1961 60. Yoneya S, Tso MOM, Shimizu K: Patterns of the choriocapillaris: A method to study the choroidal vasculature
of the enucleated human eye. Int Ophthalmol 6:95, 1983 61. Yoneya S, Tso MOM: Angioarchitecture of the human choroid. Arch Ophthalmol 105:681, 1987 62. Hogan MJ, Feeney L: Electron microscopy of the human choroid, part II. The choroidal nerves. Am J Ophthalmol 51:1072, 1961 63. Funk R, Rohen JW: Scanning electron microscopic study on the vasculature of the human anterior
eye segment, especially with respect to the ciliary processes. Exp Eye Res 51:651, 1990 64. Taniguchi Y: Fine structure of blood vessels of the ciliary body. Jpn J Ophthalmol 6:93, 1962 65. Dollery CT: Dynamic aspects of the retinal microcirculation. Arch Ophthalmol 79:536, 1968 66. McDonald DA: Blood Flow in Arteries. Baltimore, Williams & Wilkins, 1974 67. Whitmore RL: Rheology of the Circulation. Elmsford, NY, Pergamon Press, 1968 68. Charm SE, Kurland GS: Blood Flow and Microcirculation. New York, John Wiley & Sons, 1974 69. Friedman E, Smith TR, Kuwabara T: Retinal microcirculation in vivo. Invest Ophthalmol 3:217, 1964 70. Bill A: A method for quantitative determination of the blood flow through the cat
uvea. Arch Ophthalmol 67:156, 1962 71. Cohan BE, Cohas S: Flow and oxygen saturation of blood in the anterior ciliary vein of the
dog eye. Am J Physiol 205:60, 1963 72. Elgin SS: Arteriovenous oxygen difference across the uveal tract of the dog eye. Invest Ophthalmol 3:417, 1964 73. Seaman AJ, Rullman DR, Lutcher CL et al: The living extracorpeal eye. Scand J Clin Lab Invest (suppl) 84:101, 1965 74. Kety S, Schmidt DF: The nitrous oxide method for the quantitative determination of cerebral
blood flow in man: Theory, procedure, and normal values. J Clin Invest 27:476, 1948 75. Detweiler DK: Circulation. In Brobeck JR, ed: Best and Taylor's Physiological
Basis of Medical Practce, 9th ed. Baltimore, Williams & Wilkins, 1973 76. Pilkerton R. Bulle PH, O'Rourke J: Uveal blood flow determined by the nitrous oxide method. Invest Ophthalmol 3:227, 1964 77. Friedman E, Kopald HH, Smith TR: Retinal and choroidal blood flow determined with krypton-85 in anesthesized
animals. Invest Ophthalmol 3:539, 1964 78. Alm A, Bill A: The oxygen supply to the retina, part 1. Effects of changes in intraocular
and arterial blood pressures and in arterial PO2 and pCO2 on the oxygen tension in the vitreous body of the cat. Acta Physiol Scand 84:261, 1972 79. O'Day DM, Fish MB, Aronson SB et al: Ocular blood flow measurements by nuclide labelled microspheres. Arch Ophthalmol 86:205, 1971 80. Alm A, Bill A: The oxygen supply to the retina, part 11. Effects of high intraocular pressure
and of increased arterial carbon dioxide tension on uveal and
RBF in cats: A study with labeled microspheres including flow determinations
in brain and some other tissues. Acta Physiol Scand 84:306, 1972 81. Alm A, Bill A: Ocular and optic nerve blood flow at normal and increased intraocular pressures
in monkeys (Alacaca Irus): A study with radioactively labelled microsphere including flow determinations
in brain and some other tissues. Exp Eye Res 15:15, 1973 82. Alm A: The effect of stimulation of the sympathetic chain on retinal oxygen tension
and uveal, retinal, and cerebral blood flow in cats. Acta Physiol Scand 88:84, 1973 83. Alm A, Bill A, Young FA: The effects of pilocarpine and neostigmine on the blood flow through the
anterior uvea in monkeys: A study with radioactively labelledmicrospheres. Exp Eye Res 15:31, 1973 84. Weiter J, Schacher RA, Ernest T: Control of intraocular blood flow, part I. Intraocular pressure. Invest Ophthalmol 12:327, 1973 85. Williamson TH, Harris A: Ocular blood flow measurement. Br J Ophthalmol 78:939–945, 1994 86. Riva C, Ross B, Benedek GB: Laser Doppler measurements o blood flow in capillary tubes and retinal
arteries. Invest Ophthalmol 11:936, 1972 87. Stern MD: In vivo evaluation of microcirculation by coherent light scattering. Nature 254:56, 1975 88. Tanaka T, Riva C, Ben-Sira I: Blood velocity measurements in human retinal vessels. Science 186:830, 1974 89. Schlegal WA, Lawrence C: Doppler measurements of vortex blood flow in animals. Invest Ophthalmol 8:201, 1969 90. Sebag J, Delori FC, Feke GT, Weiter JJ: Effects of optic atrophy on RBF and oxygen saturation in humans. Arch Ophthalmol 107:222–226, 1989 91. Delori FC, Fitch KA, Feke GT, et al: Evaluation of micrometric and microdensitometric methods for measuring
the width of retinal vessel images on fundus phtotographs. Graefes Arch Clin Exp Ophthalmol 226:393–399, 1988 92. Chung HS, Harris A, et al: Peripupillary RBF in normal tension glaucoma. Br J Ophthalmol 83:466–469, 1999 93. Harris A, Chung HS, Ciulla TA et al: Progress in measurement of ocular blood flow and relevance to our understanding
of glaucoma and age-related macular degeneration. Prog Ret Eye Res 18:669–687, 1999 94. Schmetterer L, Dallinger S, Findl O et al: Noninvasive investigations of the normal ocular circulation in humans. Invest Opththalmol Vis Sci 39:1210–1220, 1998 95. Hickam JB, Frayser R: Studies on the retinal circulation in man: Observations on vessel diameter, arteriovenous
oxygen difference, and mean circulation time. Circulation 33:302, 1966 96. Hickam JB, Frayser R: A photographic method for measuring the mean retinal circulation time using
fluorescein. Invest Ophthalmol 4:876, 1965 97. Oberhoff P. Evans PY, Delaney JF: Cinematographic documentation of retinal circulation times. Arch Ophthalmol 74:77, 1965 98. Trokel S: Quantitative studies of choroidal blood flow by reflective densitometry. Invest Ophthalmol 4:1129, 1965 99. Gloster J: Fundus oximetry. Exp Eye Res 6:187, 1967 100. Bulpitt C, Dollery CT: Estiation of RBF by measurement of the mean circulation time. Cardiovasc Res 5:406, 1971 101. Riva C, Ben-Sira I: Two-point fluorophotometer for the human ocular fundus. Appl Optics 14:2691, 1975 102. Chandra SR, Friedman E: Choroidal blood flow, part 11. The effects of autonomic agents. Arch Ophthalmol 87:67, 1972 103. Beausang-Linder M: Effects of sympathetic stimulation on cerebral and ocular blood flow: Modification
by hypertension, hypercapnia acetayolamide, PGI-2 and papaverine. Acta Physiol Scand 114:217, 1982 104. Alm A: The effect of topic l-epinephrine on regional ocular blood flow in monkeys. Invest Ophthalmol Vis Sci 19:487, 1980 105. Bill A, Heilmann K: Ocular effects of clonidine in cats and monkeys. Exp Eye Res 21:481, 1975 106. Morgan TR, Green K, Bowman K: Effects of adrenergic agonists upon regional ocular blood flow in normal
and ganglionectomized rabbits. Exp Eye Res 32:691, 1981 107. Morrison JC, Van Buskirk EM: Microanatomy and modulation of the ciliary vasculature. Trans Ophth Soc UK 105:131, 1986 108. Laties AM, Jacobowitz D: A comparative study of the autonomic innervation of the eye in monkey, cat
and rabbit. Anat Rec 156:383, 1966 109. Wolter JR: Nerves of the normal human choroid. Arch Ophthalmol 64:120, 1960 110. Alm A, Bill A: Blood flow and oxygen extraction in the cat uvea at normal and high intraocular
pressures. Acta Physiol Scand 80:19, 1970 111. Landers MB III: Retinal oxygenation over the choroidal circulation. Trans Am Ophthalmol Soc 76:528, 1978 112. Zuckerman R, Weiter JJ: Oxygen transport in the bullfrog retina. Exp Eye Res 30:117, 1980 113. Weiter JJ, Zuckerman R: The influence of the photoreceptor-RPE complex on the inner retina: An
explanation of the beneficial effects of photocoagulation. Ophthalmology 87:1133, 1980 114. Ernest JT: The effect of systolic hypertension on rhesus monkey eyes after ocular
sympathectomy. Am J Ophthalmol 84:341, 1977 115. Schachar RA, Weiter JJ, Ernest JT: Control of intraocular blood fow, part III. Effect of chemical sympathectomy. Invest Ophthalmol 12:848, 1973 116. Grunwald JE, Furubayashi C: Effect of topical timolol maleate on the ophthalmic artery blood pressure. Invest Ophthalmol Vis Sci 30:1095, 1989 117. Feke GT, Tagawa H, Deupree DM et al: Blood flow in the normal human retina. Invest Ophthalmol Vis Sci 30:58, 1989 118. Riva CE, Grunwald JE, Sinclair SH et al: Blood velocity and volumetric flow rate in human retinal vessels. Invest Ophthalmol Vis Sci 26:1124, 1985 119. Harris A, Ciulla TA, Chung HS et al: Regulation of retinal and optic nerve blood flow. Arch Ophthalmol 116:1491–1495, 1998 120. Riva CE, Sinclair SH, Grunwald JE: Autoregulation of retinal circulation in response to decrease of perfusion
pressure. Invest Ophthalmol Vis Sci 21:34, 1981 121. Feke GT, Zuckerman R, Green GJ et al: Response of human RBF to light and dark. Invest Ophthalmol Vis Sci 24:136, 1983 122. Schmetterer L, Findl O, Strenn K et al: Role of NO in the O2 and CO2 responsiveness of cerbral and ocular circulation in humans. Am J Physiol 273:R2005–2012, 1997 123. Kelly PA, Buckley CH, Ritchie IM et al: Possible role for nitric oxide releasing nerves in the regulation of ocular
blood flow in the rat. Br J Ophthalmol 82:1199–1202, 1998 124. Alm A: Effects of norepinephrine, angiotensin, dihydroergotamine, papaverine, isoproterenol, histamine, nicotinic acid, and xantinol nicotinate on retinal
oxygen tension in cats. Acta Ophthalmol (Copenh) 50:707, 1972 125. Ffytche TJ, Bulpitt CJ, Archer D et al: Effects of papaverine on the retinal microcirculation. Br J Ophthalmol 57:910, 1973 126. Eaton AM, Hatchell DL: Measurement of retinal blood vessel width using computerized image analysis. Invest Ophthalmol Vis Sci 29:1258, 1988 127. Eliakim M, Mor I, Michaelson IC: Assessment of pharmacologic effects on the retinal circulation of hypertensive
subjects by a quantitative method. Microvasc Res 4:374, 1972 128. Frayser R. Hickam JB: Effectof vasodilator drugs on the RBF in man. Arch Ophthalmol 73:640, 1965 129. Ramalho PS, Dollery CT: Hypertensive retinopathy: Caliber changes in retinal blood vessels following
blood pressure reductions and inhalation of oxygen. Circulation 37:580, 1968 130. Dollery CT, Hill DW, Hodge JV: The response of normal retinal blood vessels to angiotensin and noradrenaline. J Physiol (Lond) 165:500, 1963 131. Forster BA, Ferrari-Dileo G. Anderson DR: Adrenergic alpha I and alpha 2 binding sites are present in bovine retinal
blood vessels. Invest Ophthalmol Vis Sci 28:1741, 1987 132. Ferrari-Dileo G, Davis EB, Anderson DR: Response of retinal vasculature to phenylephrine. Invest Ophthalmol Vis Sci 31:1181, 1990 133. Ferrari-Dileo G, Davis EB, Anderson DR: Biochemical evidence for cholinergic activity in retinal blood vessels. Invest Ophthalmol Vis Sci 30:473, 1989 134. Rockwood EJ, Fantes F, Davis EB et al: The response of retinal vasculature to angiotensin. Invest Ophthalmol Vis Sci 28:676, 1987 135. Ferrari-Dileo G, Davis EB, Anderson DR: Angiotensin binding sites in bovine and human retinal blood vessels. Invest Ophthalmol Vis Sci 28:1747, 1987 136. Tomidokoro A, Araie M, Tamaki Y et al: Effects of topical carteolol and timolol on tissue circulation in the iris
and choroid. Curr Eye Res 18:381–390, 1999 137. Yu DY, Su EN, Cringle SJ et al: Systemic and ocular vascular roles of the antiglaucoma agents beta-adrenergic
antagonists and Ca2+ entry blockers. Surv Ophthalmol 43:Suppl 1:S214–222, 1999 138. Pillunat LE, Bohm AG, Koller AU et al: Effect of topical darzolamide on optic nerve head blood flow. Graefes Arch Clin Exp Ophthalmol 237:495–500, 1999 139. Grunwald JE: Effect of topical timolol on the human retinal circulation. Invest Ophthalmol Vis Sci 27:1713, 1986 140. Martin XD, Rabineau PA: Vasoconstrictive effect of topical timolol on human retinal arteries. Graefes Arch Clin Exp Ophthalmol 227:526, 1989 141. Hoste AM, Boels PF, Brutsaert DL et al: ffect of alpha1 and beta agonist on contraction of bovine retinal resistance
arteries in vitro. Invest Ophthalmol Vis Sci 30:44, 1989 142. Nielsen PJ, Nyborg CB: Adrenergic responses in isolated bovine retinal resistance arteries. Int Ophthalmol 13:103, 1989 143. Ferrari-Dileo G: Beta 1 and beta 2 adrenergic binding sites in bovine retina and retinal
blood vessels. Invest Ophthalmol Vis Sci 29:695, 1988 144. Sullivan PM, Davies GE, Caldwell G et al: RBF during hyperglycemia. Invest Ophthalmol Vis Sci 31:2041, 1990 145. Grunwald JE, Riva CE, Martin DB et al: Effect of an insulin-induced decrease in blood glucose on the human diabetic
retinal circulation. Ophthalmology 94:1614, 1987 146. Fallon TJ, Sleightholm MA, Merrick C et al: The effect of acute hyperglycemia on flow velocity in the macular capillaries. Invest Ophthalmol Vis Sci 28:1027, 1987 147. Kohner EM: The problems of RBF in diabetes. Diabetes 25:839, 1976 148. Yoshida A, Feke GT, Morales-Stopello et al: RBF alterations during progression of diabetic retinopathy. Arch Ophthalmol 101:225, 1983 149. Grunwald JE, Riva CE, Sinclair SH et al: Laser Doppler velocimetry study of retinal circulation in diabetes mellitus. Arch Ophthalmol 104:991, 1986 150. Feke GT, Tagawa H, Yoshida A et al: Retinal circulatory changes related to retinopathy progression in insulin
dependent diabetes mellitus. Ophthalmology 92:1517, 1985 151. Feke GT, Green GJ, Goger DG et al: Laser Doppler measurements of the effect of panretinal photocoagulation
on RBF. Ophthalmology 89:757, 1982 152. Grunwald JE, Riva CE, Brucker AJ et al: Effect of panretinal photocoagulation on RBF in proliferative diabetic
retinopathy. Ophthalmology 93:590, 1986 153. Grunwald JE, Brucker AJ, Petrig BL et al: RBF regulation and the clinical response to panretinal photocoagulation
in proliferative diabetic retinopathy. Ophthalmology 96:1518, 1989 154. Riva CE, Grunwald JE, Sinclair SE: Laser Doppler velocimetry study of the effect of pure oxygen beathing on
RBF. Invest Ophthalmol Vis Sci 24:47, 1983 155. Grunwald JE, Riva CE, Brucker AJ et al: Altered retinal vascular response to 100% oxygen breathing in diabetes
mellitus. Ophthalmology 91:1447, 1984 156. Riva CE, Grunwald JE, Petrig BL: Reactivity of the human retinal circulation to darkness: A laser Doppler
velocimetry study. Invest Ophthalmol Vis Sci 24:737, 1983 157. Bill A, Sperber GO: Control of retinal and choroidal blood flow. Eye 4:319, 1990 158. Meehan RT, Taylor GR, Rock P et al: An automated method of quantifying retinal vascular responses during exposure
to novel environmental conditions. Ophthalmology 97:875, 1990 159. Cairioli J, Sears M, Mead A: Ocular blood flow in phakic and aphakic monkey eyes. Exp Eye Res 39:1, 1984 160. Ernest JT: Optic disc blood flow. Trans Ophthalmol Soc UK 96:348, 1976 161. Riva CE, Greenwald JE, Sinclair SH: Laser Doppler measurement of relative blood velocity in the human optic
nerve head. Invest Ophthalmol Vis Sci 22:241, 1982 162. Sebag J, Feke GT, Delori FC et al: Anterior optic nerve blood flow in experimental optic atrophy. Invest Ophthalmol Vis Sci 25:1415, 1985 163. Sebag J, Delori FC, Weiter JJ et al: Anterior optic nerve blood flow decreases in clinical neurogenic optic
atrophy. Ophthalmology 93:858, 1986 164. Rizzo JF, Feke GT, Goger DG et al: Measurement of optic nerve blood velocity as a function in age in normal
subjects. Invest Ophthalmol Vis Sci (suppl) 30:245, 1989 165. Cunha-Vaz J: The blood-ocular barriers. Surv Ophthalmol 23:279, 1979 166. Schnecberger EE, Lyunch RD: Tight junctions: Their structure, composition and function. Circ Res 55:723, 1984 167. Cole DF: Ocular fluids. In Davson H, ed: The Eye. Vegetative Physiology
and Biochemistry, Vol 1a, p 269. New York, Academic Press, 1984 168. Maurice D: Drug exchange between the blood and vitreous. In Chunha-Vaz
JA, ed: The Blood-Retinal Barriers, pp 165–178. New York, Plenum, 1980 169. Bito LZ: The physiology and pathophysiology ofintraocular fluids. Exp Eye Res (suppl) 25:273, 1977 170. Szalay J, Nunziata B, Henkind P: Permeability of iridial blood vessels. Exp Eye Res 21:531, 1975 171. Raviola G: The structural basis of the blood-ocular barriers. Exp Eye Res (suppl) 25:27, 1977 172. Freddo T, Raviola G: Freeze-fracture analysis of the inter-endothelial junctions in the blood
vessels of the iris in the macaca mulatta. Invest Ophthalmol Vis Sci 23:154, 1982 173. Bill A: The blood-aqueous barrier. Trans Ophthalmol Soc UK 105:149, 1986 174. Novack GD, Leopold IH: The blood-aqueous and blood-brain barriers to permeability. Am J Ophthalmol 105:412, 1988 175. Kronfeld PC, Lin CK, Luo TH: The protein content of reformed aqueous humor in man. Am J Ophthalmol 24:264, 1941 176. Eakins KE: Prostaglandin and non-prostaglandin mediated breakdown on the blood-aqueous
barrier. Exp Eye Res (suppl) 25:483, 1977 177. Vane J, Botting R: Inflammation and the mechanism of action of anti-inflammatory drugs. FASEB J 1:89, 1987 178. Neufeld AH, Cavis RM, Sears ML: Degeneration release of norepinephrine causes transient ocular hyperemia
mediated by prostaglandins. Invest Ophthalmol Vis Sci 12:167, 1973 179. Neufeld AH, Jampol LM, Sears ML: Aspirin prevents the disruption of the blood-aqueous barrier in the rabbit
eye. Nature 238:158, 1972 180. Van Haeringeng NJ, Glasius E, Oosterhuis JA et al: Drug prevention of blood-aqueous barrier disruption. Ophthalmic Res 15:180, 1983 181. Sanders DR, Kraff M: Steroidal and nonsteroidal antiinflammatory agents: Effect on postsurgical
inflammation and blood-aqueous humor barrier breakdown. Arch Ophthalmol 102:1453, 1984 182. Cristiansson J, Palm E: The exchange of substances in the anterior part of the vitreous body, bordering
upon the lens. Acta Ophthalmol 32:199, 1954 183. Peyman GA, Bok D: Peroxidase diffusion in the normal and laser-coagulated primate retina. Invest Ophthalmol 11:35, 1972 184. Tornquist P, Alm A, Bill A: Permeability of ocular vesselsand transport across the blood-retinal barrier. Eye 4:303, 1990 185. Cunha-Vaz JA, Maurice DM: The active transport of fluorescein by the retinal vessels and retina. J Physiol 191:467, 1967 186. Bill A: Blood circulation and fluid dynamics in the eye. Physiol Rev 55:383, 1975 187. Bill A: A method to determine osmotically effective albumin and gamma globulin
concentrations in tissue fluids, its application to the uvea and a note
on the effects of capillary “leaks” on tissue fluid dynamics. Acta Physiol Scand 73:511, 1968 188. Laties AM, Rapoport S: The blood-ocular barriers under osmotic stress: Studies on the freeze-dried
eye. Arch Ophthalmol 94:1086, 1976 189. Grayson MC, Laties AM: Ocular localization of sodium fluorescein. Arch Ophthalmol 85:600, 1971 190. MacMahon RT, Tso MOM, McLean W: Histologic localization of sodium fluorescein in human ocular tissues. Am J Ophthalmol 80:1058, 1975 191. Flage T: The distribution of intravenously administered peroxidase in the optic
nerve head of rabbit and monkey. Acta Ophthalmol (Copenh) 53:801, 1975 192. Payman GA, Apple D: Peroxidase diffusion processes in the optic nerve. Arch Ophthalmol 88:650, 1972 193. Tsa MOM, Shih C-Y, McLean IW: Is there a blood-brain barrier at the optic nerve head? Arch Ophthalmol 93:815, 1975 |