1. Sommer A: Epidemiology and Statistics for the Ophthalmologist, p 3. New
York, Oxford University Press, 1980 2. Epidemiology and public health ophthalmology. Int J Epidemiol 21:1200, 1992 3. Heldt J-P: Public health ophthalmology: a comprehensive model for the prevention of
blindness in developing nations. Ophthalmic Surg 18:835, 1987 4. Thylefors B, Négrel A-D, Pararajasegaram R, Dadzie KY: Available data on blindness (update 1994). Ophthalm Epidemiol 2:5, 1995 5. Thylefors B, Négrel A-D, Pararajasegaram R, et al: Global data on blindness. Bull WHO 73:115, 1995 6. The World Health Report 1996: Fighting disease, fostering development. World
Health Forum 18:1–8, 1997 7. Tielsch JM, Sommer A, Katz J et al: Socioeconomic status and visual impairment among urban Americans. Arch Ophthalmol 109:637, 1991 8. Klein R, Klein BEK, Jensen SC, et al: The relation of socioeconomic factors to age-related cataract, maculopathy, and
impaired vision. The Beaver Dam Eye Study. Ophthalmology 101:1969, 1994 9. Hirschberg J (Blodi FC, trans): The History of Ophthalmology, p 74. Bonn: J.P. Wayenborgh, 1982 10. Bolsinger A: Erblindungsursachen im Wandel der Zeiten. In von Haugwitz
T (ed): Ophthalmology in the German-speaking Countries During the 20th
Century, p 144. Bonn, J.P. Wayenborgh, 1994 11. Krumpaszky HG, Klauss V: Epidemiology of blindness and eye disease. Ophthalmologica 210:1, 1996 12. Evans TH, Murray CJL: A critical re-examination of the economics of blindness prevention under
the onchocerciasis control programme. Soc Sci Med 25:241, 1987 13. Sartwell PE: The incubation period and the dynamics of infectious disease. Am J Epidemiol 83:204, 1966 14. Mackenbach JP: The epidemiological transition theory. J Epidemiol Community Health 48:329, 1994 15. Gregg NM: Congenital cataract following German measles in the mother. Trans Ophthalmol Soc Aust 3:35, 1941 16. Rubella Surveillance. National Communicable Disease Center, U.S. Department
of Health, Education, and Welfare, June, 1969 17. Centers for Disease Control and Prevention: Case definitions for infectious
conditions under public health surveillance. MMWR 46(RR-10):1, 1997 18. Wilde WR: Observations on the epidemic ophthalmia which has prevailed in
the workhouses and schools of the Tipperary and Athlone Unions. London
J Med Jan, 1851 19. Wilhelmus KR, Liesegang TJ, Osato MS, Jones DB: Cumitech 13A, Laboratory
Diagnosis of Ocular Infections. Washington DC, American Society for
Microbiology, 1994 20. Buehler JW, Finton RJ, Goodman RA: Epidemic keratoconjunctivitis: Report of an outbreak in an ophthalmology
practice and recommendations for prevention. Infect Control 5:390, 1984 21. McGowan JE Jr, Metchock BG: Basic microbiologic support for hospital epidemiology. Infect Control Hosp Epidemiol 17:298, 1996 22. Dolin PJ: Molecular epidemiology and ophthalmology. Br J Ophthalmol 78:808, 1994 23. Speaker MG, Milch FA, Shah MK, et al: Role of external bacterial flora in the pathogenesis of acute postoperative
endophthalmitis. Ophthalmology 98:639, 1991 24. Schouten HJ: Estimating kappa from binocular data and comparing marginal probabilities. Stat Med 12:2207, 1993 25. Alderson MR: Mortality, Morbidity, and Health Statistics. New York: Stockton
Press, 1988 26. Hyder AA, Rotllant G, Morrow RH: Measuring the burden of disease: Healthy life-years. Am J Public Health 88:196, 1998 27. Goh KT, Ooi PL, Miyamura K, et al: Acute haemorrhagic conjunctivitis: Seroepidemiology of coxsackievirus A24 variant
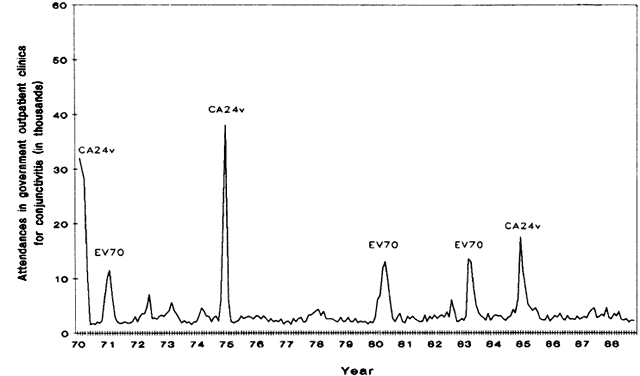
and enterovirus 70 in Singapore. J Med Virol 31: 245, 1990 28. Ostler HB, Okumoto M: The changing pattern of the etiology of central bacterial corneal (hypopyon) ulcer. Trans Pac Coast Otoophthalmol Soc 57:235, 1976 29. Upadhyay MP, Karmacharya PC, Koirala S, et al: Epidemiological characteristics, predisposing
factors, and etiologic diagnosis of corneal ulceration
in Nepal. Am J Ophthalmol 111, 1991 30. Falkow S: What is a pathogen? Developing a definition of a pathogen requires looking
closely at the many complicated relationships that exist among organisms. ASM News 63: 359, 1997 31. Egger SF, Huber-Spitzy V, Scholda C, et al: Bacterial contamination during extracapsular cataract extraction. Prospective
study on 200 patients. Ophthalmologica 208:77, 1994 32. Benenson AS, ed.: Control of Communicable Diseases Manual. Washington DC: American
Public Health Association, 1995 33. Quelmalz ST: De caecitate infantum fluoris albi materni ejusque virulenti
pedissequa desserit. Leipsig, 1750 34. Rawley W: A Treatise on One Hundred and Eighteen Principal Diseases of
the Eye and Eyelids. London, 1790 35. Von Graefe A: Über die diphtheritische Conjunctivitis und die Anwendung des Causticums
bei akuten Entzündungen. Arch Ophthalmol 1:168, 1854 36. Howe L: On the influence of the flies in the spread of Egyptian ophthalmia. Acta
Concil Ophthalmol 7, 1888 37. May RM, Anderson RM: Transmission dynamics of HIV infection. Nature 326:137, 1987 38. Weems JJ Jr: Candida parapsilosis: Epidemiology, pathogenicity, clinical manifestations, and antimicrobial
susceptibility. Clin Infect Dis 14:756, 1992 39. McMinn PC, Stewart J, Burrell CJ: A community outbreak of epidemic keratoconjunctivitis in central Australia
due to adenovirus type I. J Infect Dis 164:1113, 1991 40. Hope-Simpson RE: Infectiousness of communicable diseases in the househould (measles, chickenpox, and
mumps). Lancet 2:549, 1952 41. Evans AS: Causation and disease: The Henle-Koch postulates revisited. Yale J Biol Med 49:175, 1976 42. Huebner RJ: The virologist's dilemma. Ann NY Acad Sci 67:430, 1957 43. Hill AB: The environment and disease: Association or causation? Proc R Soc Med 58:295, 1965 44. Fredricks DN, Relman DA: Sequence-based identification of microbial pathogens: A reconsideration
of Koch's postulates. Clin Microbiol Rev 9:18, 1996 45. Sutter MC: Assigning causation in disease: Beyond Koch's postulates. Perspect Biol Med 39:581, 1996 46. Labarthe DR, Stallones RA: Epidemiological inference. In Rothman K (ed): Causal
Inference. Chestnut Hill MA, Epidemiology Resources Inc., 1988 47. Susser D: What is a cause and how do we know one? A grammar for pragmatic epidemiology. Am J Epidemiol 133:635, 1991 48. Sackett DL: Bias in analytic research. J Chron Dis 32:51, 1979 49. Miettinen OS: Confounding and effect modification. Am J Epidemiol 100:350, 1974 50. Seigel D: Designs for clinical research. Arch Ophthalmol 105:1647, 1987 51. Fletcher RH, Fletcher SW: Clinical research in general medical journals. A 30-year perspective. N Engl J Med 302:180, 1979 52. McDonnell PJ, Nobe J, Gauderman WJ: Community care of corneal ulcers. Am J Ophthalmol 114:531, 1992 53. McLeod SD, DeBacker CM, Viana MA: Differential care of corneal ulcers in the community based on apparent
severity. Ophthalmology 103:479, 1996 54. Jones DB, Visvesvara GS, Robinson NM: Acanthamoeba polyphaga keratitis and Acanthamoeba uveitis associated with fatal meningoencephalitis. Trans Ophthalmol Soc
UK 95:221, 1973. Paper presented at the Ocular Microbiology and Immunology
Group Meeting, Dallas, TX, 1973 55. Cohen EJ, Buchanan HW, Laughrea PA, et al: Diagnosis and management of Acanthamoeba keratitis. Am J Ophthalmol 100:389, 1985 56. Hamano H, Kitano J, Mitsunaga S, et al: Adverse effects of contact lens wear in a large Japanese population. CLAO J 11:141, 1985 57. Stehr-Green JK, Bailey TM, Brandt FH, et al: Acanthamoeba keratitis in contact lens wearers. A case control study. JAMA 257:57, 1987 58. Stehr-Green JK, Bailey TM, Visvesvara GS: The epidemiology of Acanthamoeba keratitis in the United States. Am J Ophthalmol 107:331, 1989 59. Dart JK: Personal Communication, 1998 60. Wilhelmus KR, Stulting RD, Sugar J, et al: Primary graft failure. A national reporting system. Arch Ophthalmol 113:1497, 1995 61. O'Day DM: Value of a centralized surveillance system during a national epidemic of
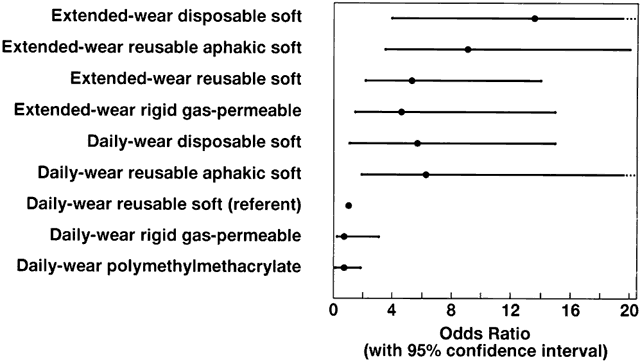
endophthalmitis. Ophthalmology 92:309, 1985 62. Pitaksiripan S, Butpongsapan S, Pravithayakarn L, et al: An outbreak of post-operative endophthalmitis in Lampang Hospital. J Med Assoc Thailand 7:S95–1995, 1995 63. Poggio EC, Glynn RJ, Schein OD, et al: The incidence of ulcerative keratitis among users of daily-wear and extended-wear
soft contact lenses. N Engl J Med 321:779, 1989 64. Methods of Assessment of Avoidable Blindness. Geneva, World Health Organization, 1980 65. The Role and Functions of National Institutes of Ophthalmology. Report
on a WHO Meeting, p 5. Cophenhagen, World Health Organization, 1979 66. International Agency for the Prevention of Blindness: World Blindness and
its Prevention, p 78. Oxford, Oxford University Press, 1980 67. International Agency for the Prevention of Blindness: World Blindness and
its Prevention, p 18. Oxford, Oxford University Press, 1984 68. International Agency for the Prevention of Blindness: World Blindness and
its Prevention, p 26. Oxford, Oxford University Press, 1988 69. Prevention of Childhood Blindness. Geneva, World Health Organization, 1992 70. Conjunctivitis of the Newborn. Prevention and Treatment at the Primary
Health Care Level, p 2. Geneva, World Health Organization, 1986 71. Brain P: Galen on Bloodletting. A Study of the Origins, Development and
Validity of his Opinions, with a Translation of the Three Works, p 91. Cambridge, Cambridge
University Press, 1986 72. Vaughn DG, Jr.: The contamination of fluorescein solutions: With special reference to Pseudomonas aeruginosa (bacillus pyocyanene). Am J Ophthalmol 39:55, 1955 73. Moy CS: Case-control designs for clinical research in ophthalmology. Arch Ophthalmol 116:661, 1998 74. Schein OD, Glynn RJ, Poggio EC: The relative risk of ulcerative keratitis among users of daily-wear and
extended-wear soft contact lenses: A case-control study. N Engl J Med 321:773, 1989 75. Gallant JE, Moor RD, Rickman DD, et al: Incidence and natural history of cytomegalovirus disease in patients with
advanced human immunodeficiency virus disease treated with zidovudine. J Infect Dis 166:1223, 1992 76. Granley JP: Epidemiologic characteristics of presumed ocular histoplasmosis. Acta Ophthalmol 119(Suppl):1, 1973 77. Hawkins BS, Ganley JP: Risk of visual impairment attributable to ocular histoplasmosis. Arch Ophthalmol 112:655, 1994 78. Leibowitz HM, Krueger DE, Maunder LR, et al: The Framingham eye study monograph: An ophthalmological and epidemiologicalal
study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and
visual acuity in a general population of 2631 adults, 1973-1975. Opthalmol 24:335, 1980 79. Klein R, Klein BEK, Lee KE: Changes in visual acuity in a population. The Beaver Dam Eye Study. Ophthalmology 103:1169, 1996 80. Erie JC, Nevitt MP, Hodge DO, Ballard DJ: Incidence of ulcerative keratitis in a defined population from 1950 through 1988. Arch Ophthalmol 111:1665, 1993 81. Coleman AL, Morgenstern H: Use of insurance claims databases to evaluate the outcomes of ophthalmic
surgery. Surv Ophthalmol 42:271, 1997 82. Chalmers TC: Ethical aspects of clinical trials. Am J Ophthalmol 79:753, 1975 83. Sommer A: Toward a better understanding of medical reports. Am J Ophthalmol 79:1053, 1975 84. Ederer F: Why do we need controls? Why do we need to randomize? Am J Ophthalmol 79:758, 1975 85. Beaman JE: Writing the protocol for a clinical trial. Am J Ophthalmol 79:775, 1975 86. Fredrickson DS: The field trial: Some thoughts on the indispensable ordeal. Bull NY Acad Med 44:985, 1968 87. Wormald R, Oldfield K: Evidence based medicine, the Cochrane Collaberation, and the CONSORT statement. Br J Ophthalmol 82:597, 1998 88. Fendrick AM, Goodman CS, Trobe JD: The effectiveness initiative. II: The spectrum of effectiveness research. Arch Ophthalmol 113:862, 1995 89. Guillié SG: Blepharoblenhorrhea contagiosa. Bibliothèque
Ophtalmologique 1:74, 1820 [The first English translation appears
in Mackenzie, W: A practical treatise on the diseases of the eye, heritage. The
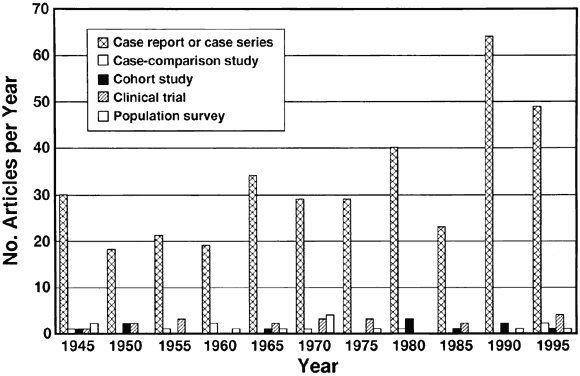
voyage of the Rôdeur. Arch Ophthalmol 71:439, 1964] 90. Schmitz H, Wigand R, Heinrich W: Worldwide epidemiology of human adenovirus infections. Am J Epidemiol 117:455, 1983 91. Ford E, Nelson KE, Warren D: Epidemiology of epidemic keratoconjunctivitis. Epidemiol Rev 9:244, 1987 92. Fitch CP, Rapoza PA, Owens S, et al: Epidemiology and diagnosis of acute conjunctivitis at an inner-city hospital. Ophthalmology 96:1215, 1989 93. Feibel RM, 1988: John Vetch and the Egyptian ophthalmia. Surv Ophthalmol 28:128, 1983 94. Wagemann M, van Bijsterveld OP: The French Egyptian campaign and its effects on ophthalmology. Doc Ophthalmol 68:135, 1988 95. Bailey R, Mabey D: HLA and trachoma. Br J Ophthalmol 181:425, 1997 96. Woolridge RL, Grayston JT, Chang IH, et al: Field trial of a monovalent and of a bivalent mineral oil adjuvant trachoma
vaccine in Taiwan school children. Am J Ophthalmol 63:1645, 1967 97. Woolridge RL, Cheng KH, Chang IH, et al: Failure of trachoma treatment with ophthalmic antibiotics and systemic
sulfonamides used along or in combination with trachoma vaccine. Am J Ophthalmol 63:1577, 1967 98. Woolridge RL, Grayston JT, Chang IH, et al: Long-term follow-up of the initial (1959-60) trachoma vaccine field trial
on Taiwan. Am J Ophthalmol 63:1650, 1967 99. Bietti GB, Guerra P, Vozza R, et al: Results of a large-scale vaccination against trachoma in East Africa (Ethiopia) 1960-1965. Am J Ophthalmol 61:1010, 1966 100. Guerra P, Buogo A, Marubini E, et al: Analysis of clinical and laboratory data of an experiment with trachoma
vaccine in Ethiopia. Am J Ophthalmol 63:1631, 1967 101. Nichols RL, Bell SD, Haddad NA, Bobb AA: Studies on trachoma. VI. Microbiological observations in a field trial
in Saudi Arabia of bivalent trachoma vaccine at three dosage levels. Am J Trop Med Hyg 18:723, 1969 102. Jones BR: The prevention of blindness from trachoma. Trans Ophthalmol Soc UK 95:16, 1975 103. Sowa S, Sowa J, Collier LH, Blyth WA: Trachoma vaccine field trials in The Gambia. J Hyg 67:699, 1969 104. Dhir SP, Agarwal LP, Detels R, et al: Field trial of two bivalent trachoma vaccines in children of Punjab Indian
villages. Am J Ophthalmol 63:1639, 1967 105. Clements C, Dhir SP, Grayston JT, Wang SP: Long term follow-up study of a trachoma vaccine trial in villages of Northern
India. Am J Ophthalmol 87:350, 1979 106. Ciribelli-Guimar'es J, Duarte A, Machado RD, et al: Tracoma. Ensaios clínicos de vacinaç'o, isolamento e identificaç'o
do agente. Resultados gerais no Pará e no Ceará 1967et1968. Rev Bras Malariol Doencas Trop 22:423, 1970 107. Trottier S, Stenberg K, von Rosen IA, et al: Haemophilus influenzae causing conjunctivitis in day-care children. Pediatr Infect Dis J 10:578, 1991 108. Schwartz B, Harrison LH, Motter JS, et al: Investigation of an outbreak of Moraxella conjunctivitis at a Navajo boarding school. Am J Ophthalmol 107:341, 1989 109. Shayegani M, Parsons LM, Gibbons WE Jr, et al: Characterization of nontypable Streptococcus pneumoniae-like organisms isolated from outbreaks of conjunctivitis. J Clin Microbiol 16:8, 1982 110. Garibaldi RA, Brodine S, Matsumiya S: Infections among patients in nursing homes: Policies, prevalence, problems. N Engl J Med 305:731, 1981 111. Ruben FL, Norden CW, Heisler B, et al: An outbreak of of Streptococcus pyogenes infections in a nursing home. Ann Intern Med 101:494, 1984 112. Barquet N, Gasser I, Domingo P, et al: Primary meningococcal conjunctivitis: Report of 21 patients and review. Rev Infect Dis 12:838, 1990 113. King S, Devi SP, Mindorff C, et al: Nosocomial Pseudomonas aeruginosa conjunctivitis in a pediatric hospital. Infect Control Hosp Epidemiol 9:77, 1988 114. Hilton E, Adams AA, Uliss A, et al: Nosocomial bacterial eye infections in intensive-care units. Lancet 1:1318, 1983 115. Brennen C, Muder RR: Conjunctivitis associated with methicillin-resistant Staphylococcus aureus in a long-term-care facility. Am J Med 88:14N, 1990 116. Ringvold A, Vik E, Bevanger LS: Moraxella lacunata isolated from epidemic conjunctivitis among teen-aged females. Acta Ophthalmol 63:427, 1985 117. Buehler JW, Holloway JT, Goodman RA, et al: Gnat sore eyes: Seasonal, acute conjunctivitis in a southern state. South Med J 76:587, 1983 118. Brazilian Purpuric Fever Study Group: Brazilian purpuric fever: Epidemic
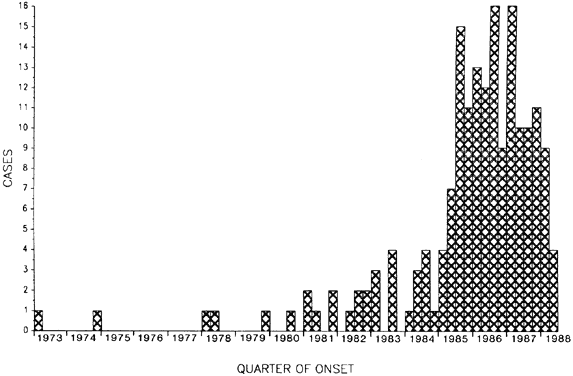
purpura fulminans associated with antecedent purulent conjunctivitis. Lancet 2:757, 1987 119. Cherian T, Steinhoff MC, Harrison LH, et al: A cluster of invasive pneumococcal disease in young children in child care. JAMA 271:695, 1994 120. Mikru FS, Molla T, Ersumo M, et al: Community-wide outbreak of Neisseria gonorrhoeae conjunctivitis in Konso district, North Omo administrative region. Ethiop Med J 29:27, 1991 121. Merianos A, Condon RJ, Tapsall JW, et al: Epidemic gonococcal conjunctivitis in central Australia. Med J Austr 162:178, 1995 122. Koppert HC, van Rij G: The phlycten, a come-back? Doc Ophthalmol 52:339, 1982 123. Alfonso E, Friedland B, Hupp S, et al: Neisseria gonorrhoeae conjunctivitis: An outbreak during an epidemic of acute hemorrhagic conjunctivitis. JAMA 250:794, 1983 124. Sangawe JL, Mtanda AT: Epidemic gonococcal ophthalmia in adults in Tanzania. Indian J Ophthalmol 32:17, 1984 125. Schwab L, Tizazu T: Destructive epidemic Neisseria gonorrhoeae keratoconjunctivitis in African adults. Br J Ophthalmol 69:525, 1985 126. Fransen L, Nsanze H, D'Costa LJ, et al: Parents of infants with ophthalmia neonatorum: A high-risk group for sexually
transmitted diseases. Sex Transm Dis 12:150, 1985 127. Najem GR, Riley HD Jr, Ordway NK, et al: Clinical and microbiologic surveillance of neonatal staphylococcal disease. Relationship
to hexachlorophene. Am J Dis Child 129:297, 1975 128. Hedberg K, Ristinen TL, Soler JT, et al: Outbreak of erythromycin-resistant staphylococcal conjunctivitis in a newborn
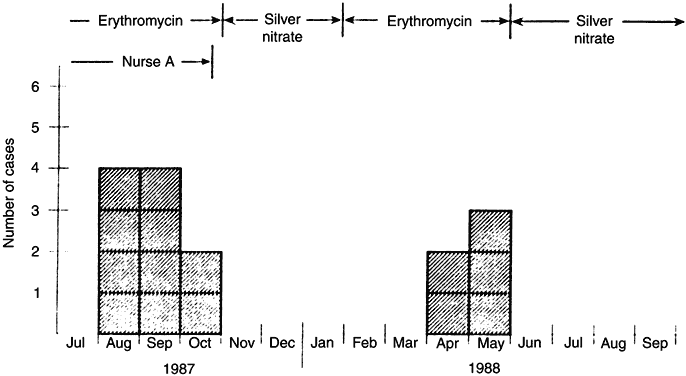
nursery. Ped Infect Dis J 9:268, 1990 129. Chowdhury MNH, Kambal AM: An outbreak of infection due to Staphylococcus aureus phage type 52 in a neonatal intensive care unit. J Hosp Infect 22:299, 1992 130. Mehta G, Kumari S: Multi-resistant Staphylococcus haemolyticus in a neonatal unit it New Delhi. Ann Trop Paed 17:15, 1997 131. Nelson JD, Dillon HC Jr, et al: A prolonged nursery epidemic associated with a newly recognized type of
group A streptococcus. J Ped 89:792, 1976 132. Drewett SE, Payne DJH, Tuke W, et al: Eradication of Pseudomonas aeruginosa infection from a special-care nursery. Lancet 1:946, 1972 133. Salminen L, Mattila L, Pitkänen Y: Iatrogene Epidemie von Ophthalmia neonatorum. Klin Monatsbl Augenheilkd 180:166, 1982 134. Traboulsi EI, Shammas IV, Rath HE, Jarudi NI: Pseudomonas aeruginosa ophthalmia neonatorum. Am J Ophthalmol 98:801, 1984 135. Garland SM, Mackay S, Tabrizi S, et al: Pseudomonas aeruginosa outbreak associated with a contaminated blood-gas analyser in a neonatal
intensive care unit. J Hosp Infect 33:145, 1996 136. Rapkin RH: Pseudomonas cepacia in an intensive care nursery. Pediatrics 57:239, 1976 137. Christensen GD, Korones SB, Reed L, et al: Epidemic Serratia marcescens in a neonatal intensive care unit: importance of the gastrointestinal
tract as a reservoir. Infect Control 3:127, 1982 138. Duggan TG, Leng RA, Hancock BM, et al: Serratia marcenscens in a newborn unit—microbiological features. Pathology 16:189, 1984 139. van Ogtrop ML, van Zoeren-Grobben D, VerbakelSalomons EMA, et al: Serratia marcescens infections in neonatal departments: Description of an outbreak and review
of the literature. J Hosp Infect 36:95, 1997 140. Callen JP: Epidemic herpes simplex virus infection. Am J Dis Child 1337:182, 1983 141. Holland EJ, Mahanti RL, Belongia EA, et al: Ocular involvement in an outbreak of herpes gladiatorum. Am J Ophthalmol 114:680, 1992 142. Yorston D, Foster A: Traditional eye medicines and corneal ulceration in Tanzania. J Trop Med Hyg 97:211, 1994 143. Landolt E, Wiesmann E: Eine Epidemie von Pseudomonas—Keratitiden bei Tragern von Dauerkontaktlinsen. Klin Monatsbl Augenheilkd 174:788, 1979 144. Schein O, Hibberd P, Kenyon KR, et al: Contact lens complications: Incidental or epidemic? Am J Ophthalmol 102:116, 1986 145. Liesegang TJ: Contact lens-related microbial keratitis: Part I: Epidemiology. Cornea 16:125, 1997 146. Ayliffe GA, Barry DR, Lowbury EJ, et al: Postoperative infection with Pseudomonas aeruginosa in an eye hospital. Lancet 1:1113, 1996 147. Nwoke BEB: The socio-economic aspects of human onchocerciasis in Africa: Present appraisal. J Hyg Epidemiol Microbiol Immun 1:337, 1990 148. Walsh JF, Molyneux DH, Birley MH: Deforestation: Effects on vector-borne disease. Parasitology 106:555, 1993 149. Burnett AJ, Shortt SG, Isaac-Renton J, et al: Multiple cases of acquired toxoplasmosis retinitis presenting in an outbreak. Ophthalmology 105:1032, 1988 150. Akstein RB, Wilson LA, Teutsch SM: Acquired toxoplasmosis. Ophthalmology 89:1299, 1982 151. McDonald JC, Gyorkos TW, Alberton B, et al: An outbreak of toxoplasmosis in pregnant women in northern Quebec. J Infect Dis 161:769, 1990 152. Javitt JC, Wang F, West SK: Blindness due to cataract: Epidemiology and prevention. Ann Rev Public Health 17: 159, 1996 153. Kattan HM, Flynn HW Jr, Pflugfelder SC, et al: Nosocomial endophthalmitis survey. Current incidence of infection after
intraocular surgery. Ophthalmology 98:227, 1991 154. Pettit TH, Olso RJ, Foos RY, et al: Fungal endophthalmitis following intraocular lens implantation. A surgical
epidemic. Arch Ophthalmol 98:1025, 1980 155. Egerer I, Sehorst W: Histopathologie der exogen mykotischen Endophthalmitis. Klin Monatsbl Augenheilkd 169:325, 1976 156. Gerding DN, Poley BJ, Hall WH, et al: Treatment of Pseudomonas endophthalmitis associated with prosthetisc intraocular lens implantation. Am J Ophthalmol 99:902, 1979 157. McCray E, Rampell N, Solomon SL, et al: Outbreak of Candida parapsilosis endophthalmitis after cataract extraction and intraocular lens implantation. J Clin Microbiol 24:625, 1986 158. Marinero Cáceres JA, de Sotello Y: Infection control in El Salvador: The Hospital Rosales experience. Infect Control 8:495, 1987 159. Klimek JJ, Ajemian E, Andrews L, et al: Outbreak of bacterial endophthalmitis after cataract surgery and lens implantation: Lack
of direct evidence for exogenous contributing factors. Am J Infect Control 14:184, 1986 160. Moore PJ, Linnemann CC Jr, Sanitato JJ, et al: Pneumococcal endophthalmitis after corneal transplantation: Control by
modification of harvesting techniques. Infect Control Hosp Epidemiol 10:102, 1989 161. Centers for Disease Control and Prevention: Outbreaks of postoperative
bacterial endophthalmitis caused by intrinsically contaminated ophthalmic
solutions - Thailand, 1992, and Canada, 1993. MMWR 45:491, 1996 162. Mirza GE, Doganay M, Caglayangil A: Postoperative endophthalmitis caused by an Enterobacter species. J Hosp Infect 26:167, 1994 163. Rogers NK, Fox PD, Noble BA, et al: Aggressive management of an epidemic of chronic pseudophakic endophthalmitis: Results
and literature survey. Br J Ophthalmol 78: 115, 1994 164. Arsan AK, Adisen A, Duman S, et al: Acute endophthalmitis outbreak after cataract surgery. J Cataract Refract Surg 22(S95–S98):1116, 1996 165. Swaddiwudhipong W, Tangkitchot T, Silarug N: An outbreak of Pseudomonas aeruginosa postoperative endophthalmitis caused by contaminated intraocular irrigating
solution. Trans R Soc Trop Med Hyg 89:288, 1995 166. Roy M, Chen JC, Miller M, et al: Epidemic Bacillus endophthalmitis after cataract surgery. I. Acute presentation and outcome. Ophthalmology 104:1758, 1997 167. Fridkin SK, Kremer FB, Bland LA, et al: Acremonium kiliense endophthalmitis that occurred after cataract extraction in an ambulatory
surgical center and was traced to an environmental reservoir. Clin Infect Dis 22:222, 1996 168. Orth B, Frei R, Intin PH, et al: Outbreak of invasive mycoses caused by Paecilomyces lilacinus from a contaminated skin lotion. Ann Intern Med 125:799, 1996 169. Daily MJ, Dickey JB, Packo KH: Endogenous Candida endophthalmitis after intravenous anesthesia with propofol. Arch Ophthalmol 109:1081, 1991 170. Servant JB, Dutton GB, Ong-Tone L, et al: Candidal endophthalmitis in Glaswegian heroin addicts: Report of an epidemic. Trans Ophthalmol Soc UK 104:297, 1985 171. O'Day DM, Head WS, Robinson RD: An outbreak of Candida parapsilosis endophthalmitis: Analysis of strains by enzyme profile and antifungal
susceptibility. Br J Ophthalmol 71:126, 1987 172. Clemons KV, Shankland GS, Richardson MD, Stevens DA: Epidemiological study by DNA typing of a Candida albicans outbreak in heroin
addicts. J Clin Microbiol 29:205, 1991 173. Crompton DO: Epidemic endophthalmitis. In Watts J, McK, McDonald PJ, O'Brien
PE, et al: (eds): Infection in Surgery. Basic and Clinical Aspects, p 356. Edinburgh, Churchill Livingstone, 1981 174. Wilhelmus KR, Penland RL: Does endophthalmitis have a seasonal fluctuation? Invest Ophthalmol Vis Sci 38(4): S1105, 1997 175. Thylefors B: Much blindness is avoidable. World Health Forum 12:78, 1991 176. Ganley JP, Roberts J: Eye conditions and related need for medical care among persons 1–74 years
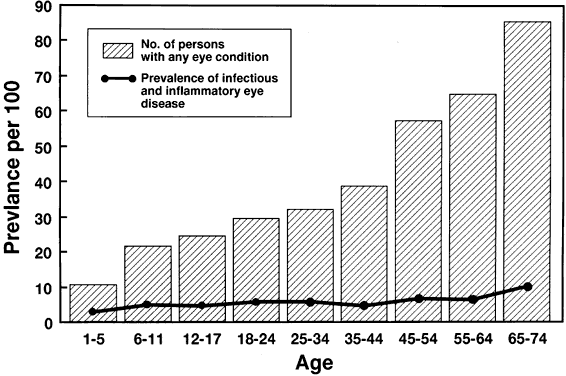
of age: United States, 1971-72. Vital Health Stat 11(228):1, 1983 177. Public Health Committee: Updated recommendations for ophthalmic practice
in relation to the human immunodeficiency virus and other infectious
agents. San Francisco, American Academy of Ophthalmology, 1992 178. Tokars JI, Culver DH, Mendelson MH, et al: Skin and mucous membrane contacts with blood during surgical procedures: Risk
and prevention. Infect Control Hosp Epidemiol 16:703, 1995 179. Kramer A, Behrens-Baumann W: Prophylactic use of topical anti-infectives in opthalmology. Opthalmologica 211: 68, 1997 180. Onchocerciasis and its Control. Report of a WHO Expert Committee on Onchocerciasis
Control. WHO Technical Report Series 852. Geneva, World Health
Organization, 1995 181. West S, Taylor HR: Community-based intervention programs for trachoma control. Int Ophthalmol 12:19, 1988 182. Thylefors B, Négrel AD: Developments for a global approach to trachoma control. Rev Int Trach Pathol Ocul Trop Subtrop Santé Pub 71:63, 1994 183. Buffington J, Chapman LE, Stobierski MG, et al: Epidemic keratoconjunctivitis in a chronic care facility: Risk factors
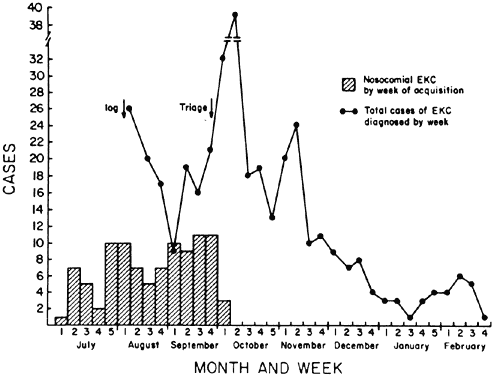
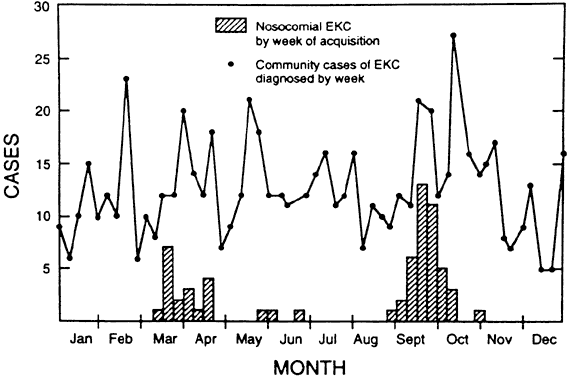
and measure for control. J Am Geriatr Soc 41:1177, 1993 184. Isenberg SJ, Apt L, Wood M: A controlled trial of povidone-iodine as prophylaxis against ophthalmia
neonatorum. N Engl J Med 332:562, 1995 185. Graefe A: Wundbehandlung bei Augen-Operationen mit besonderer Berücksichtigung
der Staar-Extraction. Operation unreifer Staare. Albrecht von Graefe Arch Ophthalmol 30:211, 1884 186. Jones BR: The need for antiviral therapy and prophylaxis of viral ocular disease. J Infect Dis 133:A93, 1976 187. Pepose JS: The potential impact of the varicella vaccine and new antivirals on ocular
disease related to varicella-zoster virus. Am J Ophthalmol 123:243, 1997 | 



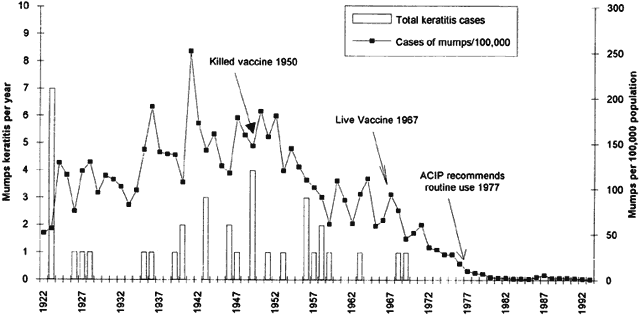
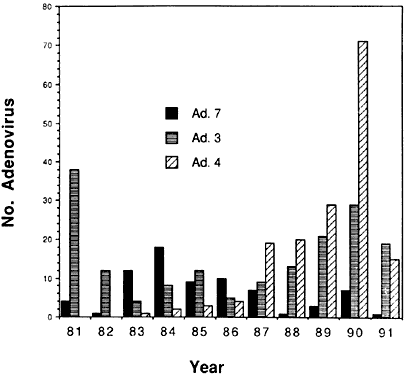
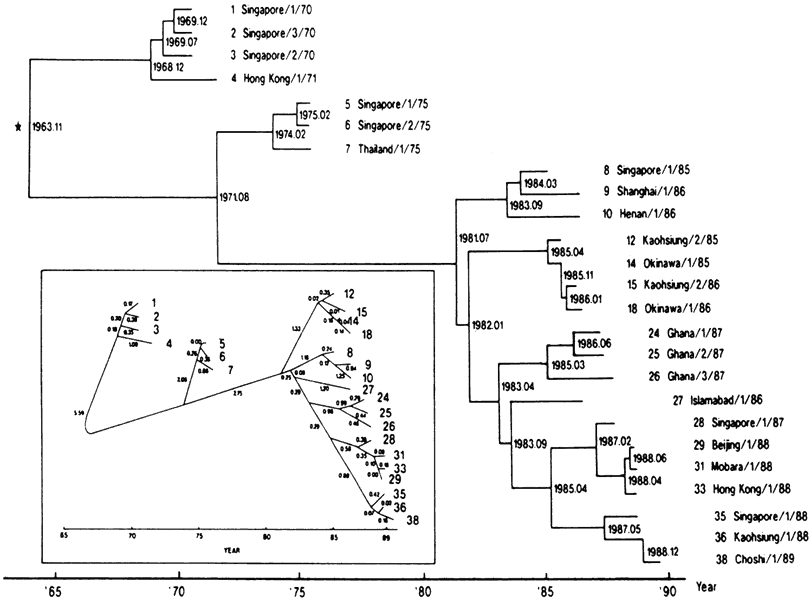
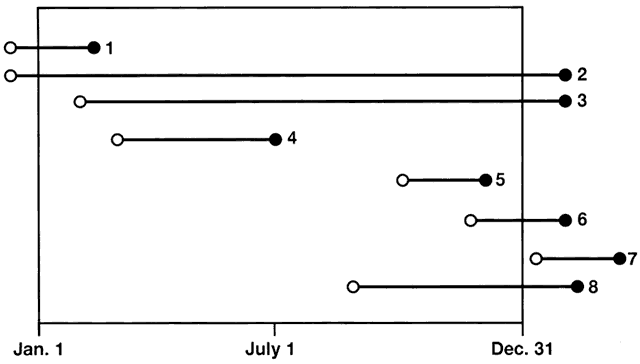
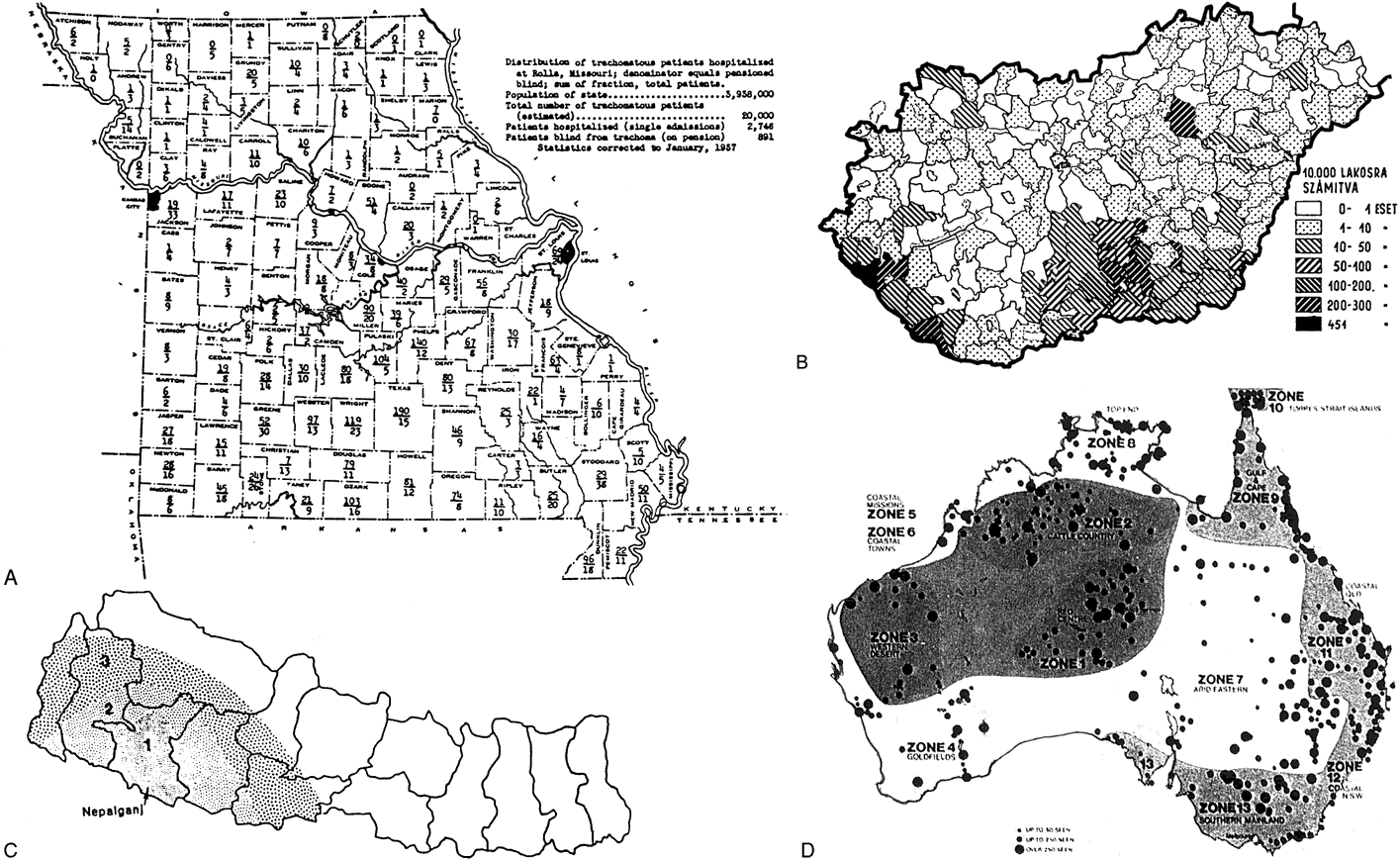
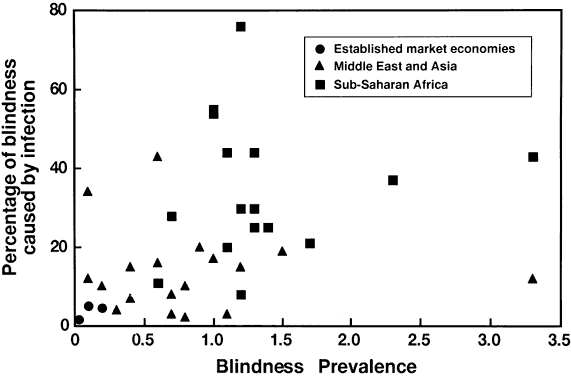
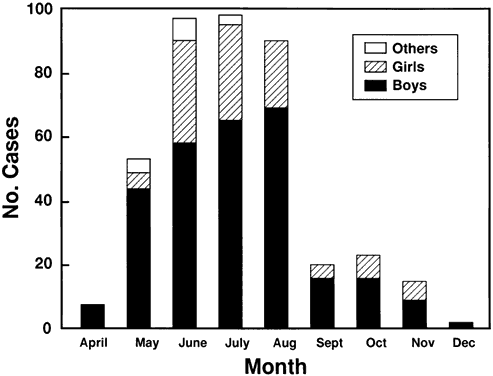
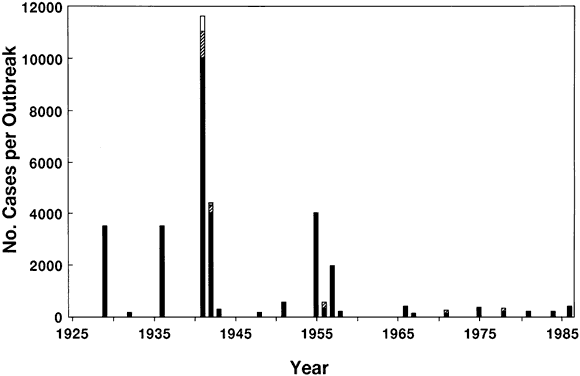
 6/18
6/18