Chapter 11:
Glaucoma
Authors:
Glaucoma is characterized by elevated intraocular pressure associated with optic disk cupping and visual field loss. In the majority of cases, there is no associated ocular disease (primary glaucoma) (Table 11-1).
Approximately 20,000 Americans are blind from glaucoma, making it the leading cause of preventable blindness in the United States. An estimated 2 million Americans have glaucoma. Primary open-angle glaucoma, the most common form, causes insidious asymptomatic progressive bilateral visual loss that is often not detected until extensive field loss has already occurred. Other forms of glaucoma are responsible for severe visual morbidity in individuals of all ages. Acute (angle-closure) glaucoma comprises 10-15% of cases in Caucasians. This percentage is much higher in Asians, particularly among the Burmese and Vietnamese in Southeast Asia.
The mechanism of raised intraocular pressure in glaucoma is impaired outflow of aqueous resulting from abnormalities within the drainage system of the anterior chamber angle (open-angle glaucoma) or impaired access of aqueous to the drainage system (closed-angle glaucoma) (Table 11-2). Treatment is directed toward reducing the intraocular pressure and, when possible, correcting the underlying cause.
Reducing aqueous production is a method of reducing intraocular pressure used in all forms of glaucoma. Several medications reduce aqueous production. Surgical procedures that reduce aqueous production are available but are generally used only after medical treatment has failed. Facilitating flow of aqueous through the trabecular meshwork is useful in open-angle glaucoma. Improving access of aqueous to the anterior chamber angle in closed-angle glaucoma may be achieved by peripheral laser iridotomy or surgical iridectomy if the cause is pupillary block, miosis if there is angle crowding, or cycloplegia if there is anterior lens displacement. Surgically bypassing the drainage system is useful in open-angle glaucoma and in angle closure that fails to respond to medical treatment. In the secondary glaucomas, consideration must always be given to treating the primary abnormality.
In all patients with glaucoma, the necessity for treatment and its effectiveness are assessed by regular determination of intraocular pressure (tonometry), inspection of optic disks, and measurement of visual fields.
The management of glaucoma is best left to the ophthalmologist, but the magnitude of the problem and the importance of detecting asymptomatic cases call for the cooperation and assistance of all medical personnel. Ophthalmoscopy (noting optic nerve changes) and tonometry should be part of the routine ophthalmologic examination of all patients old en-ough to cooperate and certainly all patients over 30 years of age. This is especially important in patients with a family history of glaucoma.
PHYSIOLOGY OF AQUEOUS HUMOR
The intraocular pressure is determined by the rate of aqueous production and the resistance to outflow of aqueous from the eye. Some knowledge of the physiology of aqueous humor is necessary for understanding glaucoma.
Composition of Aqueous
The aqueous is a clear liquid that fills the anterior and posterior chambers of the eye. Its volume is about 250 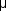 L, and its rate of production, which is subject to diurnal variation, is about 2.5
L, and its rate of production, which is subject to diurnal variation, is about 2.5 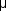 L/min. The osmotic pressure is slightly higher than that of plasma. The composition of aqueous is similar to that of plasma except for much higher concentrations of ascorbate, pyruvate, and lactate and lower concentrations of protein, urea, and glucose.
L/min. The osmotic pressure is slightly higher than that of plasma. The composition of aqueous is similar to that of plasma except for much higher concentrations of ascorbate, pyruvate, and lactate and lower concentrations of protein, urea, and glucose.
Formation & Flow of Aqueous
Aqueous is produced by the ciliary body. An ultrafiltrate of plasma produced in the stroma of the ciliary processes is modified by the barrier function and secretory processes of the ciliary epithelium. Entering the posterior chamber, the aqueous passes through the pupil into the anterior chamber (![]() Figure 11-1) and then to the trabecular meshwork in the anterior chamber angle. During this period, there is some differential exchange of components with the blood in the iris.
Figure 11-1) and then to the trabecular meshwork in the anterior chamber angle. During this period, there is some differential exchange of components with the blood in the iris.
Intraocular inflammation or trauma causes an increase in the protein concentration. This is called plasmoid aqueous and closely resembles blood serum.
Outflow of Aqueous
The trabecular meshwork is composed of beams of collagen and elastic tissue covered by trabecular cells that form a filter with a decreasing pore size as the canal of Schlemm is approached. Contraction of the ciliary muscle through its insertion into the trabecular meshwork increases pore size in the meshwork and hence the rate of aqueous drainage. Passage of aqueous into Schlemm's canal depends upon cyclic formation of transcellular channels in the endothelial lining. Efferent channels from Schlemm's canal (about 30 collector channels and 12 aqueous veins) conduct the fluid into the venous system. A small amount of aqueous passes between the bundles of the ciliary muscle and through the sclera (uveoscleral flow) (![]() Figure 11-1).
Figure 11-1).
The major resistance to aqueous outflow from the anterior chamber is the endothelial lining of Schlemm's canal and the adjacent portions of the trabecular meshwork-rather than the venous collector system. But the pressure in the episcleral venous network determines the minimum level of intraocular pressure that can be achieved by medical therapy.
Pressure Dynamics
Intraocular pressure is such an important feature of glaucoma that a review of pressure-tension-strain relationships is desirable for elucidation of the possible mechanisms of neuronal damage.
A. Pressure:
Hydrostatic pressure is the force per unit area exerted by a fluid (gas or liquid) within a closed space. With the eye, as with other fluid-filled closed systems, the pressure force is exerted normal to the structural wall (the corneoscleral wall). Average pressure in the eye is about 14 mm Hg. For calculations, centimeters of water is a more convenient unit of pressure than millimeters of mercury. To convert millimeters of mercury to centimeters of water, multiply by 1.36. In more familiar terms, the average eye pressure is about 19 cm (7.5 inches) of water, or 0.25 psi (pounds per square inch). Glaucomatous damage usually begins at roughly double that value, and the eye ruptures at about 240 times average values.
Hydrostatic pressure per se causes no damage to the delicate neurons paralleling the scleral wall. A diver lying on the ocean bottom may be compared to a neuron lying on the uveoscleral bed. The diver will perceive no discomfort at a depth of 43 meters (141 feet) even though the pressure is about 3000 mm Hg, or approximately the pressure within an eye that results in rupture. The diver's body-though subjected to about 10 tons of hydrostatic pressure-will not be pushed against the ocean floor, and a neuron is not pushed against the sclera by hydrostatic pressure.
B. Tension (Tensile Stress):
A jack supporting a car is subjected to compressive stress. A towline pulling a car is subjected to tensile stress, or tension. Stresses are assigned a magnitude of force per unit area. Tensile stress, or the tension force vector, acts parallel to the scleral wall (attempting to pull the sclera apart). In the same way, the pressure of the abdomen at right angles to a belt is almost analogous to intraocular pressure, while the tension along the belt acting to pull the belt apart is analogous to scleral tension.
Trampolines and drumheads are examples of pure tension without pressure. The pressure is the same on either side of the tensed membrane. Tension levels in the sclera, cornea, and lamina cribrosa are not equal. The tension equation for thin-walled spheres can be used to obtain a close approximation of tensions in various parts of the corneoscleral wall. Tension in the sclera is directly proportionate to the intraocular pressure multiplied by the radius of curvature of the sclera and inversely proportionate to twice the thickness of the sclera:

An inflated surgical glove or balloon (Figure 11-2) illustrates this relationship. The palm of the glove has relatively high tension and the thumb relatively low tension, though the pressure within the glove is equal at all locations. The thumb has low tension because the radius of curvature is small and the thickness large relative to the same factors at the palm. In the eye, tension is lower in the cornea or optic cup than in the sclera.
An eye under slowly increasing pressure usually ruptures beneath the lateral rectus where the sclera is thinner, as the tension equation would suggest. A precipitous pressure rise due to trauma (eg, a blow from a club) frequently ruptures the eye at the limbus owing to the anvil effect of the more viscous vitreous.
C. Strain:
Strain is stretch or displacement per unit length. A strain gauge measures displacement. Strain can result in damage and in the body can cause both pain and damage. Using the belt analogy, strain is the stretch per unit length of the belt resulting from the tension in the belt caused by the pressure of the abdomen.
Young's modulus E is used for determining the elastic properties of structures such as cables, pressure vessels, submarines, biologic cells, unicellular organisms, and eyes. E is defined as the tension required to stretch a material of unit cross section to double its original length. This is represented by the following equation:

Thus, the stretch of the sclera per unit length (strain) is derived by dividing the change in tension of the sclera by Young's modulus of the sclera E.
The belt analogy may now be used to illustrate the way in which neurons are damaged in glaucoma. Envision a very obese person wearing a large belt (sclera) with a delicate cloth liner (neurons). After fasting for several days, the obese subject feasts heavily, with the result that there is some ripping of the delicate cloth liner. The progression of the damage process is as follows: (1) The expanding abdomen (intraocular pressure) exerts gentle pressure at right angles to the belt, producing a summation tension parallel to the belt (sclera), tending to pull the belt apart. (2) The tension leads to stretching (strain) of the belt, following the rules of Young's modulus. (3) The stretching (strain) results in damage to the delicate cloth liner (neurons).
NEXT
Page: 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
10.1036/1535-8860.ch11