TABLE 2. Signs of Ocular Ischemic Syndrome and the Frequency of Their Occurrence1
| Sign | Percentage of Cases |
| Anterior Segment | |
| Rubeosis iridis | 67 |
| Neovascular glaucoma | 35 |
| Iritis | 18 |
| Posterior Segment | |
| Narrowed retinal arteries | Most |
| Dilated retinal veins | Most |
| Retinal hemorrhages | 80 |
| Neovascularization of the disc | 35 |
| Neovascularization of the retina | 8 |
| Cherry-red spot | 12 |
| Cotton-wool spots | 6 |
| Spontaneous retinal artery pulsations | 4 |
| Cholesterol emboli | 2 |
| Anterior ischemic optic neuropathy | 2 |
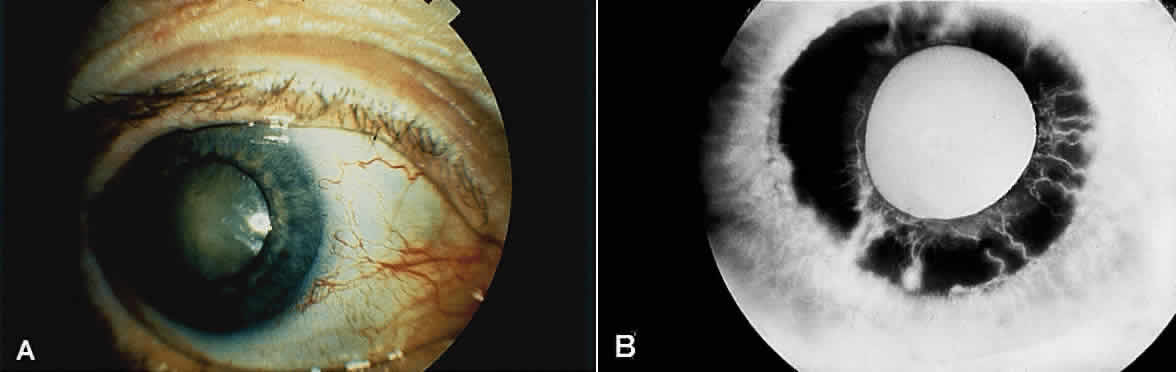
Eyes with ocular ischemic syndrome appear to be at high risk for rubeosis iridis, which is found in 67% of eyes at the time of presentation1 (Figs. 1 and 2). By comparison, only 2.8% of nonischemic CRVOs, 58% of ischemic CRVOs,14 and 15% to 20% of central retinal artery occlusions will develop neovascularization of the iris.15,16 When clinicians are faced with a patient with rubeosis iridis and no obvious etiology (i.e., there are no signs of diabetic retinopathy or retinal vascular occlusion), they should consider the possibility of ocular isch-emic syndrome. The high incidence of rubeosis iridis is probably related to the combined ischemia of both anterior and posterior segments of the globe.
|
The risk of neovascular glaucoma is likewise very high. At the time of presentation, 35% of patients will have neovascularization of the iris and an intraocular pressure greater than 22 mmHg.1 It is interesting to note that in some cases, because of poor ciliary body perfusion and decreased aqueous production, the intraocular pressure remains low, even when fibrovascular tissue completely occludes the iridocorneal angle. Eyes with normal or well-controlled intraocular pressures have been reported to have a dramatic rise in intraocular pressure after carotid endarterectomy, and it is therefore recommended that eyes be followed closely immediately after carotid surgery.
Anterior segment ischemia may cause conjunctival and episcleral vessel injection, corneal edema, and folds in Descemet's membrane (Fig. 1A).2 Aqueous flare is seen in most eyes with iris neovascularization, but a mild anterior chamber reaction is also seen in 18% of eyes with ocular ischemic syndrome, even in the absence of rubeosis iridis.1 In some cases, this anterior chamber inflammation may be the only obvious feature of the disease, and it may be easily mistaken for anterior uveitis.4 Progressive cataractous changes are often seen (Fig. 1A), presumably related to anterior segment ischemia and/or anterior chamber inflammation.
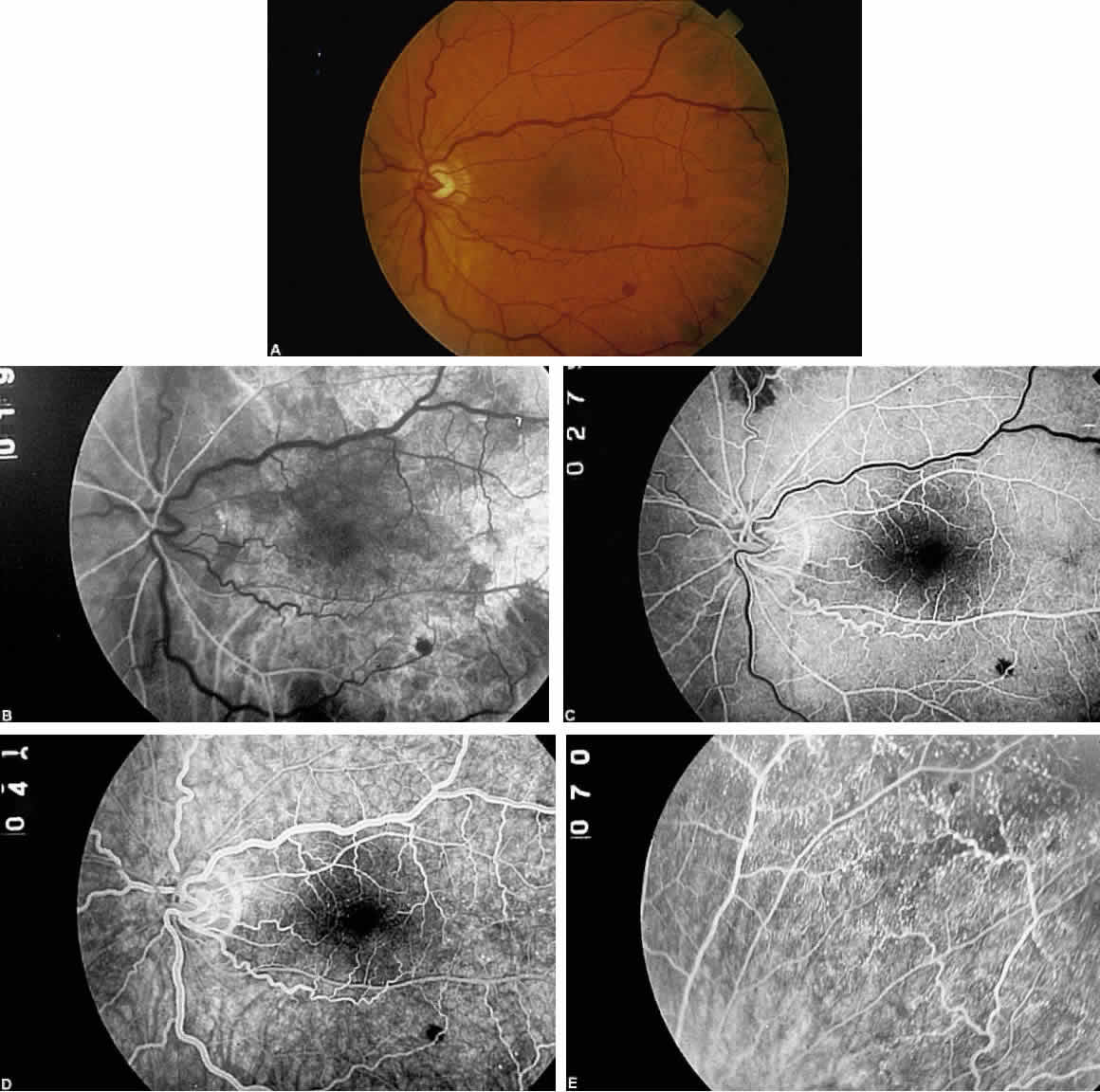
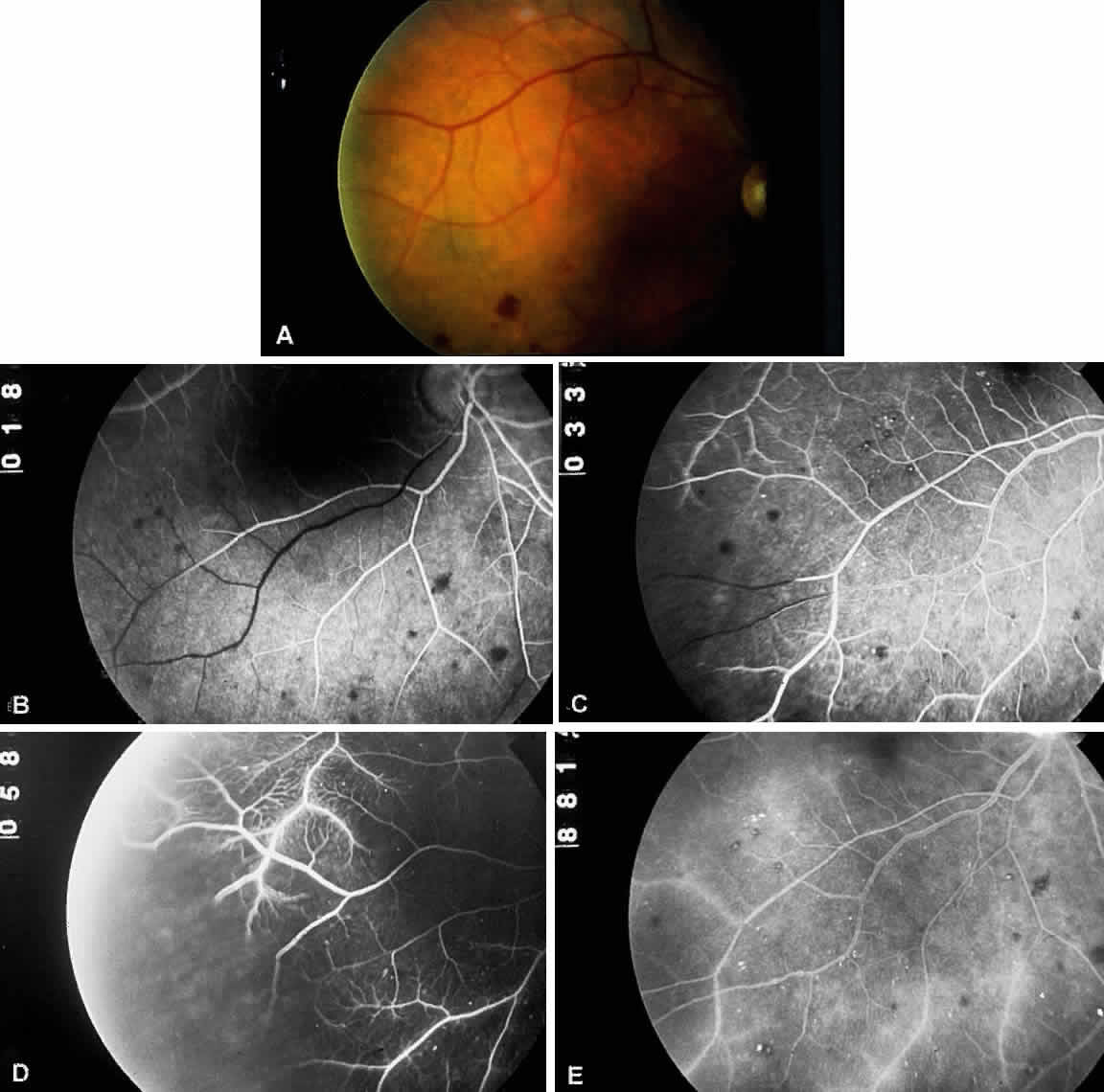
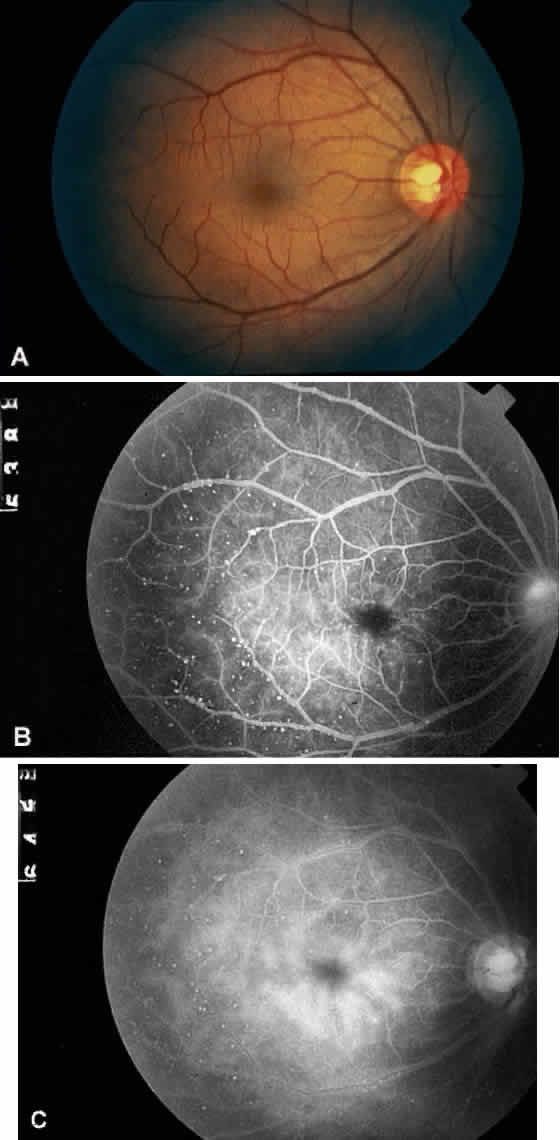
The retinal arteries are generally narrowed and straightened in eyes with ocular ischemic syndrome (Figs. 3A and 4A).3 Although such arterial narrowing can be seen in systemic hypertension or as a normal variation in the elderly, it is important to note that retinal arterial narrowing can also be caused by ocular ischemic syndrome. A significant asymmetry in retinal arterial narrowing between the two eyes may be seen in unilateral cases of ocular ischemic syndrome.
Dilated retinal veins are frequently seen in eyes with ocular ischemic syndrome (see Figs. 3A and 4A). Irregularity of the vessel caliber may be present, similar to the venous beading seen in preproliferative or proliferative diabetic retinopathy. In contrast to CRVO, the veins in eyes with ocular ischemic syndrome are generally not tortuous. The fact that ocular ischemic syndrome occurs secondary to impaired inflow, whereas CRVO is usually associated with compromised outflow, may account for the difference.
Intraretinal dot and blot hemorrhages are seen in approximately 80% of eyes with ocular ischemic syndrome (see Figs. 3A and 4A).1 As with diabetic retinopathy, these hemorrhages probably arise secondary to the rupture of microaneurysms, which, in turn, are caused by endothelial damage. Unlike background diabetic retinopathy, in which the intraretinal hemorrhages are distributed throughout the posterior pole, the hemorrhages of ocular ischemic syndrome tend to be located mainly in the midperipheral fundus. The hemorrhages of CRVO tend to be more numerous and larger than those of ocular ischemic syndrome.
Neovascularization of the retina or optic disc may occur in ocular ischemic syndrome. Optic disc neovascularization is quite common, seen in 35% of patients.1 This incidence is much higher than that seen in ischemic CRVO (5%)14 and central retinal artery occlusion (2%).15 This high incidence of disc involvement may be reflective of the generalized hypoxic status of the eye in ocular ischemic syndrome. In an older patient with no history of diabetes mellitus or vascular occlusive disease, the presence of neovascularization of the optic disc should arouse the clinical suspicion of ocular ischemic syndrome.
Neovascularization of the retina is seen in 8% of patients with ocular ischemic syndrome, and it is usually seen concurrently with neovascularization of the disc.1 Secondary vitreous hemorrhage may occur as a result of traction upon the new vessels by the vitreous gel.
A cherry-red spot is seen at presentation in approximately 12% of eyes with ocular ischemic syndrome.1 It may occur acutely because of ischemia of the inner retinal layer and whitening from emboli to the central retinal artery, or it may occur more chronically. The chronic form occurs when the intraocular pressure encroaches upon the perfusion pressure of the central retinal artery, and blood flow to the inner layer of the retina is compromised. The visual prognosis is generally grim when a cherry-red spot develops.
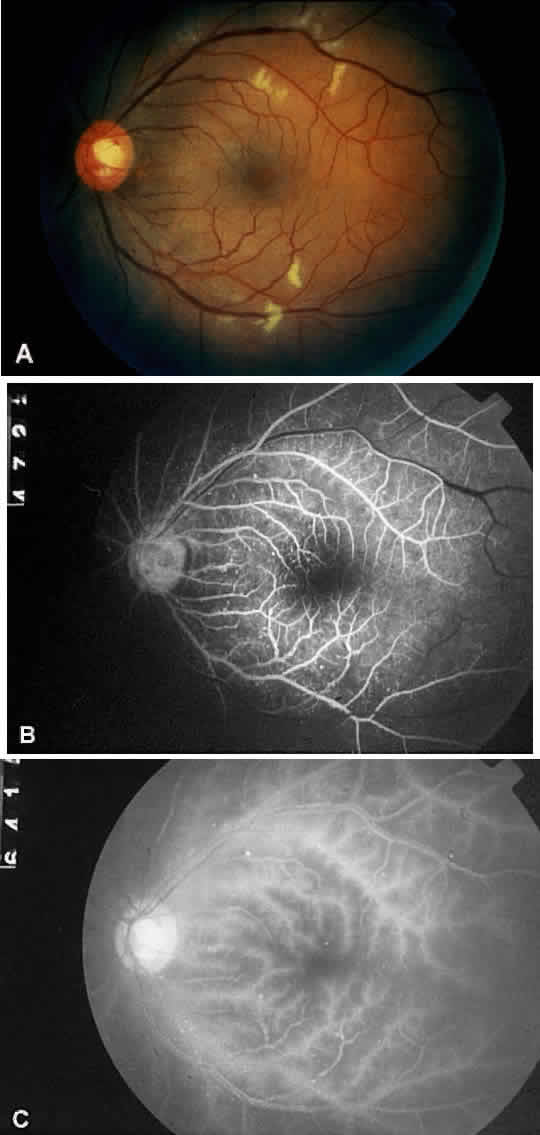
Cotton-wool spots are observed in approximately 5% of eyes with ocular ischemic syndrome (Fig. 5A).1 These spots are generally located in the posterior pole.
Spontaneous pulsations of the central retinal artery are highly suggestive of ocular ischemic syndrome. When the diastolic pressure within the central retinal artery falls below the intraocular pressure, the retinal arteries collapse during diastole, and spontaneous pulsation of the central retinal artery is observed. This phenomenon is present in 5% of eyes with ocular ischemic syndrome.1
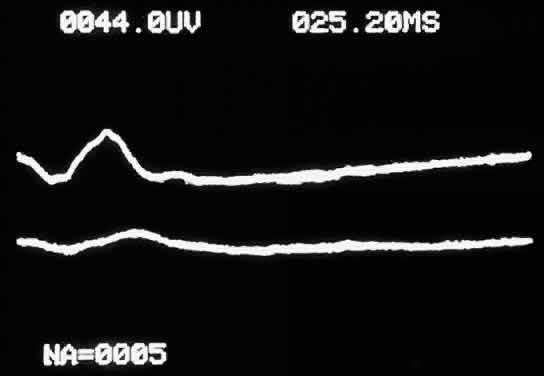
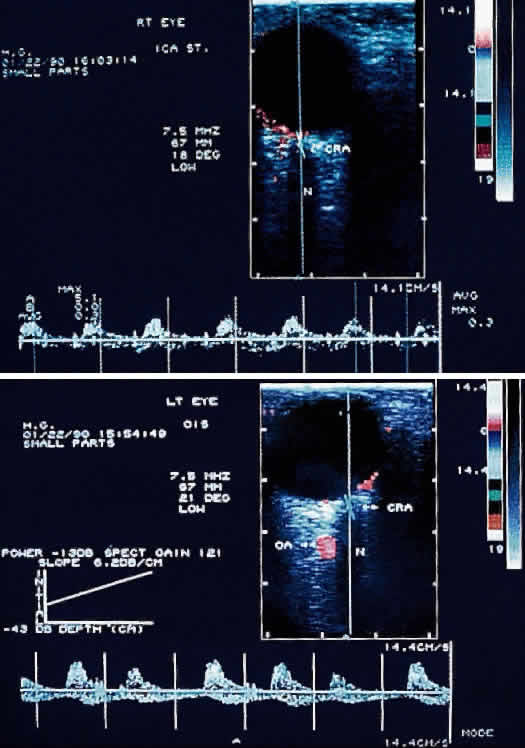
When not present spontaneously, retinal arterial pulsations may be induced by light digital pressure to the globe, thereby artificially raising the intraocular pressure above the diastolic retinal arterial pressure. With slightly more pressure, the retinal arterial tree may even be seen to collapse as the intraocular pressure is raised above the systolic retinal arterial pressure. Correspondingly, as would be expected, ophthalmodynamometry and oculoplethysmography reveal decreased readings in eyes with ocular ischemic syndrome.
Ischemic optic neuropathy has been reported in an eye with ocular ischemic syndrome.17 Hypoperfusion of the posterior ciliary circulation to the optic disc due to impaired carotid blood flow or emboli of carotid origin is the probable cause of this complication.
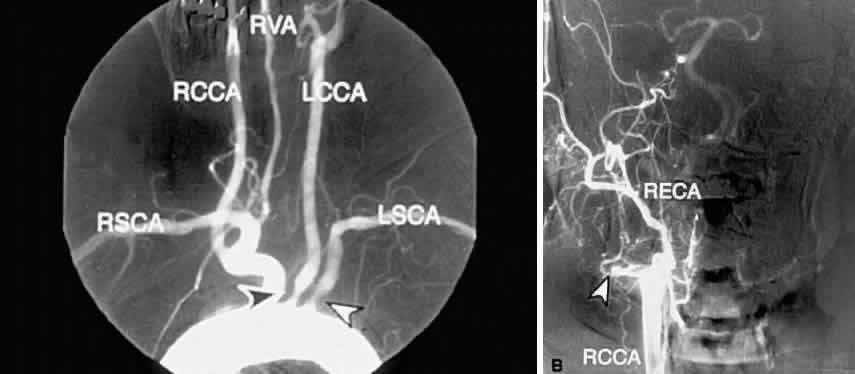
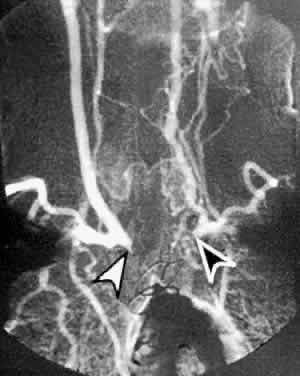
The carotid pulse is often diminished or absent in the presence of carotid occlusive disease. The finding of a cervical bruit is significant, although its absence does not exclude a significant or even a complete carotid occlusion.18,19