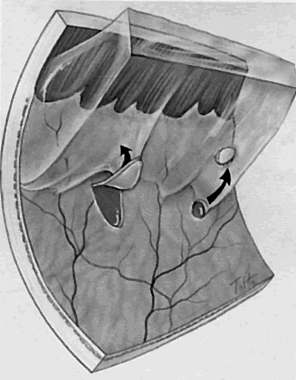
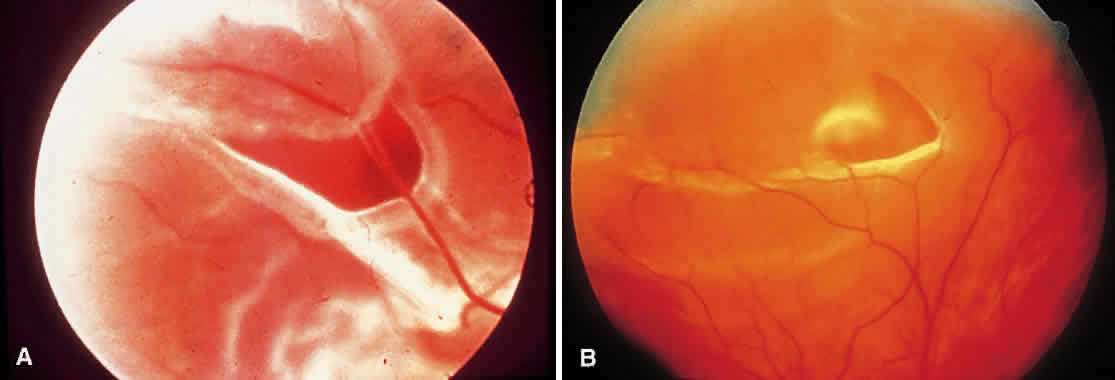
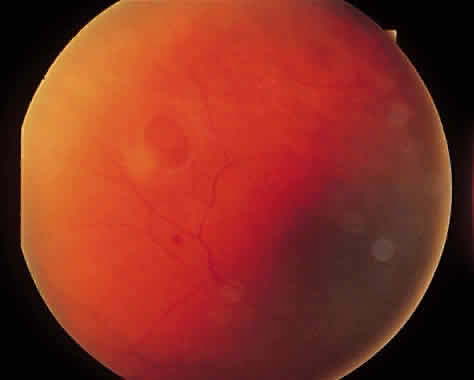
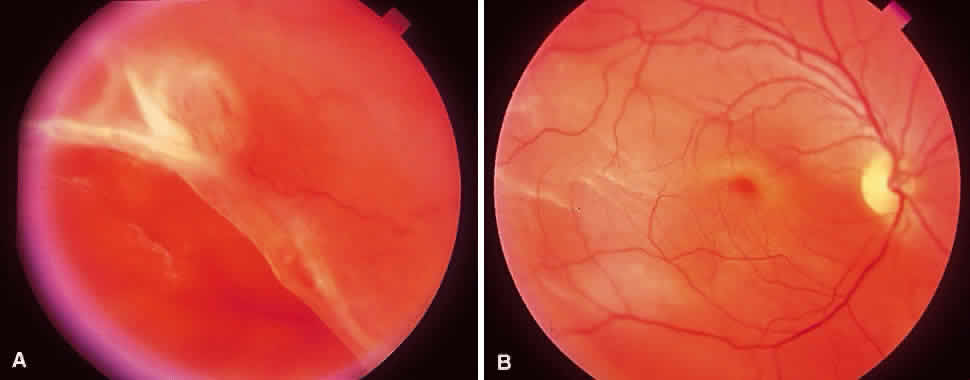
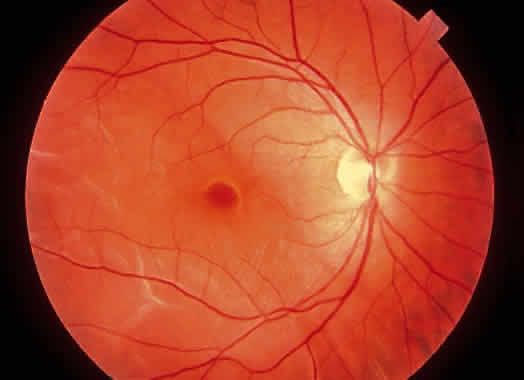
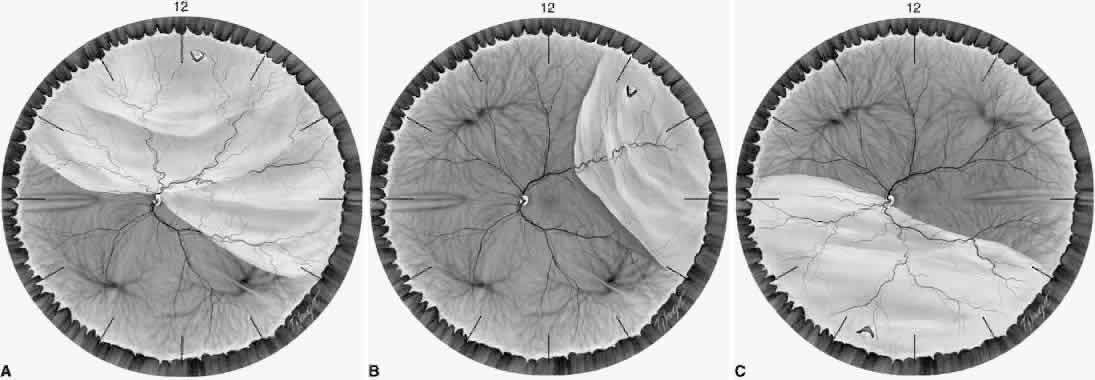
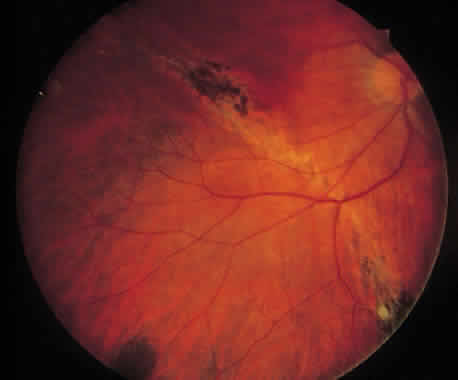
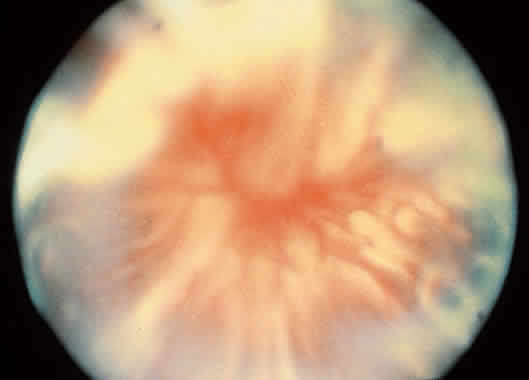
1. Foos RY: Posterior vitreous detachment. Trans Am Acad Ophthalmol Otolaryngol 76:480, 1972 2. Okun E: Gross and microscopic pathology of autopsy eyes. Part III. Retinal breaks
without detachment. Am J Ophthalmol 51:369, 1961 3. Rutnin U, Schepens CL: Fundus appearance in normal eyes. IV. Retinal breaks and other findings. Am J Ophthalmol 64:1063, 1967 4. Byer NE: Clinical study of lattice degeneration of the retina. Trans Am Acad Ophthalmol Otolaryngol 69:1064, 1965 5. Byer NE: Clinical study of retinal breaks. Trans Am Acad Ophthalmol Otolaryngol 71:461, 1967 6. Byer NE: Prognosis of asymptomatic retinal breaks. Arch Ophthalmol 92:208, 1974 7. Machemer R: The importance of fluid absorption, traction, intraocular currents, and
chorioretinal scars in the therapy of rhegmatogenous retinal detachments. Am J Ophthalmol 98:681, 1984 8. Michaelson IC, Stein R: A study in the prevention of retinal detachment. Ann Ophthalmol 1:49, 1965 9. Davis MD: Natural history of retinal breaks without detachment. Arch Ophthalmol 92:183, 1974 10. Delaney WV Jr, Oates RP: Retinal detachment in the second eye. Arch Ophthalmol 96:629, 1978 11. Folk JC, Burton TC: Bilateral phakic retinal detachment. Ophthalmology 89:815, 1982 12. Merin S, Feiler V, Hyams S et al: The fate of the fellow eye in retinal detachment. Am J Ophthalmol 71:477, 1971 13. Foos R, Wheeler N: Vitreoretinal juncture: Synchysis senilis and posterior vitreous detachment. Ophthalmology 89: 1502, 1982 14. Sebag J: Aging of the vitreous. Eye 1:254, 1987 15. Larsson L, Osterlin S: Posterior vitreous detachment—a combined clinical and physiochemical
study. Graefe Arch Clin Exp Ophthalmol 223:92, 1985 16. Wilkinson CP, Rice TA: Retinal Detachment, p 1163. St Louis: Mosby, 1997 17. Foos RY, Roth AM: Surface structure of the optic nerve head. 2. Vitreopapillary attachments
and posterior vitreous detachment. Am J Ophthalmol 76:662, 1973 18. Byer NE: Natural history of posterior vitreous detachment with early management
as the premier line of defense against retinal detachment. Ophthalmology 101:1503, 1994 19. Morse PH, Scheie HG, Aminlari A: Light flashes as a clue to retinal disease. Arch Ophthalmol 91:179, 1974 20. Tasman WS: Posterior vitreous detachment and peripheral retinal breaks. Trans Am Acad Ophthalmol Otolaryngol 72:217, 1968 21. Murakami K, Jalkh AE, Avila MP et al: Vitreous floaters. Ophthalmology 90:1271, 1983 22. Tabotabo MD, Karp LA, Benson WE: Posterior vitreous detachment. Ann Ophthalmol 12:59, 1980 23. Bohringer HR: Statistisches zur Haufigkeit und Risiko der netzhautablosung. Ophthalmologica 131:331, 1956 24. Jaffe N: Complications of acute posterior vitreous detachment. Arch Ophthalmol 79:568, 1968 25. Hikichi T, Trempe CL: Relationship between floaters, light flashes, or both, and complications
of posterior vitreous detachment. Am J Ophthalmol 117:593, 1994 26. Boldrey EE: Risk of retinal tears in patients with vitreous floaters. Am J Ophthalmol 96:783, 1983 27. Spitznas M, Hogan MJ: Outer segments of photoreceptors and the retinal
pigment epithelium: Interrelationship in the human eye. Arch Ophthalmol 1970;84:810 28. Regillo CD, Benson WE: Retinal Detachment: Diagnosis and Management, p 228. Philadelphia: Lippincott-Raven, 1998 29. Marmor MF: Retinal and retinal pigment epithelial physiology. In Regillo
CD, Brown GC, Flynn J (eds): Vitreoretinal Disease: The Essentials, pp 25–38. New
York: Thieme, 1999 30. Hageman G, Marmor M, Yao X et al: The interphotoreceptor matrix mediates
primate retinal adhesion. Arch Ophthalmol 1995;113:655 31. Bill A: Blood circulation and fluid dynamics in the eye. Physiol Rev 55:383, 1975 32. Fatt I, Shantinath K: Flow conductivity of retina and its role in retinal adhesion. Exp Eye Res 12:218, 1971 33. Orr G, Goodnight R, Lean JS: Relative permeability of retina and retinal pigment epithelium to the diffusion
of tritiated water from vitreous to choroid. Arch Ophthalmol 104:1678, 1986 34. Pederson JE, Cantrill HL, Cameron JD: Experimental retinal detachment. II. Role of the vitreous. Arch Ophthalmol 100:1155, 1982 35. Gonin J: Le traitment de decollement retinien. Ann Ocul 158:175, 1921 36. Colyear BHJ, Piscel KK: Clinical tears in the retina without detachment. Am J Ophthalmol 41:773, 1956 37. Lewis H, Kreiger AE: Rhegmatogenous retinal detachment. In Tasman W, Jaeger
EA (eds): Clinical Ophthalmology, p 12. Vol 3. Philadelphia: JB Lippincott, 1991 38. Brown GC, Tasman WS: Familial retinal dialysis. Can J Ophthalmol 15:193, 1980 39. Kinyoun JL, Knobloch WH: Idiopathic retinal dialysis. Retina 4:9, 1984 40. Smiddy WE, Green WR: Retinal dialysis: pathology and pathogenesis. Retina 2:94, 1982 41. Byer NE: The natural history of asymptomatic retinal breaks. Ophthalmology 89:1033, 1982 42. Neumann E, Hyams S: Conservative management of retinal breaks: A follow-up study of subsequent
retinal detachment. Br J Ophthalmol 56:482, 1972 43. Gass J: Reappraisal of biomicroscopic classification of stages of development of
a macular hole. Am J Ophthalmol 119:752, 1995 44. Johnson RN, Gass JD: Idiopathic macular holes: Observations, stages of formation, and implications
for surgical intervention. Ophthalmology 95:917, 1988 45. Zhang CF, Hu C: High incidence of retinal detachment secondary to macular hole in a Chinese
population [letter]. Am J Ophthalmol 94:817, 1982 46. Morita H, Ideta H, Ito K et al: Causative factors of retinal detachment in macular holes. Retina 11:281, 1991 47. Byer NE: Lattice degeneration of the retina. Surv Ophthalmol 23:213, 1979 48. Halpern JI: Routine screening of the retinal periphery. Am J Ophthalmol 62:99, 1966 49. Straatsma BR, Zeegen PD, Foos RY et al: Lattice degeneration of the retina. Am J Ophthalmol 77:619, 1974 50. Ashrafzadeh MT, Schepens CL, Elzeneiny IH et al: Aphakic and phakic retinal detachment. Arch Ophthalmol 89: 476, 1973 51. Benson WE, Morse PH: The prognosis of retinal detachment due to lattice degeneration. Ann Ophthalmol 10: 1197, 1978 52. Tillery WV, Lucier AC: Round atrophic holes in lattice degeneration: an important cause of phakic
retinal detachment. Trans Am Acad Ophthalmol Otolaryngol 81:509, 1976 53. Byer NE: Long-term natural history of lattice degeneration of the retina. Ophthalmology 96:1396, 1989 54. Akiba J: Prevalence of posterior vitreous detachment in high myopia. Ophthalmology 100:1384, 1993 55. Wilkes SR, Beard CM, Kurland LT et al: The incidence of retinal detachment in Rochester, Minnesota, 1970-1978. Am J Ophthalmol 94:670, 1982 56. Javitt JC, Vitale S, Canner JK et al: National outcomes of cataract extraction. I. Retinal detachment after inpatient
surgery. Ophthalmology 98:895, 1991 57. Nielsen NE, Naeser K: Epidemiology of retinal detachment following extracapsular cataract extraction: A
follow-up study with an analysis of risk factors. J Cataract Refractive Surg 19:675, 1993 58. Ninn-Pedersen K, Bauer B: Cataract patients in a defined Swedish population, 1986-1990. V. Postoperative
retinal detachments. Arch Ophthalmol 114:382, 1996 59. Vail D: After-results of vitreous loss. Am J Ophthalmol 59:573, 1965 60. Fastenberg DM, Schwartz PL, Lin HZ: Retinal detachment following neodymium-YAG laser capsulotomy. Am J Ophthalmol 97:288, 1984 61. Ficker LA, Vickers S, Capon MR et al: Retinal detachment following Nd:YAG posterior capsulotomy. Eye 1:86, 1987 62. Javitt JC, Tielsch JM, Canner JK et al: National outcomes of cataract extraction. Increased risk of retinal complications
associated with Nd:YAG laser capsulotomy. The Cataract Patient
Outcomes Research Team. Ophthalmology 99: 1487, 1992 63. Ackerman AL, Seelenfreund MH, Freeman HM, Schepens CL: Cataract extraction following retinal detachment surgery. Arch Ophthalmol 84:41, 1970 64. Benson WE, Grand MG, Okun E: Aphakic retinal detachment. Management of the fellow eye. Arch Ophthalmol 93:245, 1975 65. Everett WG, Katzin D: Meridional distribution of retinal breaks in aphakic retinal detachment. Am J Ophthalmol 66:928, 1968 66. Phelps CD, Burton TC: Glaucoma and retinal detachment. Arch Ophthalmol 95:418, 1977 67. Wilkinson CP: Phakic retinal detachments in the elderly. Retina 15:220, 1995 68. Yoshida A, Ogasawara H, Jalkh AE et al: Retinal detachment after cataract surgery. Predisposing factors. Ophthalmology 99:453, 1992 69. Griffith RD, Ryan EA, Hilton GF: Primary retinal detachments without apparent breaks. Am J Ophthalmol 81: 420, 1976 70. Campbell CJ, Rittler MC: Cataract extraction in the retinal detachment prone patient. Am J Ophthalmol 73:17, 1972 71. Jagger JD, Cooling RJ, Fison LG et al: Management of retinal detachment following congenital cataract surgery. Trans Ophthalmol Soc UK 103:103, 1983 72. Cox MS, Schepens CL, Freeman HM: Retinal detachment due to ocular contusion. Arch Ophthalmol 76:678, 1966 73. Cox M, Freeman H: Traumatic retinal detachment. In Freeman H (ed): Ocular
Trauma, pp 285–293. New York: Appleton-Century-Crofts, 1979 74. Winslow RL, Tasman W: Juvenile rhegmatogenous retinal detachment. Ophthalmology 85:607, 1978 75. Tasman W: Peripheral retinal changes following blunt trauma. Trans Am Ophthalmol Soc 70:190, 1972 76. Cox MS, Freeman HM: Retinal detachment due to ocular penetration. Arch
Ophthalmol 1976;96:1354 77. Yoon YH, Marmor MF: Rapid enhancement of retinal adhesion by laser photocoagulation. Ophthalmology 95: 1385, 1988 78. Hagler WS, North AW: Retinal dialyses and retinal detachment. Arch Ophthalmol 79:376, 1968 79. Sellors PJ, Mooney D: Fundus changes after traumatic hyphaema. Br J Ophthalmol 57:600, 1973 80. De Juan E Jr, Sternberg P Jr, Michels RG: Penetrating ocular injuries: Types of injuries and visual results. Ophthalmology 90:1318, 1983 81. Meredith TA, Gordon PA: Pars plana vitrectomy for severe penetrating injury with posterior segment
involvement. Am J Ophthalmol 103:549, 1987 82. Percival SPB: Late complications from posterior segment intraocular foreign bodies. Br J Ophthalmol 56:462, 1972 83. Regillo CD, Vander JF, Duker JS et al: Repair of retinitis-related retinal detachments with silicone oil in patients
with acquired immunodeficiency syndrome. Am J Ophthalmol 113:21, 1992 84. Doden W, Stark N: Retina and vitreous findings after serious indirect eye trauma. Klin Monatsbl Augenheilkd 614:32, 1974 85. Regillo CD, Tasman WS, Brown GC: Surgical management of complications associated with X-linked retinoschisis. Arch Ophthalmol 111:1080, 1993 86. Wang K, Hilton GF: Retinal detachment associated with coloboma of the choroid. Trans Am Ophthalmol Soc 83: 49, 1985 87. Machemer R, Norton EW: Experimental retinal detachment in the owl monkey. I. Methods of production
and clinical picture. Am J Ophthalmol 66:388, 1968 88. Machemer R: Experimental retinal detachment in the owl monkey. II. Histology of retina
and pigment epithelium. Am J Ophthalmol 66:396, 1968 89. Cook B, Lewis GP, Fisher SK, Adler R: Apoptotic photoreceptor degeneration in experimental retinal detachment. Invest Ophthalmol Vis Sci 36:990, 1995 90. Nork TM, Millecchia LL, Strickland BD et al: Selective loss of blue cones and rods in human retinal detachment. Arch Ophthalmol 113:1066, 1995 91. Machemer R: Experimental retinal detachment in the owl monkey. IV. The reattached retina. Am J Ophthalmol 66:1075, 1968 92. Kroll AJ, Machemer R: Experimental retinal detachment in the owl monkey. 3. Electron microscopy
of retina and pigment epithelium. Am J Ophthalmol 66:410, 1968 93. Lincoff H, Gieser R: Finding the retinal hole. Arch Ophthalmol 85:565, 1971 94. Benson WE, Nantawan P, Morse PH: Characteristics and prognosis of retinal detachments with demarcation lines. Am J Ophthalmol 84:641, 1977 95. Felder KS, Brockhurst RJ: Retinal neovascularization complicating rhegmatogenous retinal detachment
of long duration. Am J Ophthalmol 93:773, 1982 96. Netland PA, Mukai S, Covington HI: Elevated intraocular pressure secondary to rhegmatogenous retinal detachment. Surv Ophthalmol 39:234, 1994 97. Schwartz A: Chronic open-angle glaucoma secondary to rhegmatogenous retinal detachment. Trans Am Ophthalmol Soc 70:178, 1972 98. Havener WH: Massive vitreous retraction. Ophthalmic Surg 4:22, 1973 99. Morse PH: Fixed retinal star folds in retinal detachment. Am J Ophthalmol 77:760, 1974 100. Machemer R, Aaberg TM, Freeman HM et al: An updated classification of retinal detachment with proliferative vitreoretinopathy. Am J Ophthalmol 112:159, 1991 101. Campochiaro PA: Pathogenic mechanisms in proliferative vitreoretinopathy. Arch Ophthalmol 115:237, 1997 102. Ryan SJ: Traction retinal detachment. Am J Ophthalmol 115:1, 1993 103. Clarkson JG, Green WR, Massof D: A histopathologic review of 168 cases of preretinal membrane. Am J Ophthalmol 84:1, 1977 104. Machemer R, Laqua H: Pigment epithelium proliferation in retinal detachment (massive periretinal
proliferation). Am J Ophthalmol 80:1, 1975 105. Machemer R, van Horn D, Aaberg TM: Pigment epithelial proliferation in human retinal detachment with massive
periretinal proliferation. Am J Ophthalmol 85:181, 1978 106. Singh AK, Glaser BM, Lemor M, Michels RG: Gravity-dependent distribution of retinal pigment epithelial cells dispersed
into the vitreous cavity. Retina 6:77, 1986 107. Byer NE: Clinical study of senile retinoschisis. Arch Ophthalmol 79:36, 1968 108. Straatsma BR, Foss RY: Typical and reticular degenerative retinoschisis. Am J Ophthalmol 75:551, 1973 109. Chignell AH, Shilling J: Prophylaxis of retinal detachment. Br J Ophthalmol 57:291, 1973 110. Morse PH, Scheie HG: Prophylactic cryoretinopexy of retinal breaks. Arch Ophthalmol 92:204, 1974 111. Nadel AJ, Gieser RG: The treatment of acute horseshoe retinal tears by transconjunctival cryopexy. Ann Ophthalmol 7:1568, 1975 112. Robertson DM, Norton EW: Long-term follow-up of treated retinal breaks. Am J Ophthalmol 75:395, 1973 113. Pollak A, Oliver M: Argon laser photocoagulation of symptomatic flap tears and retinal breaks
of fellow eyes. Br J Ophthalmol 65:469, 1981 114. Hyams SW, Meir E, Ivry M et al: Chorioretinal lesions predisposing to retinal detachment. Am J Ophthalmol 78: 429, 1974 115. Byer NE: Changes in and prognosis of lattice degeneration of the retina. Trans
Am Acad Ophthalmol Otolaryngol 78:OP114, 1974 116. Hyams SW, Neumann E, Friedman Z: Myopia-aphakia. II. Vitreous and peripheral retina. Br J Ophthalmol 59:483, 1975 117. Folk JC, Arrindell EL, Klugman MR: The fellow eye of patients with phakic lattice retinal detachment. Ophthalmology 96:72, 1989 118. McCuen BWd, Azen SP, Stern W et al: Vitrectomy with silicone oil or perfluoropropane gas in eyes with severe
proliferative vitreoretinopathy. Silicone Study Report 3. Retina 13:279, 1993 119. Anderson DH, Guerin CJ, Erickson PA et al: Morphological recovery in the reattached retina. Invest Ophthalmol Vis Sci 27:168, 1986 120. Hartz AJ, Burton TC, Gottlieb MS et al: Outcome and cost analysis of scheduled versus emergency scleral buckling
surgery. Ophthalmology 99:1358, 1992 121. Hilton GF, Grizzard WS: Pneumatic retinopexy. A two-step outpatient operation without conjunctival
incision. Ophthalmology 93:626, 1986 122. Hilton GF, Kelly NE, Salzano TC et al: Pneumatic retinopexy: A collaborative report of the first 100 cases. Ophthalmology 94:307, 1987 123. Tornambe PE, Hilton GF, Brinton DA et al: Pneumatic retinopexy: A two-year follow-up study of the multicenter clinical
trial comparing pneumatic retinopexy with scleral buckling. Ophthalmology 98:1115, 1991 124. Tornambe PE, Hilton GF: Pneumatic retinopexy. A multicenter randomized controlled clinical trial
comparing pneumatic retinopexy with scleral buckling. The Retinal Detachment
Study Group. Ophthalmology 96:772, 1989 125. Hilton GF, Tornambe PE: Pneumatic retinopexy. An analysis of intraoperative and postoperative complications. The
Retinal Detachment Study Group. Retina 11:285, 1991 126. Benson WE, Chan P, Sharma S et al: Current popularity of pneumatic retinopexy. Retina 19:238, 1999 127. Lincoff HA, Kreissig I, Hahn YS: A temporary balloon buckle for the treatment of small retinal detachments. Ophthalmology 86:586, 1979 128. Kreissig I, Failer J, Lincoff H, Ferrari F: Results of a temporary balloon buckle in the treatment of 500 retinal detachments
and a comparison with pneumatic retinopexy. Am J Ophthalmol 107:381, 1989 129. Schoch LH, Olk RJ, Arribas NP et al: The Lincoff temporary balloon buckle. Am J Ophthalmol 101:646, 1986 130. Escoffery RF, Olk RJ, Grand MG, Boniuk I: Vitrectomy without scleral buckling for primary rhegmatogenous retinal
detachment. Am J Ophthalmol 99:275, 1985 131. Campo RV, Sipperley JO, Sneed SR et al: Pars plana vitrectomy without scleral buckle for pseudophakic retinal detachments. Ophthalmology 106:1811, 1999 132. Vrabec TR: Laser photocoagulation repair of macula-sparing cytomegalovirus-related
retinal detachment. Ophthalmology 104:2062, 1997 133. Davis JL, Hummer J, Feuer WJ: Laser photocoagulation for retinal detachments and retinal tears in cytomegalovirus
retinitis. Ophthalmology 104:2053, 1997 |