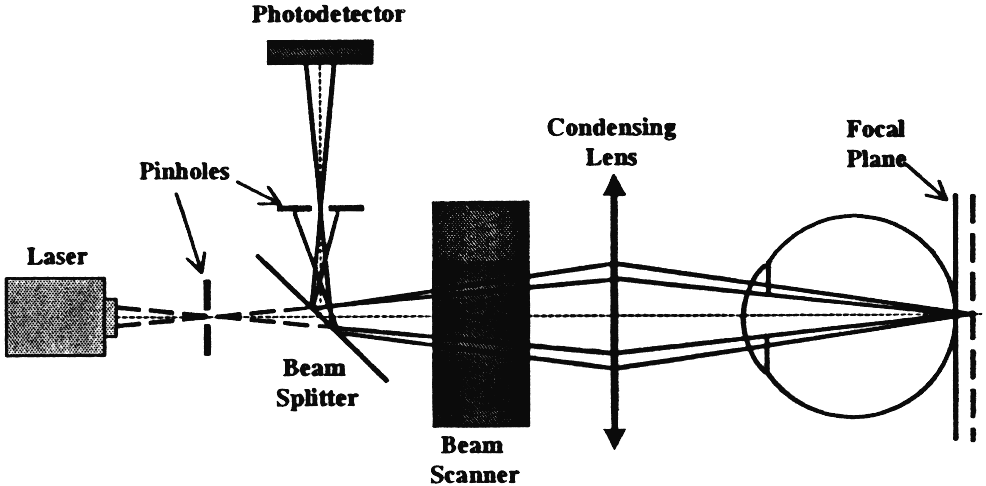
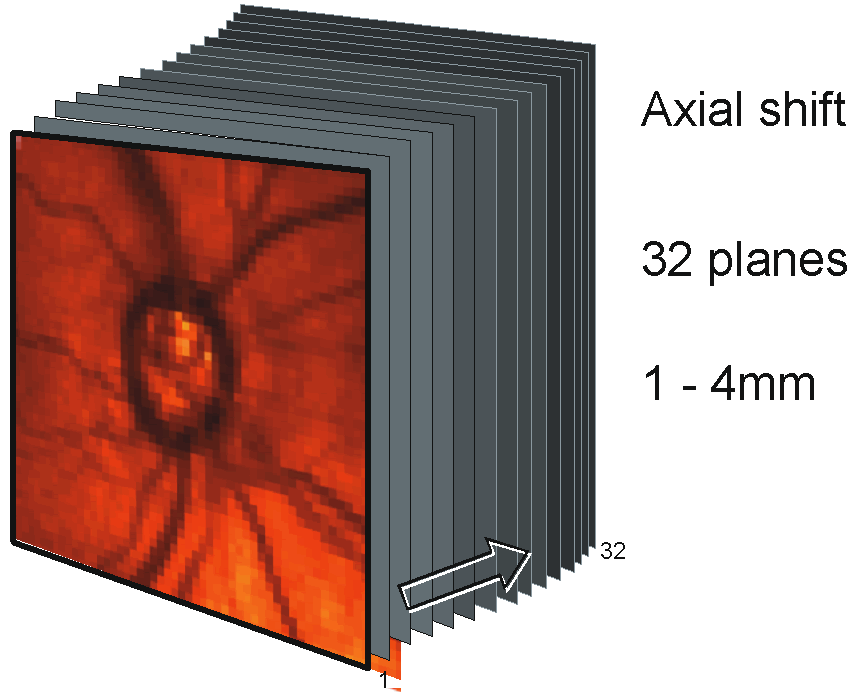
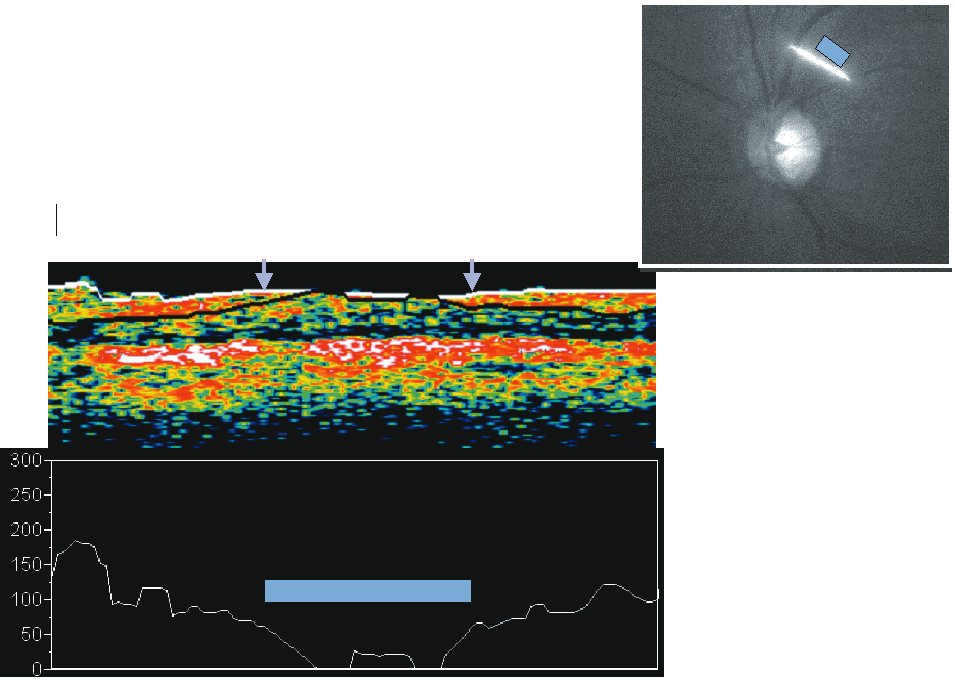
A series of two-dimensional confocal optical section images can be recorded sequentially by changing the location of the focal plane along the optical axis, progressing from anterior to the ONH, through the ONH and ending posterior to the lamina cribrosa. A tomographic series typically consists of 32 sequentially scanned optical section images, allowing the object to be resolved spatially in three dimensions. A mean topography image is typically produced from averaging three sets of three-dimensional images. With a current commercially available version, the Heidelberg Retina Tomograph (HRT) II (Heidelberg Engineering, Heidelberg, Germany), the field of view is fixed at 15 × 15 degrees, and digitization is performed in frames of 384 × 384 pixels with a spatial resolution of 10 μm per pixel. Image acquisition takes approximately 1 second. The scan depth, set by the operator, may range from 1 to 4 mm. The software stacks the series of 32 images so that corresponding points on the retina are aligned horizontally and vertically to compensate for eye movement during image acquisition (Fig. 2).
|
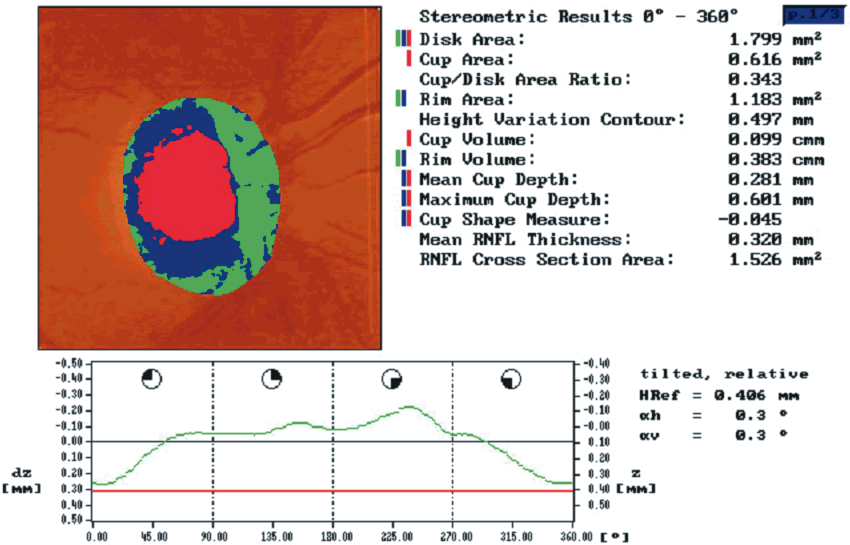
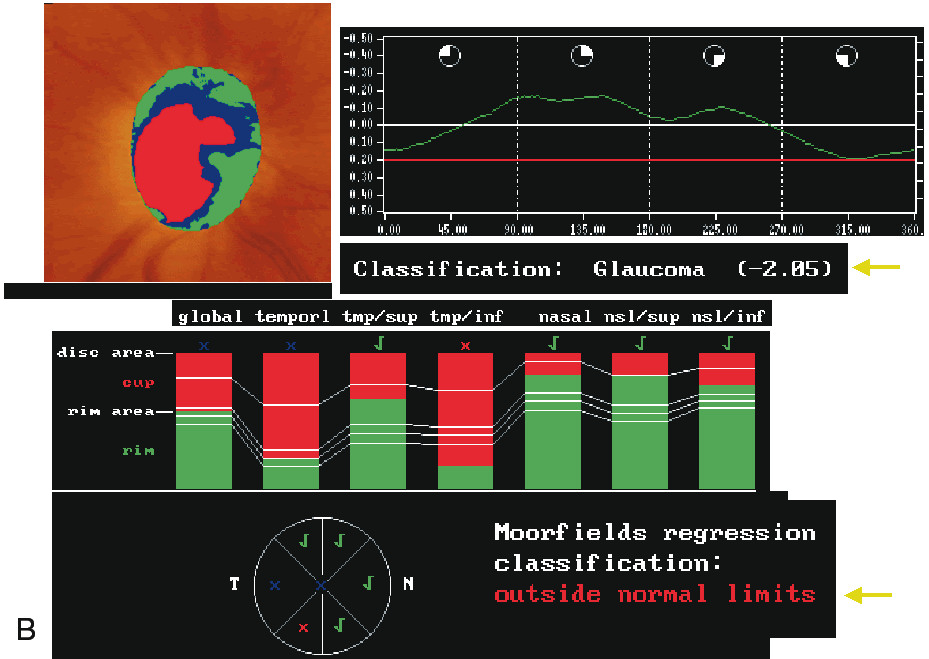
To evaluate the topographic information a contour line defining the disc margin is drawn by the examiner around the inner margin of the peripapillary scleral ring. The HRT software then establishes a reference plane 50 μm posterior to the retinal surface at the papillomacular bundle. The reference plane is established at this location because the fibers in the papillomacular bundle are thought to be least affected as glaucoma progresses, thereby providing a stable reference plane. As displayed in Figure 3, all structures below the reference plane are considered to belong to the optic cup (red) and all structures above the reference plane and within the contour line are considered to belong to neural rim (blue).
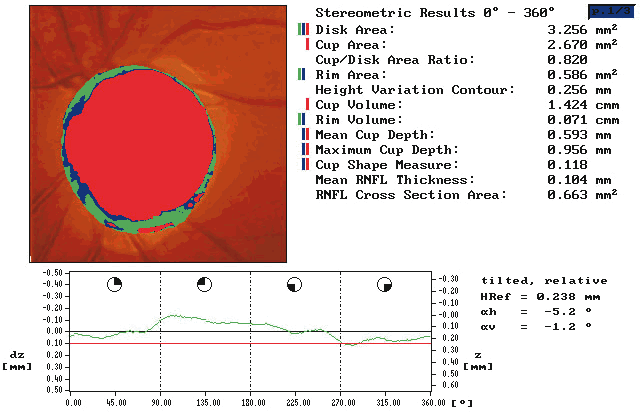
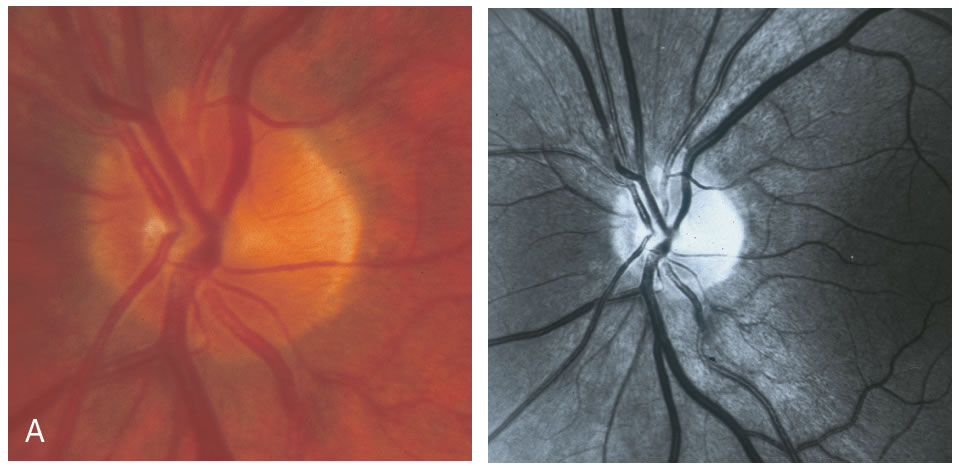
Stereometric parameters of optic nerve head topography are generated relative to the reference plane and include rim area, rim volume, cup area, cup volume, cup-to-disc ratio, mean RNFL thickness, and RNFL cross-sectional area. Parameters independent of the reference plane include mean and maximum cup depth, height variation contour, and cup shape measure (the statistical third moment of the distribution of all cup depth measurements). A characteristic sign in normal discs is the configuration of the contour line height display that demonstrates a double hump pattern corresponding to the thicker distribution of nerve fibers along the superior and inferior poles of the ONH (Fig. 4). Glaucomatous structural damage is characterized by a reduction of parameters that describe rim structures (area, volume) and indicate tissue loss (cup shape measure, cup volume, cup-to-disc ratio, cup steepness). As shown in Figure 5, glaucomatous alterations are typically associated with an asymmetrical or diffuse flattening of the contour line, or localized depressions corresponding to disc notches.
REPRODUCIBILITY
The reproducibility of a measurement is critical to determine the validity of a single measurement or to detect change in repeated measurements over time. With regard to optic nerve imaging, reproducibility is defined as the ability to obtain the same values in the absence of structural change. The magnitude of change that is detectable by the instrument is related to its reproducibility; the better the reproducibility the smaller the change that can be detected. Test-retest variability must, therefore, be small to detect small but real changes of the ONH to determine interval progression of glaucomatous damage. Various investigators have reported high levels of reproducibility with CSLO.19–24 The reproducibility of height measurements has been shown to be between 10 and 20 μm and the coefficients of variation of the stereometric parameters been reported to be approximately 5%. Mean standard deviation of test-retest variability was found to be significantly greater for glaucoma patients versus normal controls.25 Test-retest variability was also influenced by age, and was greater in older patients. Because glaucomatous discs tend to have a greater degree of sloping and excavation of the neural rim, and because the computer algorithm used to align the images has a higher error in areas of steep topographic slope, it is not surprising that glaucoma patients were found to have a higher degree of test-retest variability. The age effect was largely attributed to media opacities. No significant differences were found between short-term and long-term variability estimates, indicating that measurements are not influenced by the time interval between measurements.26
ACCURACY
Histologic validation of measurements obtained with CSLO has been reported. Good correlation was found between in vivo optic disc topographic measures and histomorphometric measurements of optic nerve fiber number in primate eyes with laser-induced glaucoma.27 Plastic models have also been developed which have shown high levels of accuracy with CSLO.28 However, extrapolation of these results to a living human eye is difficult because of the different and complex optical properties of living human tissue.
Clinical correlation with CSLO measurements has concentrated on associations between topographic measures with visual function. A significant association between RNFL cross-sectional area and regional and hemifield measures of visual field loss has been reported.29 Peripapillary height,30 rim area and cup shape measure31,32 have also been demonstrated to be highly correlated with global indices of visual function. Cup shape measure summarizes the structure of the cup and takes into account its depth variation and steepness of the cup walls, independent of both disc size and a retinal reference plane. This parameter is a good indicator of the severity of disc damage and appears to have parameter the strongest relationship with visual field loss.
SENSITIVITY AND SPECIFICITY
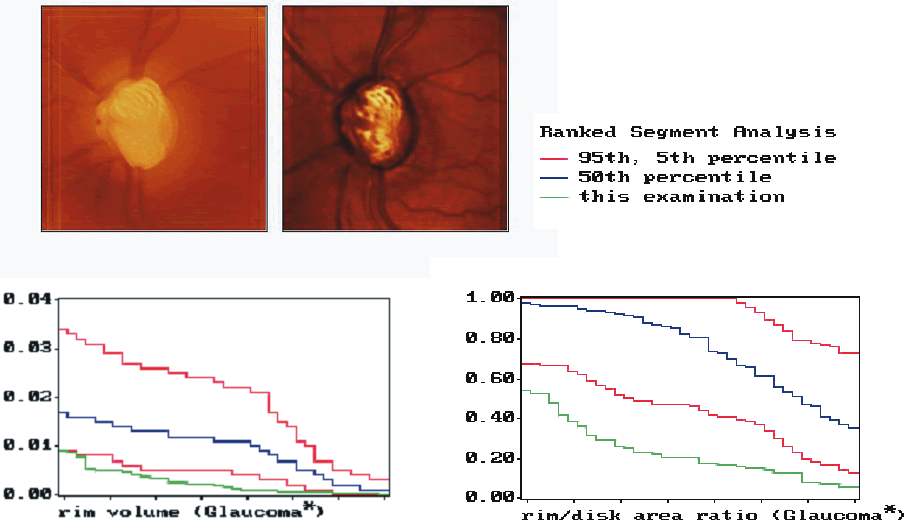
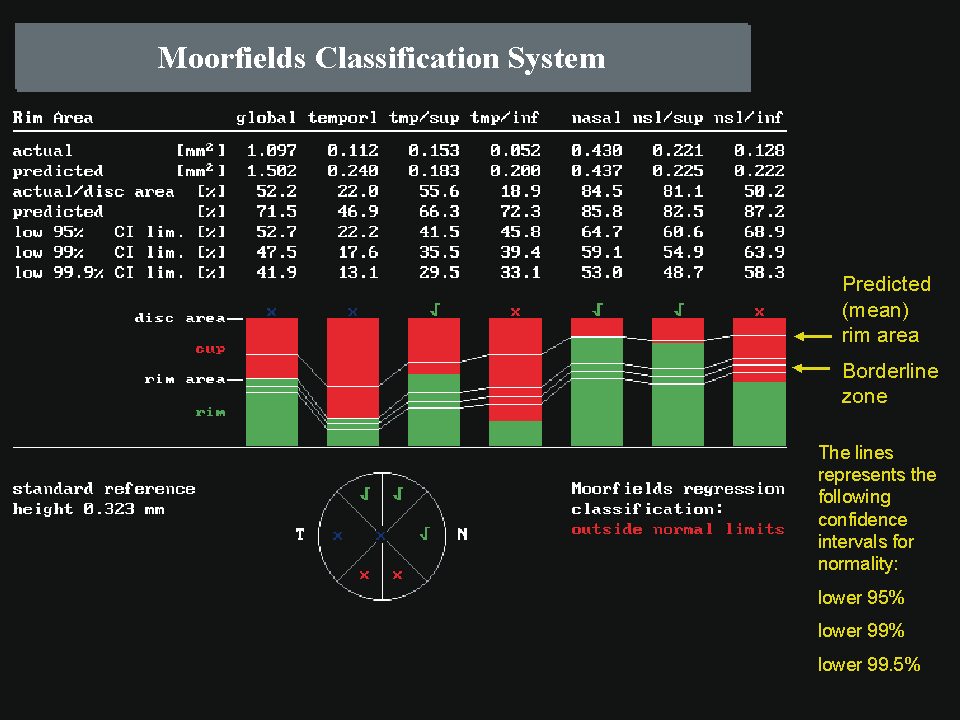
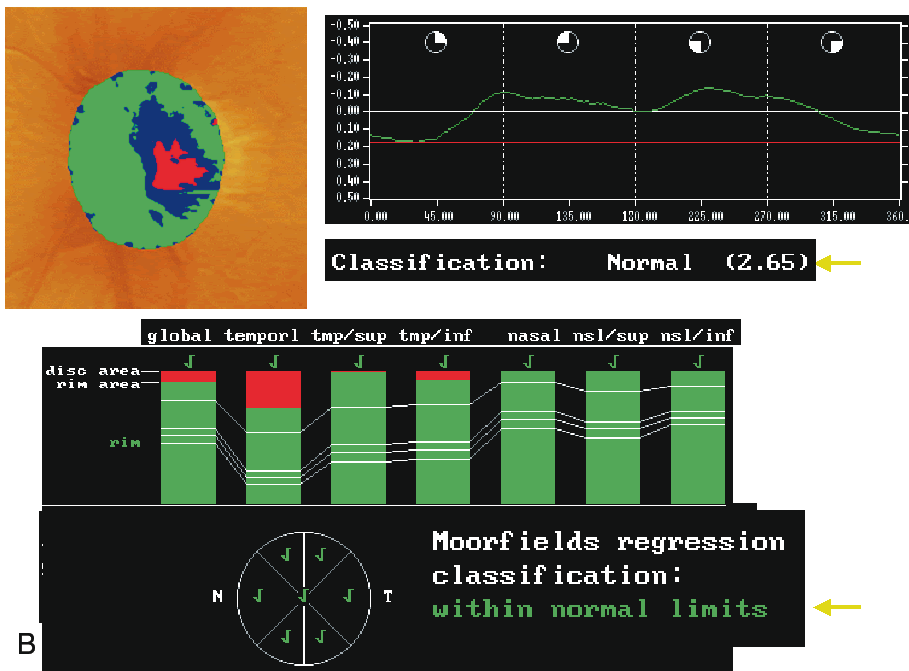
Because of the large interindividual variability of normal optic nerve head topography, there is no technique, qualitative or quantitative, that will provide perfect discrimination between normal and glaucomatous eyes. It is therefore not surprising that there is considerable overlap in individual optic disc parameter measurements between healthy eyes and eyes with early to moderate glaucoma. Currently, the HRT uses software with various statistical analyses to improve the discriminating ability between normal and glaucomatous eyes. These techniques include multivariate discriminant analysis, ranked segment distribution curves (Fig. 6), and regression analysis using a normative database (Fig. 7).
Multivariate analysis studies of the HRT parameters take into account combinations of parameters. It has been found by several investigators that cup shape measure, rim volume, and retinal height variation contour are the most important parameters to differentiate between normal and glaucomatous optic discs.33-35 A linear combination of these parameters has been used to compute the Mikelberg discriminant function for classification of optic discs as normal or glaucomatous. With ranked segment distribution (RSD) curves, the optic nerve head is divided into 36 segments of 10 degrees, the stereometric parameters of which are then plotted in descending order. Each individual ranked segment analysis can then be compared to the normal range. An eye with glaucomatous damage is shown in Figure 6. The RSD curves are far outside the fifth percentile for normal eyes. This eye can therefore been classified as being glaucomatous with a probability of more than 95%.
Regression analysis was used to generate the Moorfield's regression classification score, which is based on the relationship between the neuroretinal rim area and the optic disc area. This classification system, as depicted in Figure 7, determines whether a disc is within or outside the normal reference limits derived from a normative database. Approximately 67% of eyes with early glaucoma are classified as outside normal limits and a further 20% are borderline.36 Abnormalities other than glaucoma, such as tilted discs, may cause measurements to fall outside the normal range. The sensitivity and specificity of the HRT to discriminate between healthy and glaucomatous eyes has been investigated and varies widely, ranging from 62% to 84% and 80% to 96%, respectively (Table 1).33,35–38 This wide variability in discriminating ability may be explained in part by different sample sizes, definitions of glaucoma, and varying degrees of glaucomatous optic nerve damage. It should be emphasized that the characteristics of the study population markedly influence the discriminating ability of any technique involved in differentiating glaucomatous from healthy eyes. For any technique, an instrument will appear to be more sensitive if it is used to separate healthy eyes from those with advanced glaucoma compared to those with only mild glaucoma.
Table 1. Confocal Scanning Laser Ophthalmoscopy: Previously Published Results
of Sensitivity and Specificity
| Sensitivity | Specificity | MD (dB) | |
| Mikelberg et al. (1995) | 87% | 84% | -5.5 |
| Iester et al. (1997) | 74% | 88% | -8.3 |
| Bathija et al. (1998) | 62% | 94% | <-10 |
| Caprioli et al. (1998) | 85% | 80% | -4.8 |
| Wollstein et al. (2000) | 84% | 96% | -23.6 |
ABILITY TO DETECT CHANGE OVER TIME
Quantitative methods that facilitate longitudinal analysis of topographic information are under active development. Longitudinal studies are underway to identify the best summary measurements to detect change and to determine whether CSLO can improve our ability to detect progression of glaucoma. Because progression of glaucoma is slow, years of follow-up are needed to answer this question. Follow-up studies in monkeys39 and in patients after surgery40,41 or medical reduction of intraocular pressure42 can be used as surrogates to more rapidly determine which analysis strategies are best to identify clinically relevant progression of glaucoma. Currently, there are several automated analysis strategies on the HRT to detect change in optic disc topography over time. These include topographic difference images, probablility map analysis and statistical changes in stereometric parameters.
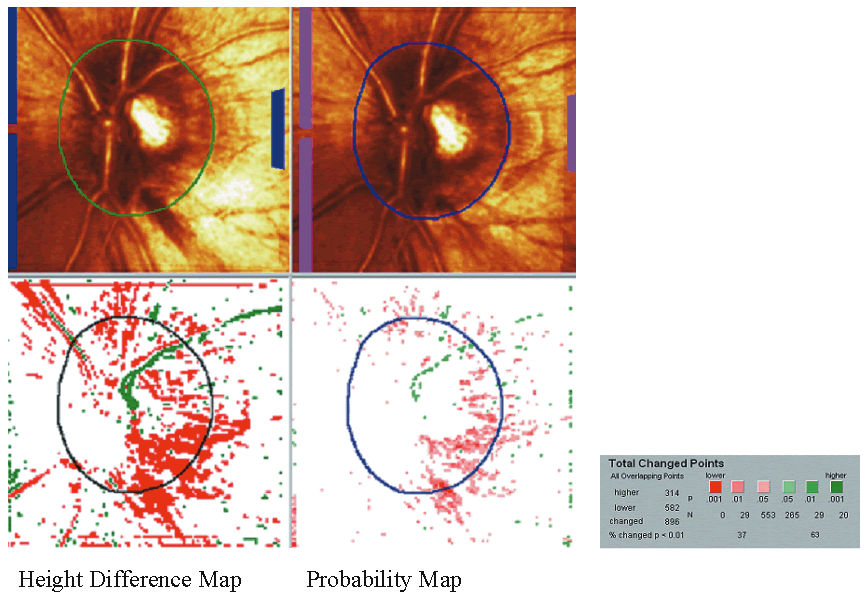
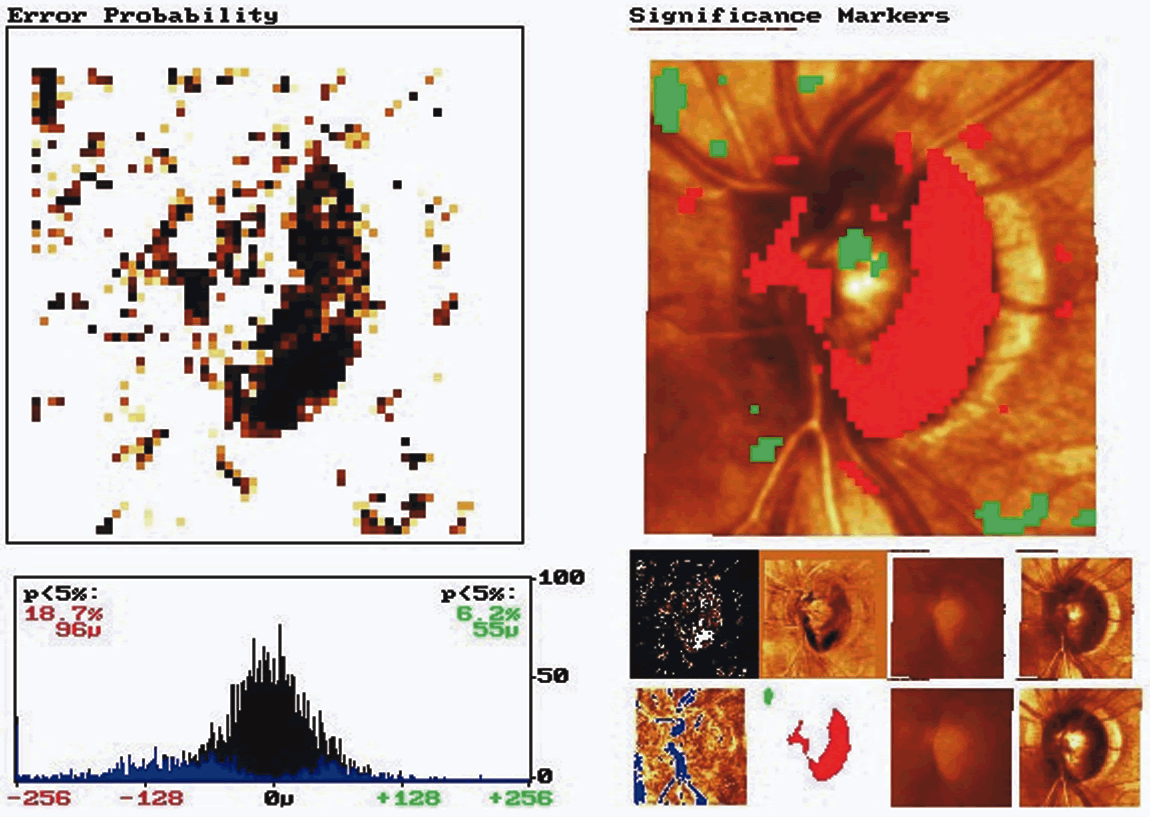
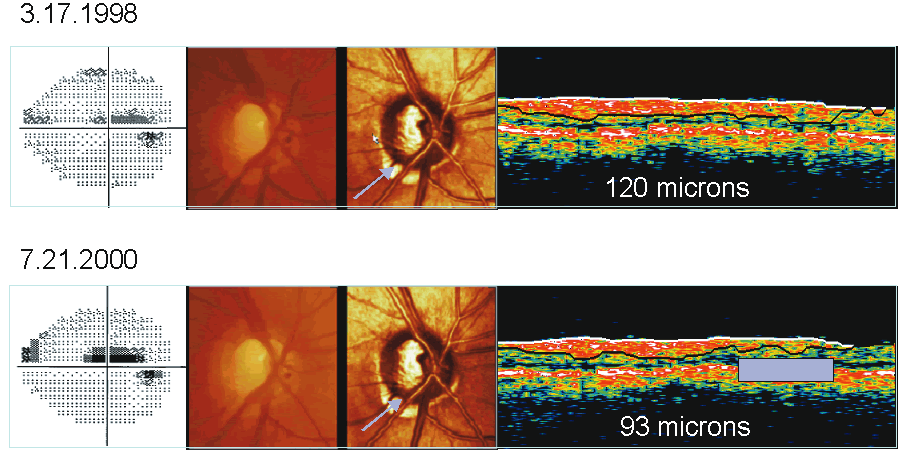
Topographic difference images are determined from the mean topographic images of at least two examinations after automatic correction for shift, rotation, and tilt between the images. Based on multiple image acquisitions during each visit, the software also determines the reproducibility for each individual examination at each location in the image by calculating the standard deviation of the height measurements. This allows the significance of a detected local height change to be determined and appropriate confidence intervals developed. Figure 8 shows the baseline and follow-up examination of a glaucomatous eye. The red areas on the height difference map are depressed more than 50 μm on the follow-up examination. The probability map, on the right, displays the significance of the detected change. The sensitivity for detecting change is influenced by the reproducibility of the height measurements. Therefore, in areas of greater variability, such as along the slope of the cup and over the blood vessels, the confidence limits of the measurement will be larger, and thus requires a greater change before it can be detected reliably.
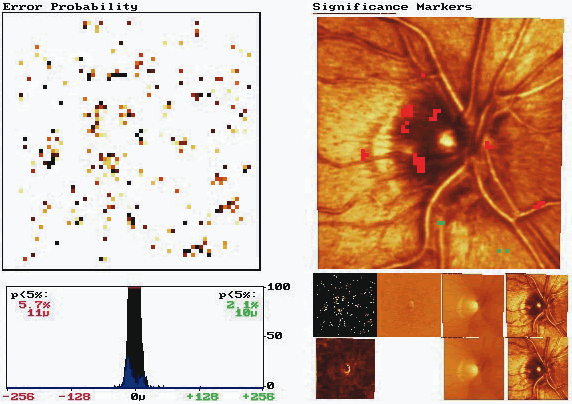
Probability map analysis is based on the evaluation of arrays of condensed superpixels from individual topographic images from which location-specific confidence intervals have been determined. The analysis includes a color-coded image of change in condensed pixels over time that can be viewed with the probability map to determine which areas of change reach statistical significance. In the example in Figure 9, the baseline and follow-up examination of a normal eye are shown. This probability map is rather clean, with approximately 5% of the superpixels randomly spread over the entire image exhibiting significant change, reflecting a relatively small amount of noise inherent to the image. Figure 10 shows the probability map analysis of a glaucoma patient with 5 years between baseline and follow-up examinations. The red markers on the right in Figure 10 indicate diffuse glaucomatous progression of the neuroretinal rim. The advantage of topographic difference images and probability map analyses include their provision of location-specific change information. In addition, there is no need to outline the disc margin or to determine a reference plane.
Global and regional changes in stereometric optic disc parameters can be monitored over time. Analysis strategies that normalize parameters for graphical presentation with values of 0 for normal and 1 for glaucoma have recently been introduced, with most eyes assigned as intermediate value depending on whether they appear more normal or more glaucomatous.
ADVANTAGES
CSLO provides objective, reproducible, quantitative assessment of the ONH and RNFL. Real-time three-dimensional topographic maps of the ONH can be obtained in approximately 1 second. Images can be obtained even with moderate cataract and do not require pupillary dilation. No mechanical storage is necessary and multiple topographic summary parameters are obtainable with automated techniques.
DISADVANTAGES
The high cost has limited the generalized use of this technology in clinical practice. In addition, the considerable variation in optic disc topography among normal eyes necessitates a large age- and race-specific normative database that has yet to established. Further studies are needed to determine the most sensitive and specific summary measurements for diagnosis and management of glaucoma. Technological limitations include the use of a standard reference plane and the need for correct placement of the disc margin by the operator, both of which can influence many of the topographic indices generated. Assessment of optic disc topography is only one aspect of the clinical evaluation for glaucoma. Other features of glaucoma including RNFL damage, peripapillary atrophy, and disc hemorrhages can be missed by sole reliance on this technique for monitoring of glaucoma.