Since 1971, various terms have been used to describe ARN including Kirasawa's uveitis,4 necrotizing vaso-occlusive retinitis,5 peripheral retinal necrosis,6 and bilateral ARN.7 Behçet's disease, a noninfectious retinal vasculitis with systemic involvement of mucous membranes including recurrent oral and genital ulcers, has an extended course with numerous exacerbations and remissions and for these reasons is a distinct entity. ARN classically consists of uveitis and peripheral necrotizing retinitis often with secondary retinal vasculitis and optic nerve inflammation. In Behçet's disease, retinal vasculitis is the primary cause of retinal disease.
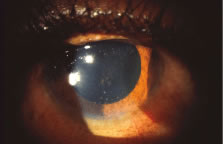
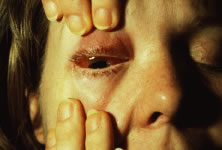
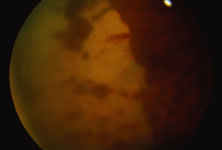
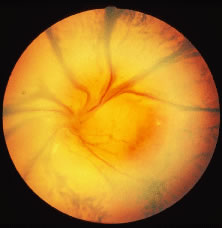
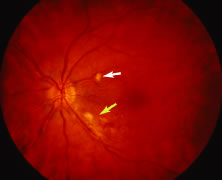
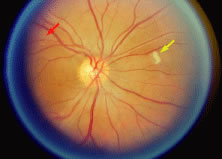
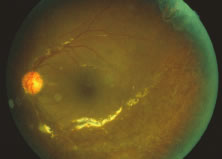
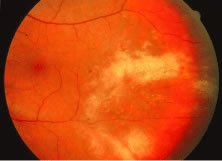
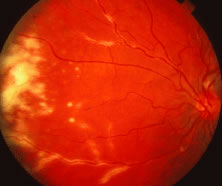
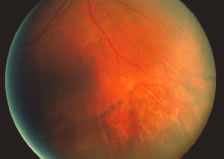
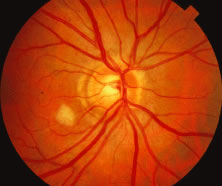
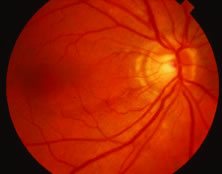
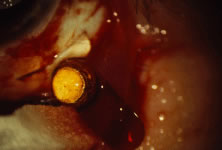
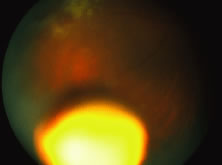
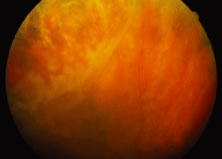
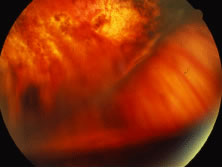
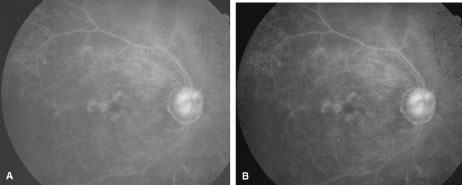
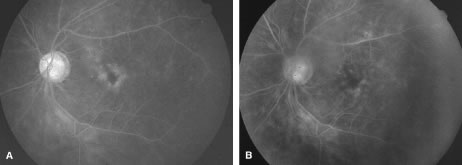
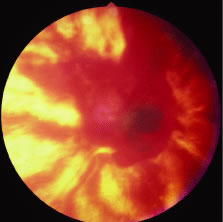
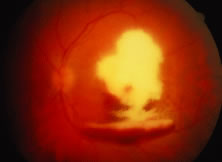
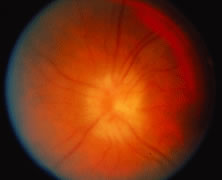
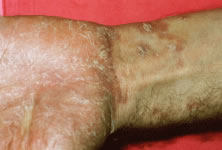
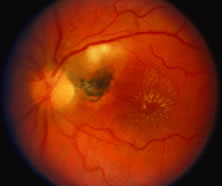
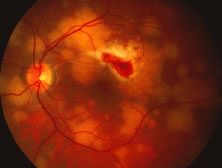
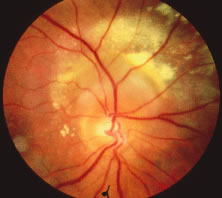
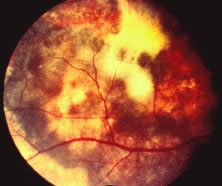
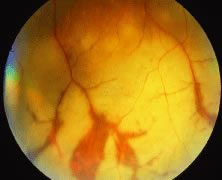
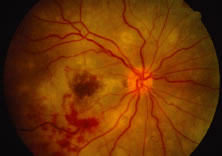
The ARN patient typically presents with progressive visual blurring in one or both eyes occurring over several weeks. These patients often are initially treated with corticosteroids, antitoxoplasmosis drugs, and other medications before arriving at the correct diagnosis. Examination reveals a prominent anterior uveitis that may be granulomatous or nongranulomatous (Fig. 1). Inflammatory signs may be prominent and cause severe pain (Fig. 2). The uveitis may be diffuse and so severe that it causes proptosis. These signs, and the diffuse vitreitis that makes the view of the retina difficult, may contribute to the high degree of delayed and/or misdiagnosis that occurs in the early stages of the disease. Significant vitreous cellular infiltration is seen in the presence of retinitis that is manifest by opacification of the retina, often most prominently in the periphery. Posterior pole involvement may include retinitis, as well as inflammation of the optic nerve head. Optic neuropathy might be the first sign of ARN with subsequent development of other retinal manifestations.8 Ultrasonography and computed tomography (CT) might be helpful in cases of ARN associated with optic nerve edema revealing enlargement of the optic nerve sheath.9 Even in ARN patients who are not immunocompromised and who have no clinical evidence of encephalitis, magnetic resonance imaging of selected cases has shown lesions of the lateral geniculate, optic tracts, and chiasma, which suggests that the virus spreads through the central nervous system (CNS) by axoplasmic transport from the retinal ganglion cells.10 A secondary retinal vasculitis is common, often accompanied by a mild number of retinal hemorrhages. Days to weeks after onset of the infection, the discrete peripheral lesions typically coalesce into a white or yellow ring of infected retina, and the associated vasculature is obliterated (Fig. 3). Necrotic retina desquamates into the vitreous resulting in vitreous sheets.3,6 Eventually, most untreated eyes can be expected to develop retinal detachment resulting from development of multiple full-thickness retinal breaks accompanied by traction or exudation.11 Giant retinal pigment epithelial tears have also been reported.12
|
|
|
In 1994, the American Uveitis Society published its criteria for the diagnosis of ARN. These criteria (classified into mandatory and supporting categories) were dependent on the clinical signs of the disease, and no laboratory or investigative results were included in these criteria.
The required clinical criteria include the following:
- One or more foci of retinal necrosis with discrete borders, located in
the peripheral retina.
- Rapid progression of the disease in the absence of therapy.
- Circumferential spread of the disease.
- Evidence of occlusive vasculopathy and arteriolar involvement.
- A prominent inflammatory reaction in the vitreous and anterior chamber.
The supporting clinical criteria include the following:
- Optic neuropathy or atrophy.
- Scleritis.
- Pain.13
A human leukocyte antigen (HLA) association between ARN and HLA-DQw7, and HLA-Bw62 and DR4 has recently been demonstrated. An immunogenetic predisposition may explain why only some individuals who have been exposed to these ubiquitous herpesviruses develop the disease.14 In Japanese patients, the risk of ARN infection increases with HLA-Aw33, B44, and DRw6.15 No predilection for sex, race, or age has been identified. ARN has been reported in patients ranging from 9 to 89 years old.16 Before knowledge of the vital etiology, there was documentation of a variety of antecedent systemic vital or bacterial infections that were suspected to have a causal role.7,17,18 A history of prior exposure to herpesviruses, particularly VSV, may be elicited in some but not all cases. In some cases, unilateral ARN may be followed by involvement of the contralateral eye up to 10 years after the first episode. Recurrences in the initially involved eye may occur but they are rare.16,19–21
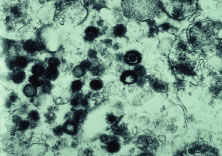
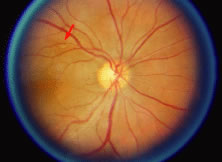
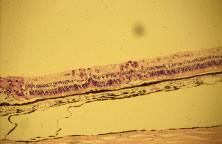
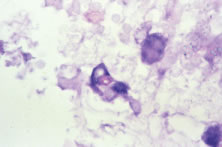
Both herpes simplex virus type 1 and herpes simplex virus type 2 (HSV-1, HSV-2) may cause ARN.20–22 In a single report, CMV particles were identified in and cultured from the retina of an enucleated eye of a nonimmunosuppressed patient suffering from bilateral ARN.23 VZV has been reported most frequently as the viral etiologic agent of ARN.2,3,24–27 We have demonstrated herpesvirus family viral particles in endoretinal biopsy specimens taken from patients in the active stage of the disease who showed an enormous viral load (Fig. 4). These studies, combined with the failure of many enucleated eyes with ARN to demonstrate evidence of viral particles, indicate that the virus is present only in the active stages of the disease and that a gliotic retina will not demonstrate the etiologic agent.28
|
Some cases of ARN follow or precede zoster dermatitis,2,29,30 leading some to conclude that the retina is infected secondary to neural spread from sensory ganglia in the CNS. However, most patients with herpes zoster ophthalmicus do not develop ARN,3 suggesting that transmission of virus to the retina associated with trigeminal nerve reactivation is uncommon. The site of latency and mechanism of viral infection remain unknown.
The polymerase chain reaction (PCR) is a highly sensitive, specific, and rapid means of detecting small amounts of viral deoxyribonucleic acid (DNA) in intraocular fluid samples. Recently, PCR-based assays have been used as aids for the diagnosis of ARN and the determination of the specific virus causing the syndrome.31–33 Ganatra and associates conducted an interesting study about the viral causes of ARN using PCR-based assay of various viruses DNA in aqueous and vitreous. They found that VZV or HSV-1 cause ARN in patients older than 25 years, whereas HSV-2 causes ARN in patients younger than 25 years. In this study, VZV DNA was recovered in 15 out of 30 eyes. HSV-2 DNA was detected in six eyes, and HSV-1 was detected in seven eyes. CMV DNA was detected in only one eye, and no virus DNA was recovered in one eye in which the sample was taken after 6 weeks of acyclovir therapy. Also, no sample was positive for DNA from more than one virus.34
Light microscopic examination of these retinas reveals full-thickness necrosis and loss of the retinal architecture.25,30,35 Within the necrotic retina, macrophages, plasma cells, and other inflammatory cells are found in addition to cells containing eosinophilic inclusions. There is a sharp demarcation between affected and nonaffected retina, suggesting cell-to-cell transmission of the virus. Arteritis manifests as endothelial cell swelling with occlusion of the vessel lumen, infiltration of the subendothelium with plasma cells, and inflammatory cell thrombi. When healing occurs, a thin glial scar replaces the necrotic neural elements. Herpesvirus capsids may be observed in electron microscopic specimens of actively infected retinal tissue but are not seen once healing has occurred and glial scar tissue replaces the necrotic retina.28,30
Given the evidence that most cases of ARN are due to VZV or HSV, acyclovir therapy is recommended.35–38 Acyclovir is taken up by infected cells and phosphorylated into a proactive form by virally encoded thymidine kinase. Subsequent phosphorylation by cell coded kinases results in formation of the active triphosphate form of acyclovir that inhibits viral replication by blocking DNA polymerase activity. Hung and coworkers39 demonstrated that oral administration of acyclovir (400 mg five times per day) to normal volunteers can produce mean plasma levels of 8.7 μm and mean aqueous levels of 3.3 μm.
Some strains of herpesviruses known to have caused ARN are inhibited only by acyclovir concentrations greater than the achievable aqueous concentrations after oral administration (400 mg five times per day) of acyclovir. Intravenous therapy with 1500 mg/m2/day in three divided doses achieves peak plasma levels of 30 to 40 μm without significant toxicity to the patient and is, therefore, the recommended regimen. Aqueous acyclovir levels of 8.7 to 11.2 μm are achievable using intravenous acyclovir (1500 mg/m2/day), and these levels are sufficient to treat most cases of ARN. Prodrugs of acyclovir are being developed that yield serum levels after oral administration that are as high as serum levels after intravenous infusions.
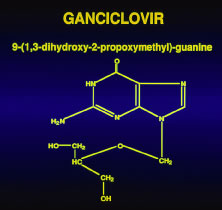
The duration of therapy is generally dependent on the response of the infection. Ten days is probably sufficient because progression of retinal lesions usually stops within several days of initiation of intravenous therapy.39 In cases that are deemed unresponsive to acyclovir, cultures of a retinal biopsy specimen may provide evidence of the presence of an acyclovir-resistant strain of VZV, HSV, or CMV, which in resistant to acyclovir but sensitive to ganciclovir.23,40
The use of steroid and/or anticoagulant therapy remains controversial. Optic nerve infiltration with inflammatory cells and occlusions of arteriolar vessels may result in optic nerve ischemia, which leads some to treat patients with oral prednisone at doses up to 120 mg daily.35,40 Some have suggested that sudden visual loss out of proportion to retinal findings is suggestive of optic nerve ischemia caused by mechanical compression and/or edema and have advocated optic nerve sheath decompression in selected cases.41 To avoid vaso-occlusion and because of a report of altered platelet aggregation in this disease,42 some investigators have advocated the use of aspirin (500 mg/day), heparin, sodium warfarin (Coumadin), or other antiplatelet agents as adjunct therapy to hopefully diminish the vascular complications of ARN.35 Finally, high-dose steroid administration, for example, methylprednisolone 250 mg intravenously every 6 hours, may diminish the generalized ocular inflammatory response resulting in less serum exudation and hence a decrease in the concentration of chemotactic agents in the vitreous that are responsible for causing migration of pigment epithelium cells. This would have the theoretic advantage of decreasing the chances for developing proliferative vitreoretinopathy. Controlled studies evaluating the efficacy of steroids in preventing proliferative vitreoretinopathy or comparing acyclovir versus acyclovir with antiplatelet therapy in preventing ARN-related optic neuropathy are lacking, and the evidence that corticosteroid use worsens the course of active herpetic corneal disease must be remembered.
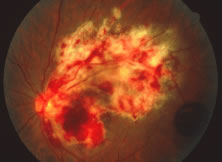
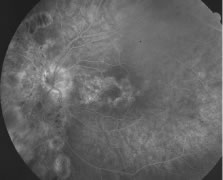
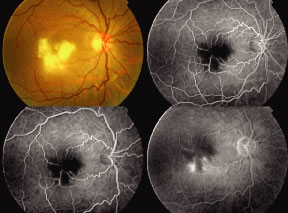
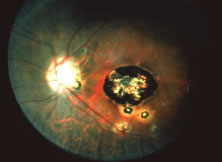
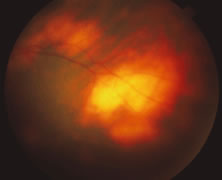
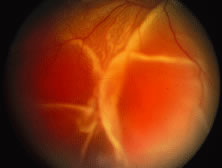
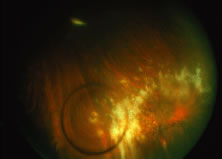
A variable number of eyes, up to 75%, develop retinal detachments (Fig. 5). Prophylactic laser for demarcating the areas of active retinitis from normal retina has been advocated to create chorioretinal adhesions that prevent retinal detachments around sites of retinal break formation (which usually occur at the zone between affected and healthy retina). Han and associates43 reported five cases treated with prophylactic laserpexy in addition to antiviral, steroid, and antiplatelet therapy. After 15 months of follow-up, no retinal detachments were noted. Sternberg and coworkers44 described a 75% decrease in the rate of retinal detachment using prophylactic photocoagulation. Some have advocated the creation of a “new ora serrata” by applying confluent rows of laser burns posterior to the areas of retinitis. However, if traction forces from vitreous organization, epiretinal membrane (ERM) formation, or proliferative vitreoretinopathy develop as they commonly do, the contractile forces will be able to overcome any increased chorioretinal adhesion created by the laserpexy. However, McDonald and associates45 reported failure of prophylactic peripheral laserpexy to prevent retinal detachment in ARN patients. In addition, many cases of ARN result in a severe vitritis, limiting the view for a planned laserpexy (Fig. 6). Therefore, vitrectomy with endolaser and concomitant encircling of the eye with a scleral buckle to reduce traction may be required in some patients.46,47 Decisions relating to the need for scleral buckling to support retinal breaks and the use of silicone oil or long-acting gases to repair retinal detachments should be made by an experienced vitreoretinal surgeon.40,48 Selection of cases to undergo operation should be made with consideration given to optic nerve function, visual potential, and medical control of retinitis.
|
The ocular complications of ARN are myriad. During the active infection, uveitis is a prominent feature. Secondary glaucoma may develop because of inflammatory debris in the trabecular meshwork. Posterior synechiae may form with resulting pupillary block and occlusion of the angle by forward movement of the peripheral iris. Pupillary membranes and cataract development have been described. Optic nerve atrophy has been noted and may be due to loss of ganglion cell bodies in the retina or direct invasion of the nerve head by the virus with subsequent inflammation and swelling. Iris neovascularization and neovascular glaucoma may occur. Disc and retinal neovascularization with subsequent vitreous hemorrhage have also been noted.16,49 Finally, a blind painful eye may develop, necessitating retrobulbar alcohol injection or enucleation.
Recently, a mild variant of ARN was described in Japan that may represent a self-limited form of the disease.1 We recently reported a series of cases in which the retinitis was limited and in which the visual prognosis was significantly better than is commonly reported.50 These reports would seem to indicate that ARN manifested by limited peripheral involvement may portend a better prognosis, particularly when treated early with appropriate antiviral therapy.
Perhaps the most significant complication of a unilateral case of ARN is subsequent involvement of the fellow eye. Sternberg and associates16 reported the cases of six patients, four of whom went on to bilateral involvement anywhere from 3 weeks to 22 months following diagnosis of ARN in the first eye. Matsuo and associates1 described the onset of ARN in the fellow eye after a 3-year interval. In one case, a 10-year quiescent interval was reported between the onset of ARN in the first eye and involvement of the fellow eye. Culbertson and coworkers25 reported that 36% of their patients eventually had bilateral ARN syndrome. Interestingly, two independent laboratories studying the electrophysiologic aspects of ARN have found that the electroretinograms of unaffected eyes may be abnormal.51 The prognostic significance for eventual contralateral involvement has not been investigated. Prolonged oral acyclovir or valacyclovir therapy probably reduces the risk of fellow eye involvement.
In summary, ARN is primarily seen in otherwise healthy persons but has been reported in immunocompromised hosts as well; in HIV patients it is termed progressive outer retinal necrosis. Patients typically present with progressive visual blurring and may carry numerous prior misdiagnoses. The infection causes a severe retinitis, vitreitis, and uveitis that may result in numerous secondary ocular complications. Although the viral infection is treatable, preferably with intravenously administered acyclovir, eventual retinal detachment occurs in most cases. Hence, surgical interventions to preserve vision are usually warranted. Prophylactic laserpexy to wall off the area of infection and prevent total retinal detachment may be helpful if adequate visualization of the area to be treated is possible. However, for patients in whom adequate visualization of the retina is not possible or if retinal detachment is identified, pars plana vitrectomy with endolaser and possible scleral buckling should be considered. Most importantly, frequent dilated retinal examination of both eyes must be performed during the convalescent period and for several years thereafter.