CONGENITAL PTOSIS
Congenital ptosis is a condition present from birth, and, by history, the eyelid malposition has remained stable. Congenital ptosis is unilateral in 75% of cases. The remaining 25% are bilateral.3 In children with congenital ptosis, one must carefully asses refractive error and visual function to identify and treat amblyopia. Amblyopia occurs in 20% of congenital ptosis patients, only 4% of which is attributed to the ptotic eyelid occluding the pupil. Other causes of amblyopia-associated ptosis include anisometropia, high astigmatism, or strabismus.2 In all cases, early assessment and treatment of ptosis is necessary when the visual axis is partially or completely obstructed, because amblyopia and permanent visual loss are severe consequences. If the patient already demonstrates significant amblyopia, there is an extremely high rate of failure of ptosis surgery. Vision is required to motivate patients to learn to use their frontalis and forehead muscles to elevate the eyelids after ptosis surgery in patients with poor elevating power of the eyelids.
Congenital Myogenic Ptosis
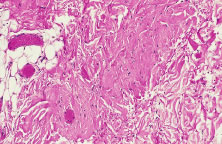
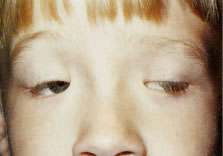
The most common type of congenital ptosis is myogenic ptosis. The pathophysiology involves dysgenesis in the levator palpebrae superioris (LPS) muscle. The skeletal muscle fibers of the LPS are replaced by fibroadipose tissue (Fig. 1). This causes decreased levator muscle function and ptosis. Both elevation and relaxation of the eyelid are impaired because of fibrosis within the muscle and the paucity of normal muscle tissue. Lagophthalmos on downgaze is the sine qui non of congenital ptosis and is caused by the fibrosis of the LPS, which restricts relaxation of the levator muscle. If the levator pulling power is markedly reduced or absent, the patient may also lack an eyelid crease (Fig. 2). In this situation, the abnormal aponeurotic fibers of the LPS do not insert onto the orbicularis and dermis. Lash ptosis may also be seen in myogenic congenital ptosis from decreased tone and loss of structural support.
|
|
Superior rectus dysfunction is found in approximately 5% of congenital myogenic ptosis cases. The superior rectus muscle and LPS share similar embryologic origins. Here, abnormal fibrofatty tissue is seen in both muscles on biopsy.3 A concomitant maldevelopment of the superior rectus muscle seen in certain cases of congenital myogenic ptosis is called double elevator palsy or monocular elevation deficiency.2 In these patients, congenital myogenic ptosis is associated with poor Bell's phenomenon or vertical strabismus. Repair of congenital myogenic ptosis is difficult, because the pathophysiology involves an abnormal muscle, which can not be corrected surgically.
Congenital Aponeurotic Ptosis
Rarely, congenital ptosis may be aponeurotic in origin. Here, the ptosis is due to failure of the levator aponeurosis to insert at its normal position on the anterior tarsus. This may result from birth trauma, especially with forceps delivery. Usually there is good eyelid excursion yet an abnormally high eyelid crease.
Congenital Mechanical Ptosis
Mechanical ptosis can be a congenital abnormality resulting from a mass effect in the upper eyelid, brow, or orbit. Patients can present at birth with a hemangioma, dermoid, or plexiform neurofibroma, which are common causes of mechanical ptosis. Amblyopia can result from astigmatism induced from the mass effect on the globe or from obstruction of the visual axis resulting from the ptotic eyelid. Hemangiomas usually resolve spontaneously or respond to steroid injections.2 Removal of the offending dermoid will usually eliminate the associated mechanical ptosis. Ptosis associated with neurofibromatosis and plexiform neurofibromas is not easily corrected. The treatment is beyond the scope of the text.
Congenital Neurogenic Ptosis
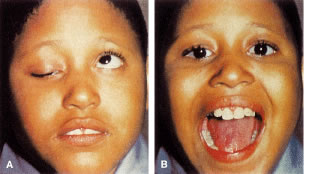
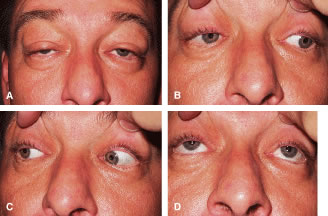
Congenital neurogenic ptosis, though rare, is caused by innervational defects during embryonic development. The Marcus Gunn (Jaw-Winking) ptosis (MGJWP) is the most common type of congenital neurogenic synkinetic ptosis. It is observed in 4% to 6% of patients with congenital ptosis. This eyelid malposition and synkinetic movement involves an abnormal innervation of a normal LPS muscle causing the unilaterally ptotic eyelid to wink with jaw movement (Fig. 3). Synkinesis occurs from aberrant connections between the motor branches of the fifth cranial nerve that normally supplies the pterygoid muscle or the muscles of mastication and the superior division of the oculomotor nerve (CN-3) that innervates the LPS and superior rectus muscles.4 The aberrant connections in the central nervous system can be bilateral, although only one side is symptomatic, demonstrating the wink.5 Clinically, these patients usually have a baseline ptosis on the affected side in primary gaze. The wink is induced by use of muscles of mastication (e.g., sucking, swallowing, chewing, or a lateral motion of the jaw to either side). The wink consists of a rapid elevation of the ptotic lid to a position higher than the unaffected side and then an equally rapid descent back to the ptotic state.6 In this situation, ipsilateral hypotropia or vertical strabismus is common because the superior rectus muscle may be involved. The ptosis is often corrected with surgery. Excision of the levator aponeurosis seems to alleviate some of the symptoms of the jaw-wink and synkinesis before ptosis surgery. Some children with the associated jaw-wink develop adaptive behavioral mechanisms and learn to avoid movements that reduce the wink reflex. This has caused some clinicians to state that MGJWP ptosis improves with age.
Congenital third nerve palsy is another type of neurogenic congenital ptosis. In these patients, blepharoptosis manifests along with deficits in adduction, elevation, and depression of the globe resulting from the innervation of the oculomotor nerve to the LPS muscle and rectus muscles. Mydriasis may be present depending on the severity of the paresis affecting the innervation to the pupillary dilator muscles.
Congenital Horner's syndrome is a rare neurogenic type of ptosis, which evidences a mild ptosis, miosis, anhidrosis, and a decreased pigmentation of the iris and areola on the affected side.7 The ptosis is due to decreased function of the sympathetically innervated Muller's muscle of the upper eyelid. Decreased sympathetic tone to the inferior tarsal muscle results in elevation of the lower eyelid, further narrowing the palpebral fissure on the involved side, the so-called reverse ptosis of the lower eyelid. Although the lesion may be anywhere on the sympathetic chain, congenital Horner's is most often the result of a second-order neuron involvement from brachial plexus injury at birth. The classification of the first (central), second (preganglionic), and third (postganglionic) order neuron lesions along the sympathetic chain are discussed later in the chapter.8
Blepharocanthal Syndrome
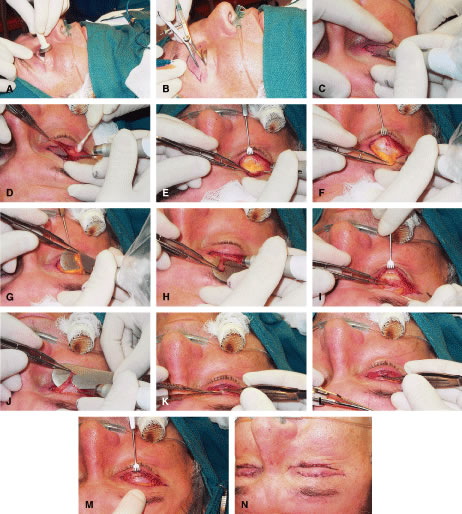
Another type of congenital ptosis that is inherited is the blepharocanthal syndrome. The inheritance is autosomal dominant and comprises about 5% of patients with congenital ptosis3 (Fig. 4).
|
The blepharocanthal syndrome includes two or more of the following features2,4:
- Ptosis with poor levator muscle function and absence of a definitive eyelid
- Epicanthus inversus—a medial canthal tendon fold most prominent in
the lower eyelid and extending from the lower to the upper eyelid
- Telecanthus—a widened intercanthal distance
- Blepharophimosis—a redundancy and lengthening of the upper eyelid
margin
- Lower eyelid ectropion
- Narrowing of the palpebral fissures
Associated findings with blepharocanthal syndrome include hypertelorism, poorly developed nasal bridge, hypoplasia of the superior orbital rims and the tarsal plate, and lop ears (upper third ear deformity of the helix causing overhanging of auricular cartilage).2 In addition, because of hypogonadism and specific hormonal deficiencies, females with this condition should be closely monitored for menstrual irregularity and infertility.9 The abnormalities of the female reproductive system, however, are hormonally related and are not sex linked. In cases of ptosis associated with coexisting congenital eyelid anomalies such as in blepharophimosis, eyelid reconstruction may be staged and ptosis repair is usually delayed until the other eyelid abnormalities are corrected. Ptosis correction at the same time of eyelid reconstruction may be necessary in the setting in which ptosis-induced amblyopia is threatening visual development of the patient.
ACQUIRED PTOSIS
Acquired ptosis is seen as a change from the patient's baseline. The incidence of acquired ptosis has increased in the recent years with heightened awareness among the patient population of diagnosis and treatment. Upper eyelid anatomy is crucial in understanding the pathogenesis of acquired ptosis. (See Chapter 72.) The levator complex of the upper eyelid includes the LPS muscle and the levator aponeurosis. Muller's muscle is sympathetically innervated muscle, which also plays a role in eyelid retraction. Ptosis of the upper eyelid can be due to a weakness of the elevating power of the eyelid retractors as a result of neuromyogenic, traumatic, or involutional insult. In addition, the levator aponeurosis is comprised of a group of collagenous sheets, or lamellae, which have attachments to the posterior orbicular fascia and the anterior tarsal surface. Among these lamellae, there may at one time be a mixture of attenuation, dehiscence, and disinsertion from their attachment sites, resulting in a ptotic eyelid.10 The various types of acquired ptosis (e.g., aponeurotic, neurogenic, myogenic, mechanical, and traumatic) are discussed.
Acquired Aponeurotic Ptosis
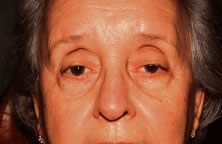
Acquired ptosis is most commonly aponeurotic in etiology. Involutional changes of aging, repetitive traction on the eyelid, or both result in a thinning of the levator aponeurosis or separation from its insertion site on the anterior tarsal surface. Though aponeurotic ptosis most often occurs in older adults, it can be seen in at any age. Younger patients may develop aponeurotic ptosis as a result of trauma, orbital or eyelid swelling, excessive eyelid rubbing, contact lens wear, pregnancy, blepharochalasis, prior ocular surgery, or chronic ocular inflammation.11 Cases of aponeurotic ptosis have even been seen in young children and infants. In these patients, the eyelid crease is usually elevated because of disinsertion from the tarsal plate with superior retraction of the levator aponeurosis (Fig. 5). The attached dermis and orbicularis move cephalad with the migration of the aponeurosis and lead to the formation or appearance of a superior sulcus deformity, a high eyelid crease, and fold and thinning of the upper eyelid.
|
At times, the history may be elusive in which case a cause may not be determined. Patients with aponeurotic ptosis often complain of eyelid and brow fatigue. The ptosis is usually worse later in the day, and this finding should not be confused with those of myopathic ptosis [i.e., myasthenia gravis (MG)]. Patients often notice a superior visual field as a result of the lower upper eyelid position. Older patients often complain of difficulty reading because the ptosis is worse on downgaze. This can be attributed to the involutional changes of the eyelids associated with aging, including loss of tone of the levator muscle and Muller's muscle and atrophy of the orbital fat, leading to a mild degree of enophthalmos.3
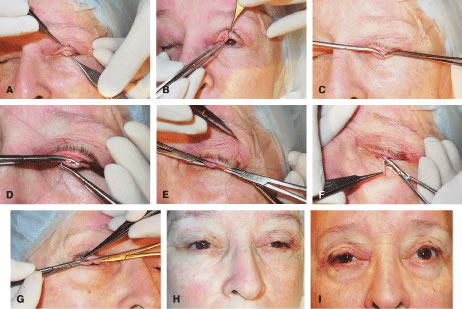
The LPS muscle function in aponeurotic ptosis is either normal or slightly reduced, as assessed by measuring in millimeters the amount of eyelid excursion from downgaze to upgaze.6 These measurements are helpful in distinguishing congenital from acquired ptosis (Table 1). LPS function in normal individuals measures 16 to 20 mm. This is reduced to 12 to 16 mm in acquired aponeurotic ptosis. Horizontal laxity in the upper eyelid is also associated with aponeurotic ptosis and can be attributed to weakness in the lateral canthal tendon or the supporting eyelid structures of the upper eyelid tarsal sling. Specifically, dehiscence of the medial aspect of Whitnall's ligament can occur, resulting in loss of medial support of the eyelid. The tarsal plate shifts laterally causing a more ptotic eyelid nasally and an abnormal eyelid contour. The lateral shift is usually associated with aging and should be recognized preoperatively, because this phenomenon may alter the surgical approach for ptosis repair. Lash ptosis can develop as a result of secondary ptosis from the hooding effect of redundant skin and soft tissue of the upper eyelid resting on the eyelashes. Lash ptosis may also be caused by aging changes in the upper eyelid, including decreased tone, loss of structural support from increasing laxity of the upper eyelid, and atrophy of the tarsus. Surgical repair in these patients with horizontal laxity is difficult and demands balance between horizontal tension of the eyelid and vertical pull of the levator aponeurosis. Correction of aponeurotic ptosis is usually by an external approach levator resection and is discussed later in this chapter.
Table 1. Comparison of Congenital Myogenic Ptosis to Acquired Aponeurotic
Ptosis
| Congenital Myogenic Ptosis | Acquired Aponeurotic Ptosis | |
| Palpebral fissure height | Mild to severe ptosis | Mild to severe ptosis |
| Upper eyelid crease | Weak/absent crease in normal position | Higher than normal crease |
| Levator function on downgaze | Reduced eyelid lag | Near normal eyelid droop |
From American Academy of Ophthalmology. Orbit, Eyelids and Lacrimal System; BCSC Section 7. San Francisco: American Academy of Ophthalmology, 2000-2001:Fig XII-1.
Acquired Neurogenic Ptosis
Acquired neurogenic ptosis is usually caused by a third cranial nerve (oculomotor nerve) palsy or by disruption of sympathetic innervation to Muller's muscle. There are two classifications of third nerve palsies: vasculopathic, from diabetes, hypertension, and arteriosclerotic disease, and compressive, from a neoplasm or an aneurysm. Vasculopathic processes rarely affect the pupillary fibers of the third nerve and generally resolve spontaneously within 3 months. If the palsy has not resolved within 6 months or if the pupil is involved in the patient's initial presentation, further workup to rule out neoplasm or aneurysm must be performed.2 An oculomotor (CN-3) palsy rarely causes an isolated LPS muscle deficit, usually involving rectus muscles. Pupillary fibers are more likely to be involved with a compressive neuropathy. The classic presentation of a compressive third nerve palsy is unilateral, rapidly evolving ptosis, exotropia, hypotropia, and mydriasis. When the pupil is spared, one should suspect a vasculopathic cause.
A patient with an oculomotor nerve palsy may develop synkinetic ptosis as a result of aberrant regeneration and misdirection of third cranial nerve fibers. Although more often congenital, this condition may also develop during recovery from an oculomotor nerve paralysis. Synkinesis can cause elevation of the eyelid with the use of the extraocular muscles, as well as paradoxical pupillary dilatation or constriction.8 The ptotic eyelid may rise as the inferior rectus, medial rectus, or superior rectus muscle contracts. If aberrant regeneration of the nerve is observed in the examination of a patient without a known history of oculomotor nerve palsy, a workup is necessary to rule out a compressive neoplasm of the cavernous sinus or an aneurysm.
Horner's syndrome, an acquired neurogenic ptosis, is caused by a disruption of sympathetic innervation to eyelid retractor muscles, to pupillary dilator muscles, and to the sweat glands in the skin on the affected side. Horner's syndrome classically presents with unilateral ptosis, miosis, and anhidrosis. Paralysis of Muller's muscle in the upper eyelid results in mild ptosis measuring approximately 1 to 2 mm. The lower eyelid may be elevated approximately 1 mm because of paresis of the inferior tarsal muscle. This further narrows the palpebral fissure and may mislead the clinician to think that the patient manifests enophthalmos. Ptosis of the lower eyelid has been called reverse ptosis. The pupil on the involved side is constricted and dilates poorly in the dark as compared with the pupil of the unaffected eye. There also may be lack of sweating on the affected side of the face and neck, as well as depigmentation of the iris.8
Various lesions located along the course of the sympathetic chain cause Horner's syndrome. Tumors, inflammatory processes, aneurysms, and injuries are the most frequent causes of Horner's syndrome.3 The anatomic location of the lesion determines if it is situated along a first-order, second-order, or third-order neuron. The central or first-order neuron courses from the hypothalamus to the cervical portion of the spinal cord.12 Lesions in the brain, spinal cord, or brain stem may result in a first-order insult. The second-order, or preganglionic, neuron passes from the cervical portion of the spinal cord along the sympathetic chain through the upper thorax and neck to synapse in the superior cervical ganglion. Apical lung tumors (Pancoast tumors), metastasis, thoracic surgery, thoracic aortic aneurysms, or trauma to the brachial plexus are examples of second-order lesions that can cause disruption of the sympathetic chain. The third-order, or postganglionic, neuron travels with the sympathetic fibers along the internal carotid artery to the orbit. These lesions are most often of a vascular etiology, such as a carotid artery dissection. Third-order lesions also result from head and neck trauma, tumors, infections, and lesions of the cavernous sinus.13 Localization of the lesion along the sympathetic chain is essential in proper diagnosis and treatment of Horner's syndrome.
Topical pharmacologic testing is used to clinically localize the lesion causing the Horner's syndrome as first, second, or third order. Topical hydroxyamphetamine 1%, or Paredrine, is used to differentiate first and second order, or preganglionic lesions from a third order or postganglionic lesion.14 In a normal eye and in a preganglionic Horner's syndrome, the postganglionic neuron is intact and norepinephrine is present. The action of topical hydroxyamphetamine releases the norepinephrine from the presynaptic terminal into the myoneural junction of the pupillary dilator muscle fibers resulting in dilation of the pupil. In addition, hydroxyamphetamine acts similarly to stimulate Muller's muscle, which will raise the ptotic upper eyelid. A poor pupillary response or no improvement of ptosis usually indicates a third-order lesion, in which the postsynaptic terminal has degenerated and no norepinephrine is present.
Topical 4% to 10% cocaine is another pharmacologic test that localizes third-neuron Horner's syndrome by assessing pupillary response. Cocaine acts to block the reuptake of norepinephrine at the neuromuscular junction, which stimulates the pupillary dilator muscle. This blockade results in pupillary dilation in the normal eye or in a first- and second-order lesion where norepinephrine is present. Cocaine similarly activates Muller's muscle, consequently elevating the ptotic eyelid 1 to 2 mm. In a third-order neuron lesion, there is an absence of norepinephrine in the synaptic cleft and cocaine will have no effect. The typical response after the application of cocaine is a lack of pupillary dilation and failure of eyelid ptosis to resolve. However, addition of epinephrine or hydroxyamphetamine topically can dilate the pupil and elevate the ptotic eyelid in a third-order lesion.
Horner's syndrome is often associated with other neurologic signs, especially when caused by tumor, aneurysm, or inflammation. The etiology must be identified before initiating proper treatment. Surgery to correct eyelid ptosis is only considered once a thorough evaluation, proper diagnosis, and treatment has been implemented.
Myasthenia gravis (MG) is a B-cell mediated autoimmune disease and is a neurogenic acquired ptosis. MG is characterized by weakness of the affected muscles. Patients often initially present to an ophthalmologist with complaints of diplopia or ptosis or both, because the extraocular muscles, particularly the LPS, are often the first to be affected in new onset MG.3 In MG, antibodies to acetylcholine (ACH) receptors cause a functional blockade of the ACH receptors at the neuromuscular junction. The histopathology in MG also demonstrates a flattening and marked loss of the folds present in the postsynaptic membrane. In the normal membrane, these folds increase the surface area available for ACH-receptor binding.15 The reduced number and availability of receptors causes a decrease in the total binding of ACH in the synapses, reducing neural transmission and resulting in a weakness of the levator muscle and ptosis. The unbound ACH in the synaptic cleft is then degraded. Ptosis may be exacerbated by prolonged upgaze, which causes a saturation of the few remaining ACH receptors.
MG often initially presents as a neurogenic ptosis and must always be considered in the differential diagnosis of a patient presenting with an acquired ptosis. The eyelid ptosis often begins unilaterally but may variably affect the other eyelid. Other accompanying findings include dysphagia; hoarseness; dysarthria; dyspnea; weakness of the jaw, neck trunk, and arms; and fatigue with walking.12 Dysphagia and dyspnea can be life threatening. Patients often complain of minimal symptoms in the morning with progressive worsening of symptoms resulting from fatigue toward evening.3 Symptoms are exacerbated after exertion and improve with rest. Patients diagnosed with MG sometimes have associated autoimmune diseases. Five percent of patients with MG are also diagnosed with Grave's disease. Other patients with MG have been found to have hyperactivity of the thymus gland. Thymus hyperplasia is thought to play a role in the immune mechanism in MG.
Ocular myasthenia is myasthenia isolated to the periocular muscles, including the LPS, the orbicularis oculi, and the rectus muscles. Their symptoms are usually localized to include ptosis, diplopia, and fatigue of the periocular muscles. In this type of myasthenia, the ACH-receptor antibody normally found in myasthenic patients is often absent. This difference is theorized to represent the antigenic differences in the synaptic terminals of ocular muscles and other skeletal muscles. The extraocular muscles differ from other skeletal muscles in that they have multiple end plates for each muscle fiber and a myoneural junction that occurs within (hypolemmal) instead of on the muscle fiber. Ocular myasthenia may occasionally be seen with other autoimmune diseases, such as Hashimoto's disease, hyperthyroidism, multiple sclerosis, systemic lupus erythematosus, polymyositis, or hemolytic anemia.16
The classic diagnostic test for myasthenic ptosis is the Tensilon (edrophonium chloride) test. Tensilon is an antiacetylcholinesterase drug that is administered intravenously. Pharmacologically, it acts to overcome the antibody receptor blockade in MG and inhibits the breakdown of ACH. A positive test in a patient with myasthenia resolves the weakness of the affected muscle (e.g., LPS or rectus muscle) and results in resolution of symptoms (e.g., ptosis or diplopia). Patients must be forewarned of the potential yet short-acting adverse effects of the Tensilon test, which can be dramatic including fasciculations, nausea, lacrimation, salivation, flushing, abdominal cramping, bradycardia, and even respiratory arrest. Atropine sulfate (0.4 to 0.6 mg) is a muscarinic antagonist and must be available to administer in case of the adverse reactions of Tensilon. Atropine sulfate blocks the action of ACH by binding to postsynaptic muscarinic receptors. Some physicians treat prophylactically with atropine by injecting the patient with 0.6-mg intramuscular (IM) before administering Tensilon.
The Tensilon test can be administered in an office setting, provided that the clinician and ancillary help are comfortable and equipped to handle potential adverse reactions. Safer environments include the operating room (OR), ambulatory surgery center, or emergency room where resuscitation is easily performed. Whenever a Tensilon test is planned, the patient should be semireclined, and resuscitation equipment should be readily available. The patient's vital signs, including blood pressure and pulse, should be monitored throughout the procedure. Tensilon is initially administered as a test dose, 2 mg (0.2 cc) and is slowly injected intravenously through a butterfly needle. The patient is observed for 60 seconds for elevation of the ptotic eyelid or improvement of extraocular movements. If there is improvement of symptoms after 1 minute, it is a positive test, and, thus, the test is terminated. If no response occurs, the remaining 8 mg is slowly administered and the patient is observed for 1 to 5 minutes. This dose can be divided into two 4-mg doses, which seems to cause fewer adverse effects.12
Prostigmin (neostigmine methylsulfate) is another anticholinesterase drug that can be used as a pharmacologic diagnostic test for MG. Although the adverse reactions are similar to Tensilon, the more frequent side effects are less severe (e.g., salivation, fasciculations, gastrointestinal discomfort). This is useful in children and adults who may require a longer observation period than with the Tensilon test. Neostigmine is administered intramuscularly and is concurrently injected with atropine. The dosage formula is: (weight (kg) × adult dose) divided by 70. The adult dose typically measures 1.5 mg with 0.4 mg atropine. Resolution of ptosis or diplopia in a positive test is expected to occur over a 30- to 45-minute time period.
Other clinical tests for MG include the rest test and the ice pack test. The rest, or sleep test, is a clinical diagnostic test.17 This involves taking precise measurements of the patient's degree of ptosis on presentation, then allowing the patient to rest quietly with eyelids closed for up to 30 minutes. Immediately after the patient is aroused, the ptosis measurements are repeated. Improvement of the ptosis with rest is highly suggestive of MG, because the receptors not occupied by antibodies are free to bind newly released ACH.12 The ice pack test is a safe diagnostic clinical test for MG that is easily performed in the office. This entails placing an ice pack over the affected ptotic eye for 2 minutes and then reevaluating the patient for resolution of ptosis. Myasthenic weakness is known to improve with lowering of the temperature of skeletal muscle. Cold is thought to affect the neuromuscular junction by decreasing cholinesterase activity and by promoting efficiency of ACH by eliciting depolarizations at the end plate.18 Kubis and colleagues17 demonstrated that in 90% of patients with MG ptosis, there is an improvement of their ptosis with the ice test. Recent studies concur that the ice test is more effective than the rest test alone in diagnosing the ptosis of MG.19
Another diagnostic marker for MG is a serum assay for antiacetylcholine receptor antibodies (ACHR Ab test). Binding antibodies are usually measured, because they are detected in approximately 90% of patients with generalized MG and 70% of patients with ocular MG.12 There have, however, been false-positive reports with this test in cases of thymoma in patients without myasthenia. These include patients with Lambert-Eaton myasthenic syndrome and small cell lung cancer, as well as one third of the population older than 70 years.20 If the patient has not yet been diagnosed with MG or if the ophthalmologist is the first physician to suspect MG, the patient should be further evaluated by his or her internist for systemic involvement. Referral to a neurophthalmologist is usually appropriate for a patient suspected to have MG. Serologic screening tests for thyroid dysfunction and autoimmune diseases should be performed because there is a high association of MG with thyroidopathy and other autoimmune diseases.12 Radiographic studies looking for thymomas should also be performed on MG patients, because they are visible on computed tomography (CT) scan in 10% of patients with MG.
Patients with MG should undergo appropriate treatment with anticholinesterase medication (e.g., Mestinon and Prostigmin), corticosteroids, or other immunosuppressant agents under supervision of their internist or neurologist. In patients with enlargement of the thymus or a thymoma, a thymectomy is usually indicated. Some patients, especially younger individuals have gone into remission of the disease following thymectomy.3 The systemic manifestations of MG can be life threatening and must be managed before considering surgical treatment of blepharoptosis. Patients often demonstrate poor eyelid closure resulting from weakness of the involved orbicularis muscles, which may lead to problems at surgery. Patients must also be warned that the ptosis may fluctuate and correction of ptosis is difficult.
Acquired Myogenic Ptosis
Myogenic ptosis is a rare type of acquired of blepharoptosis and can be found in localized or diffuse muscular disease, such as the muscular dystrophies. The muscular dystrophies are distinguishable by their mode of inheritance and clinical features. Blepharoptosis is a clinical sign of certain muscular dystrophies, including myotonic dystrophy, chronic progressive ophthalmoplegia (CPEO), and oculopharyngeal muscular dystrophy.
Myotonic dystrophy is an autosomal dominant (chromosome 19) multisystem disorder with an onset usually in the second decade of life. Histologically, myotonic dystrophy is characterized by degenerative changes of skeletal muscle including disruption of myofilaments and sarcoplasmic reticulum, focal accumulation of mitochondria, and eventually atrophy and fibrosis of muscle.21 The distal musculature is usually the first to be affected. Myotonia is often the initial symptom, which results from delayed relaxation of skeletal muscle after contraction. Symptoms are exacerbated with cold, excitement, and fatigue. Patients often develop a characteristic myopathic facies, presenting with blepharoptosis, frontal balding, and wasting of the temporalis masseter muscle. Ocular findings include ptosis, blepharospasm, ophthalmoplegia (similar to CPEO), pigmentary retinopathy, “Christmas tree” cataracts, and miotic pupils.2 Associated systemic findings include low intelligence, insulin resistance, cardiac conduction abnormalities, and testicular and uterine atrophy. Electromyogram (EMG) is a diagnostic test demonstrating myotonic discharges.
Chronic progressive ophthalmoplegia (CPEO) is another muscular dystrophy with several variants, all of which result from mutations in mitochondrial DNA. Most cases are autosomal dominant and maternally inherited. Inherited CPEO has been linked to specific families of Italian and Finnish descent. One form of CPEO, however, has been found to occur as a sporadic mutation. On muscle biopsy, histopathologic examination reveals ragged red fibers, which represent accumulations of abnormal mitochondria within the myofibers.21 EMG findings can also be abnormal. Because the disorder is mitochondrial in origin, it tends to affect tissues with high oxidative needs: cardiac muscle, skeletal muscle, and the central nervous system.22
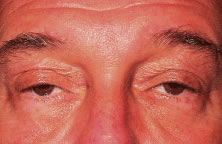
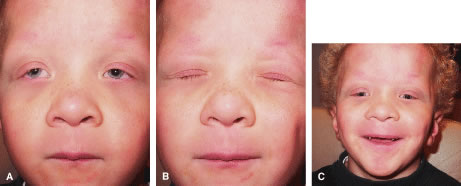
CPEO usually presents in the second to fourth decade often initially with bilateral progressive ptosis (Fig. 6A). In addition to LPS muscle involvement, patients also develop a weakness of other periocular muscles including the rectus muscles and the orbicularis oculi muscles (see Fig. 6B to D). Despite limited ductions from weakness of rectus muscles, binocular vision is usually preserved. Various types of CPEO are associated with systemic deficits such as retinal pigment degeneration,hyperacusia, dysphagia, ataxia, and proximal muscle weakness. The retinal pigment changes differ from retinitis pigmentosa in that they are mainly at the posterior pole with a mottled appearance of the pigment epithelium. Visual changes are mild if any.21 There is also a pediatric variant of CPEO, known as Kearns-Sayre syndrome, which includes cardiac conduction abnormalities (a significant surgical risk), retinal pigment degeneration, and bilateral ptosis.22
|
Oculopharyngeal muscular dystrophy is a disorder characterized by progressive atrophy and weakness of the hypopharyngeal and ocular muscles. The disorder results from a mutation of the PAB2 protein that binds to messenger ribonucleic acid (mRNA).21 It usually presents in the third to fourth decade with dysphagia or regurgitation of food resulting from lack of coordination of the pharyngeal muscles. The dysphagia can progress to intolerance of liquids and the patient is at risk from aspiration pneumonia and death. Surgical intervention such as cricopharyngostomy is sometimes necessary. The dysphagia usually precedes the ptosis by several years. The ptosis observed is bilateral, but usually asymmetric, and the extraocular muscles may also be involved.
Other rare causes of myogenic ptosis include Guillain-Barré syndrome or iatrogenic botulism. Injection of botulinum toxin for the treatment of rhytids or blepharospasm may result in a temporary ptosis usually lasting a few weeks.
Correction of acquired myogenic ptosis can be difficult, because most patients with myogenic ptosis have a progressive disease making the surgical outcome unpredictable. These patients are at increased risk for complications following ptosis surgery because of poor eye-protective mechanisms (EPMs) including inadequate blink, poor Bell's phenomenon, and consequently exposure keratitis. The surgical approach is most often based on the amount of levator muscle function present, and the patient is instructed on the potential variability following surgical correction. This is discussed in further detail later in the chapter.
Mechanical Acquired Ptosis
Mechanical acquired ptosis is seen with any abnormality that results in pulling down of the upper eyelid resulting in ptosis despite normal muscular function. This could include dermatochalasis, tumors of the lid or orbit, chalazion, postsurgical or traumatic edema, a lost contact lens in the superior fornix, or a scar.23 Eliminating the mass effect of the lesion usually alleviates mechanical ptosis.
Traumatic Acquired Ptosis
Traumatic acquired ptosis of the upper eyelid can result from blunt or sharp injury usually to the LPS muscle or the levator aponeurosis either by trauma or surgery. If the insult is traumatic, the underlying etiology may be myogenic, neurogenic, mechanical, cicatricial, aponeurotic, or even a combination of these. Exposure of orbital fat in the upper eyelid after injury typically demonstrates violation of the orbital septum. In this case, the upper eyelid should be explored for possible injury to the levator muscle and repaired at this time. Posttraumatic or postsurgical ptosis often results following orbital, ocular, and neurosurgical procedures. It is important to initially observe these patients without surgical intervention, because acute factors such as hemorrhage, neuropraxia, and edema are temporary. Spontaneous resolution or improvement often occurs in these patients over the course of 6 or more months. Surgical repair of patients with traumatic ptosis is highly variable based on the etiology of the injury and existing elevating power of the upper eyelid.
Pseudoptosis
Pseudoptosis should be differentiated from true blepharoptosis. Various conditions may make an upper eyelid appear low, including a hypertropia on the contralateral side, enophthalmos, microphthalmos, anophthalmos, blepharochalasis, phthisis bulbi, dermatochalasis, or a superior sulcus defect secondary to trauma or cicatrix. In addition, widening of the palpebral fissures on the contralateral side can give the appearance of pseudoptosis and may be due to eyelid retraction from Grave's disease, axial proptosis, congenital eyelid retraction, or high myopia.
Pseudoptosis is often manifested in aging patients as a result of redundancy and laxity of the skin and tissue of the upper eyelid and brow. In evaluating eyelid ptosis, it is important to distinguish the independently functioning units of the eyebrow, redundant eyelid skin, and the elevating power of the eyelid. In brow ptosis, recruitment of the frontalis muscle is needed to elevate the eyebrow and eyelid. Relaxation or fatigue of the forehead muscles that can be elicited during examination will manifest the brow ptosis and may induce mechanical ptosis of the eyelid. Dermatochalasis, a commonly seen condition, in which excess skin overhangs the eyelid margin, can also cause a mechanical pseudoptosis or a secondary ptosis. This “hooding” effect can be corrected during the examination by manual elevation of the redundant eyelid skin, allowing visualization of the true palpebral aperture. In brow ptosis and dermatochalasis, the levator muscle function is usually intact. Surgery to correct brow ptosis requires a brow lift, and correction of dermatochalasis requires blepharoplasty not ptosis surgery. In aging patients especially, involutional ptosis can occur in conjunction with brow ptosis or dermatochalasis, in which case ptosis surgery is indicated. Each factor plays an important role in the symptomatic ptosis patient and should be individually examined and recognized.