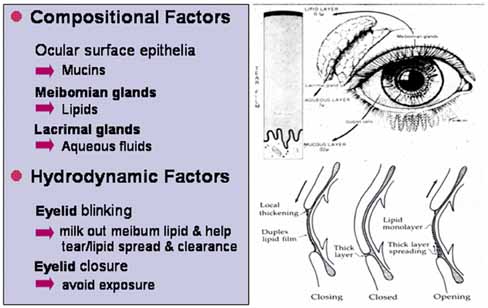
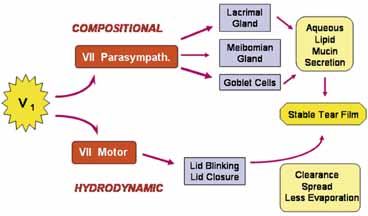
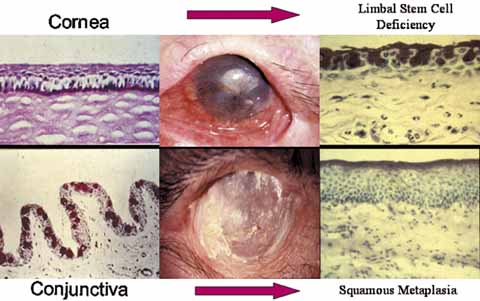
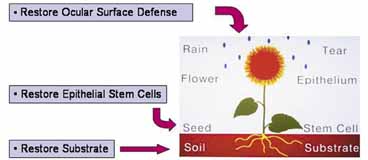
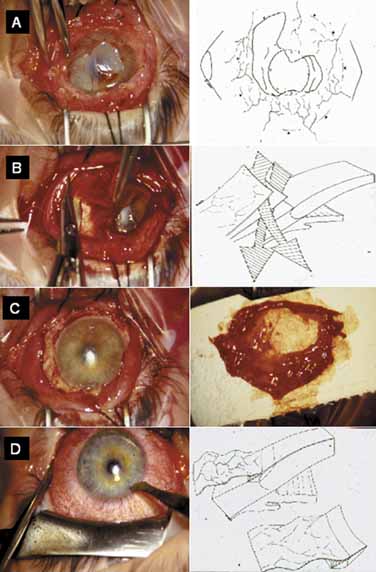
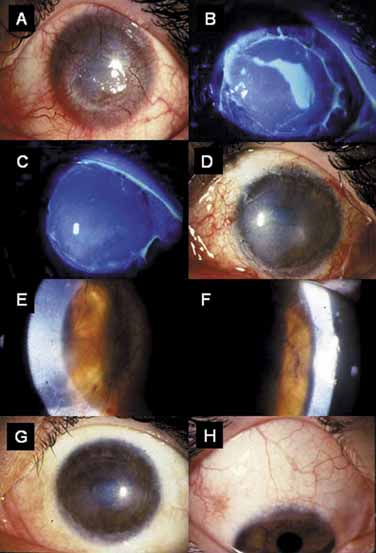
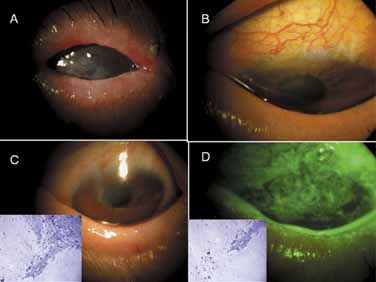
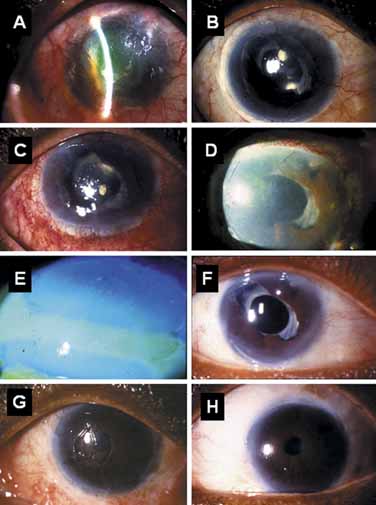
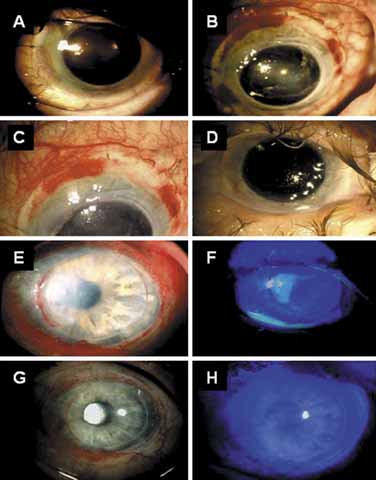
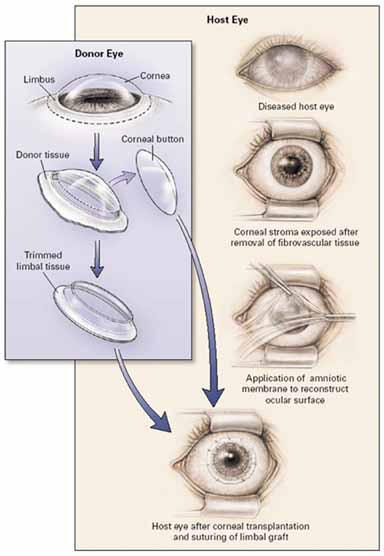
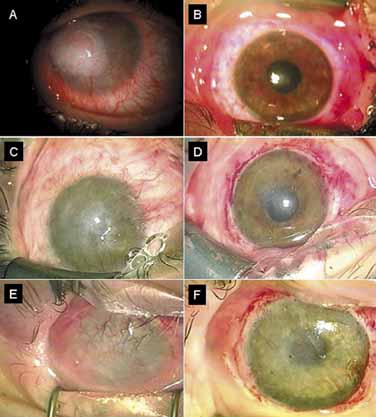
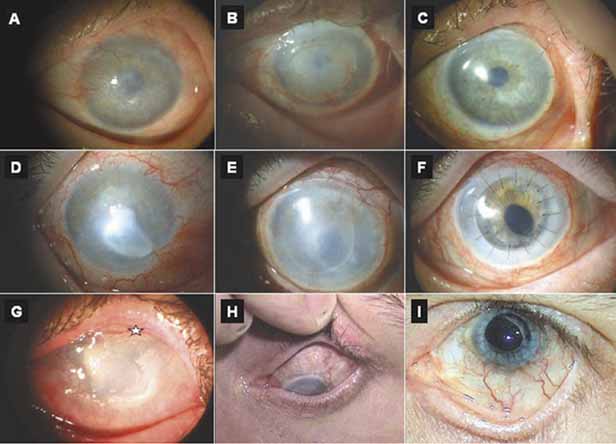
1. Tseng SCG, Tsubota K: Important concepts for treating ocular surface and tear disorders. Am J Ophthalmol 124:825, 1997 2. Lemp MA: Report of the National Eye Institute/Industry Workshop on clinical
trials in dry eyes. CLAO J 21:4, 1995 3. Tseng SCG: Staging of conjunctival squamous metaplasia by impression cytology. Ophthalmology 92:728, 1985 4. Hatchell DL, Sommer A: Detection of ocular surface abnormalities in experimental vitamin A deficiency. Arch Ophthalmol 102:1389, 1989 5. Wittpenn JR, Tseng SCG, Sommer A: Detection of early xerophthalmia by impression cytology. Arch Ophthalmol 104:237, 1986 6. Nelson JD: Ocular surface impression using cellulose acetate filter material. Ocular
pemphigoid. Surv Ophthalmol 27:67, 1982 7. Nelson JD, Wright JC: Conjunctival goblet cell densities in ocular surface disorders. Arch Ophthalmol 102:1049, 1984 8. Puangsricharern V, Tseng SCG: Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology 102:1476, 1995 9. Schermer A, Galvin S, Sun T-T: Differentiation-related expression of a major 64K corneal keratin
in vivo and in culture suggests limbal location of corneal epithelial
stem cells. J Cell Biol 103:49, 1986 10. Nishida K, Kinoshita S, Ohashi Y, et al: Ocular surface abnormalities in aniridia. Am J Ophthalmol 120:368, 1995 11. Dua HS, Gomes JAP, Singh A: Corneal epithelial wound healing. Br J Ophthalmol 78:401, 1994 12. Huang AJW, Tseng SCG, Kenyon KR: Alteration of epithelial paracellular permeability during corneal epithelial
wound healing. Invest Ophthalmol Vis Sci 31:429, 1990 13. Fujishima H, Shimazaki J, Tsubota K: Temporary corneal stem cell dysfunction after radiation therapy. Br J Ophthalmol 80:911, 1996 14. Schwartz GS, Holland EJ: Iatrogenic limbal stem cell deficiency. Cornea 7:31, 1998 15. Pires RTF, Chokshi A, Tseng SCG: Amniotic membrane transplantation or limbal conjunctival autograft for
limbal stem cell deficiency induced by 5-fluorouracil in glaucoma
surgeries. Cornea 19:284, 1999 16. Gass JDM: The syndrome of keratoconjunctivitis, superficial moniliasis, idiopathic
hypoparathyroidism and Addison's disease. Am J Ophthalmol 54:660, 1962 17. Espana EM, Raju V, Tseng SC: Focal limbal stem cell deficiency corresponding to an iris coloboma. Br J Ophthalmol 86:1451, 2002 18. Espana EM, Grueterich M, Romano AC, et al: Idiopathic limbal stem cell deficiency. Ophthalmology 109:2004, 2002 19. Kruse FE, Joussen AM, Rohrschneider K, et al: Cryoperserved human amniotic membrane for ocular surface reconstruction. Graefe's Arch Clin Exp Ophthalmol 238:68, 2000 20. Dua HS, Azuara-Blanco A: Amniotic membrane transplantation. Br J Ophthalmol 83:748, 1999 21. Kruse FE, Voelcker HE, Rohrschneider K: Transplantation of amniotic membrane for reconstruction of the eye surface. Ophthalmologe 95:114, 1998 22. Sippel KC, Ma JJK, Foster CS: Amniotic membrane surgery. Curr Opin Ophthalmol 12:269, 2001 23. Tseng SCG, Tsubota K: Amniotic membrane transplantation for ocular surface reconstruction. In Holland EJ, Mannis MJ, eds. Ocular Surface Diseases: Medical and Surgical Management. New York: Springer, 2001:226–231 24. Dua HS: The conjunctiva in corneal epithelial wound healing. Br J Ophthalmol 82:1407, 1998 25. Anderson DF, Ellies P, Pires RTF, et al: Amniotic membrane transplantation for partial limbal stem cell deficiency: long
term outcomes. Br J Ophthalmol 85:567, 2001 26. Meller D, Pires RTF, Mack RJS, et al: Amniotic membrane transplantation for acute chemical or thermal burns. Ophthalmology 107:980, 2000 27. John T, Foulks GN, John ME, et al: Amniotic membrane in the surgical management of acute toxic epidermal necrolysis. Ophthalmology 109:351, 2002 28. Wei Z-G, Wu R-L, Lavker RM, et al: In vitro growth and differentiation of rabbit bulbar, fornix, and palpebral
conjunctival epithelia. Implication on conjunctival epithelial transdifferentiation
and stem cells. Invest Ophthalmol Vis Sci 34:1814, 1993 29. Holland EJ, Schwartz GS: The evolution of epithelial transplantation for severe ocular surface disease
and a proposed classification system. Cornea 15:549, 1996 30. Kenyon KR, Tseng SCG: Limbal autograft transplantation for ocular surface disorders. Ophthalmology 96:709, 1989 31. Ronk JF, Ruiz-Esmenjaud S, Osorio M, et al: Limbal conjunctival autograft in a subacute alkaline corneal burn. Cornea 13:465, 1994 32. Meallet MA, Espana EM, Grueterich M, et al: Amniotic membrane transplantation for recipient and donor eyes undergoing
conjunctival limbal autograft for total limbal stem cell deficiency. Ophthalmology 110:1585, 2003 33. Daya SM, Ilari L: Living related conjunctival limbal allograft for the treatment of stem
cell deficiency. Ophthalmology 108:126, 2001 34. Tsubota K, Satake Y, Kaido M, et al: Treatment of severe ocular surface disorders with corneal epithelial stem-cell
transplantation. N Engl J Med 340:1697, 1999 35. Solomon A, Ellies P, Anderson DF, et al: Long-term outcome of kerarolimbal allograft with or without penetrating
keratoplasty for total limbal stem cell deficiency. Ophthalmology 109:1159, 2002 36. Ikari L, Daya SM: Long-term outcomes of keratolimbal allograft for the treatment of
severe ocular surface disorders. Ophthalmology 109:1278, 2002 37. Shimazaki J, Shimmura S, Fujishima H, et al: Association of preoperative tear function with surgical outcome in severe
Stevens-Johnson syndrome. Ophthalmology 107:1518, 2000 38. Tsubota K, Satake Y, Ohyama M, et al: Surgical reconstruction of the ocular surface in advanced ocular cicatricial
pemphigoid and Stevens-Johnson syndrome. Am J Ophthalmol 122:38, 1996 39. Mita T, Yamashita H, Kaji Y, et al: Functional difference of TGF-β isoforms regulating corneal wound
healing after excimer laser keratectomy. Exp Eye Res 68:513, 1999 40. Schwartz GS, Gomes JAP, Holland EJ: Preoperative Staging of Disease Severity. In Holland EJ, Mannis MJ, eds. Ocular Surface Disease. New York: Springer-Verlag, 2002:158–167 41. Shimazaki J, Yang H-Y, Tsubota K: Amniotic membrane transplantation for ocular surface reconstruction in
patients with chemical and thermal burns. Ophthalmology 104:2068, 1997 42. Tseng SCG, Prabhasawat P, Barton K, et al: Amniotic membrane transplantation with or without limbal allografts for
corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol 116:431, 1998 43. Holland EJ, Schwartz GS: Changing concepts in the management of severe ocular surface disease over
twenty-five years. Cornea 19:688, 2000 44. Espana EM, Di Pascuale M, Grueterich M, et al: Keratolimbal allograft for corneal surface reconstruction. Eye 110:481, 2003 45. Shimazaki J, Maruyama F, Shimmura S, et al: Immunologic rejection of the central graft after limbal allograft transplantation
combined with penetrating keratoplasty. Cornea 20:149, 2001 46. Pellegrini G, Traverso CE, Franzi AT, et al: Long-term restoration of damaged corneal surface with autologous
cultivated corneal epithelium. Lancet 349:990, 1997 47. Rama P, Bonini S, Lambiase A, et al: Autologous fibrin-cultured limbal stem cells permanently restore
the corneal surface of patients with total limbal stem cell deficiency. Transplantation 72:1478, 2001 48. Tsai RJF, Li L-M, Chen J-K: Reconstruction of damaged corneas by transplantation of autologous limbal
epithelial cells. N Engl J Med 343:86, 2000 49. Schwab IR: Cultured corneal epithelia for ocular surface disease. Trans Am Ophthalmol Soc 97:891, 1999 50. Schwab IR, Reyes M, Isseroff RR: Successful transplantation of bioengineered tissue replacements in patients
with ocular surface disease. Cornea 19:421, 2000 51. Grueterich M, Espana EM, Touhami A, et al: Phenotypic study of a case with successful transplantation of ex vivo expanded
human limbal epithelium for unilateral total limbal stem cell
deficiency. Ophthalmology 109:1547, 2002 52. Koizumi N, Inatomi T, Suzuki T, et al: Cultivated corneal epithelial transplantation for ocular surface reconstruction
in acute phase of Stevens-Johnson syndrome. Arch Ophthalmol 119:298, 2001 53. Koizumi N, Inatomi T, Suzuki T, et al: Cultivated corneal epithelial stem cell transplantation in ocular surface
disorders. Ophthalmology 108:1569, 2001 |