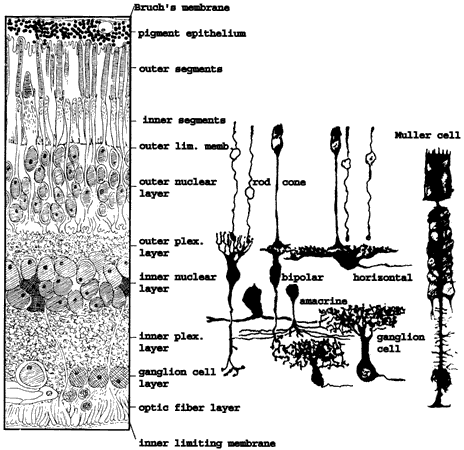
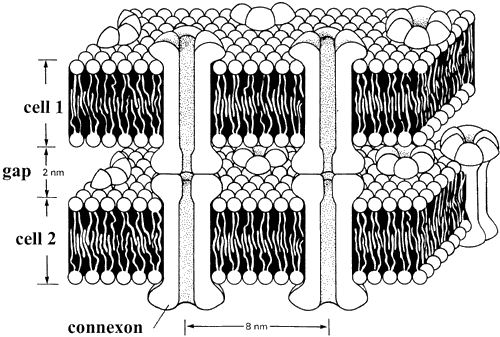
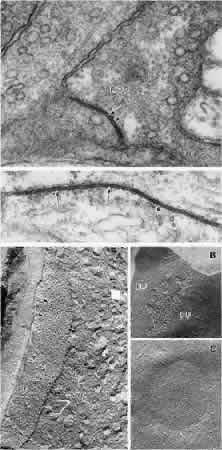
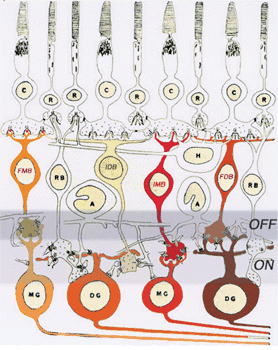
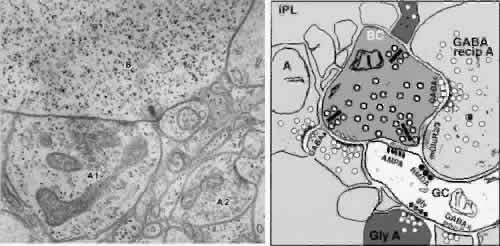
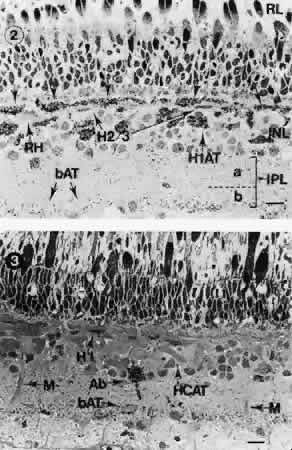
1. Ramón y Cajal S: The Structure of the Retina. Springfield, IL, Charles
C. Thomas, 1972 2. Levitan IB: Modulation of ion channels by phosphorylation. How the brain works. Advances in Second Messenger and Phosphoprotein Research 33:3, 1999 3. Fuxe K, Agnati LF, eds: Volume Transmission in the Brain. Advances in Neuroscience, vol 1. New
York, Raven Press, 1991 4. Polyak S: The Retina. Chicago, University of Chicago Press, 1941 5. Curcio CA, Sloan KR, Packer O et al: Distribution of cones in human and monkey retina: individual variability
and radial asymmetry. Science 236:579, 1987 6. Adams CK, Perez JM, Hawthorne MN: Rod and cone densities in the rhesus monkey. Invest Ophthalmol Vis Sci 13:885, 1974 7. Kolb H, Linberg KA, Fisher SK: Neurons of the human retina: a golgi study. J Comp Neurol 318:147, 1992 8. Dacey DM: The mosaic of midget ganglion cells in the human retina. J Neurosci 13:5334, 1993 9. Perry VH, Oehler R, Cowey A: Retinal ganglion cells that project to the dorsal lateral geniculate nucleus
in the macaque monkey. Neuroscience 12:1101, 1984 10. Tauchi M, Masland RH: The shape and arrangement of the cholinergic neurons
in the rabbit retina. Proc R Soc Lond B223:101, 1984 11. Crook JM, Lange-Malecki B, Lee BB, Valberg A: Visual resolution of macaque retinal ganglion cells. J Physiol Lond 396:205, 1988 12. Pirenne MH: Visual acuity. In: Davson H, ed: The Eye, vol. 2, The Visual
Process, pp 175–195. New York, Academic Press, 1962 13. Goodenough DA: Bulk isolation of mouse hepatocyte gap junctions. J Cell Biol 61:557, 1974 14. Revel JP, Karnovsky MJ: Hexagonal array of subunits in intercellular junctions of the mouse heart
and liver. J Cell Biol 33:C7, 1967 15. Witkovsky P, Shakib M, Ripps H: Interreceptoral junctions in the teleost retina. Invest Ophthalmol Vis Sci 13:996, 1974 16. Witkovsky P, Owen WG, Woodwoth M: Gap junctions among the perikarya, dendrites, and axon terminals of the
luminosity-type horizontal cell of the turtle retina. J Comp Neurol 216:359, 1984 17. Bruzzone R, Ressot C: Connexins, gap junctions and cell-cell signalling in the nervous system. Eur J Neurosci 9:1, 1997 18. Dermietzel R, Kremer M, Paputsoglu G et al: Molecular and functional diversity of neural connexins in the retina. J Neurosci 20:8331, 2000 19. Guldenagel M, Sohl G, Plum A et al: Expression patterns of connexin genes in mouse retina. J Comp Neurol 425:193, 2000 20. Verselis VK, Ginter CS, Bargiello TA: Opposite voltage gating polarities
of two closely related connexins. Nature 368, 1994 21. Bennett MVL: Gap junctions as electrical synapses. J Neurocytol 26:349, 1997 22. Xin D, Bloomfield SA: Dark- and light-induced changes in coupling between horizontal cells in
the rabbit retina.J Comp Neurol 383:512, 1998 23. Hampson ECGM, Vaney DI, Weiler R: Dopaminergic modulation of gap junction permeability between amacrine cells
in mammalian retina. J Neurosci 12:4911, 1992 24. Baylor DA, Fuortes MGF: Electrical responses of single cones in the retina of the turtle. J Physiol 207:77, 1970 25. Mastronarde DN: Correlated firing of retinal ganglion cells. Trends Neurosci 12:75, 1989 26. Zucker RS: Exocytosis: a molecular and physiological perspective. Neuron 17:1049, 1996 27. Augustine GJ, Betz H, Bommert K et al: Molecular pathways for presynaptic
calcium signaling. In: Stjarne L, Greengard P, Grillner S et al: Molecular
and Cellular Mechanisms of Neurotransmitter Release. New York, Raven
Press, 1994 28. Ziff EB: Enlightening the postsynaptic density. Neuron 19:1163, 1997 29. O'Brien RJ, Kamboj S, Ehlers MD et al: Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron 21:1067, 1998 30. Neher E: Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter
release.Neuron 20:389, 1998 31. Llinas R, Sugimori M, Silver RB: The concept of calcium concentration microdomains in synaptic transmission. Neuropharmacology 34:1443, 1995 32. Golovina VA, Blaustein MP: Spatially and functionally distinct Ca2+ stores in sarcoplasmic and endoplasmic reticulum. Science 275:1643, 1998 33. Rao-Mirotznik R, Harkins A, Buchsbaum G, Sterling P: Mammalian rod terminal: architecture of a binary synapse. Neuron 14:561, 1995 34. Chun MH, Grunert U, Martin PR, Wässle H: The synaptic complex of cones in the fovea and in the periphery of the
macaque monkey retina. Vision Res 36:3383, 1996 35. Marc RE, Liu W-LS, Kallionatis M et al: Patterns of glutamate immunoreactivity in the goldfish retina. J Neurosci 10:4006–4034, 1990 36. Ayoub GS, Copenhagen DR: Application of a fluorometric method to measure glutamate release from
single retinal photoreceptors. J Neurosci Meth 37:7, 1991 37. Copenhagen DR, Jahr CE: Release of endogenous excitatory amino acids from turtle photoreceptors. Nature 342:536, 1989 38. Lasater EM, Dowling JE: Carp horizontal cells in culture respond selectively to L-glutamate and
its agonists. Proc Natl Acad Sci USA 79:936, 1982 39. Vardi N, Morigiwa K, Wang T-L et al: Neurochemistryof the mammalian cone/synaptic complex. Vision Res 38:1359, 1998 40. Morigiwa K, Vardi N: Differential expression of ionotropic glutamate receptor subunits in the
outer retina. J Comp Neurol 405:173, 1999 41. Vardi N, Morigiwa K: ON cone bipolar cells in rat express the metabotropic receptor mGluR6. Vis Neurosci 14:789, 1997 42. Brandstätter JH, Koulen P, Wässle H: Diversity of glutamate receptors in the glutamate retina. Vision Res 38:1385, 1997 43. Schmitz Y, Witkovsky P: Dependence of photoreceptor glutamate release on a dihydropyridine-sensitive
calcium channel. Neuroscience 48:1209, 1997 44. Schmitz Y, Witkovsky P: Glutamate release by the intact light-responsive potoreceptor layer of
the Xenopus retina. J Neurosci Meth 68:55, 1996 45. Wilkinson MF, Barnes S: The dihydropyridine-sensitive calcium channel subtype in cone photoreceptors. J Gen Physiol 107:621, 1996 46. Taylor WR, Morgans C: Localization and properties of voltage-gated calcium channels in cone photoreceptors
of Tupaia belangeri. Vis Neurosci 15:541, 1998 47. Hollman M, Heinemann S: Cloned glutamate receptors. Ann Rev Neurosci 17:31, 1994 48. Rosenmund C, Stern-Bach Y, Stevens CF: The tetrameric structure of a glutamate receptor channel. Science 280:1596, 1998 49. Pin J-P, Duvoisin R: The metabotropic glutamate receptors: structure and functions. Neuropharmacology 34:1, 1995 50. Qin P, Pourcho RG: Distribution of AMPA-selective glutamate receptor subunits in cat retina. Brain Res 710:303, 1996 51. Nomura A, Shigeomoto R, Nakamura Y et al: Developmentally regulated postsynaptic localiztion of a metabotropic glutamate
receptor in rat rod bipolar cells. Cell 77:361, 1994 52. Vardi N, Duvoisin R, Wu G, Sterling P: Localization of mGluR6 to dendrites of ON bipolar cells in primate retina. J Comp Neurol 423:402, 2000 53. Haverkamp S, Grunert U, Wässle H: The cone pedicle, a complex synapse in the retina. Neuron 27:85, 2000 54. Haverkamp S, Wässle H: Immunocytochemical analysis of the mouse retina. J Comp Neurol 424:1, 2000 55. Braithwaite SP, Meyer G, Henley JM: Interactions between AMPA receptors and intracellular proteins. Neuropharmacology 39:919, 2000 56. Rauen T, Rothstein JD, Wässle H: Differential expression of three glutamate transporter subtypes in the
rat retina. Cell Tiss Res 286:325, 1996 57. Kolb H, Boycott BB, Dowling JE: A second type of midget bipolar cell in
the primate retina. Philos Trans R Soc Lond Biol 255, 1969 58. Kouyama N, Marshak DW: Bipolar cells specific for blue cones in the macaque retina. J Neurosci 12:1233, 1992 59. Jacoby RA, Wiecmann AF, Amara SG et al: Diffuse bipolar cells provide input to OFF parasol ganglion cells in the
macaque retina. J Comp Neurol 416:6, 2000 60. Peichl L, Sandmann D, Boycott BB: Comparative anatomy and function of mammalian
horizontal cells. In Chalpua & Finlay, eds: Development and
Organization of the Retina, pp 147–172. New York, Plenum Press, 1998 61. Peichl L, Gonzales-Soriano J: Morphological types of horizontal cell in rodent retinae: A comparison
of rat, mouse, gerbil and guinea pig. Vis Neurosci 11:501, 1994 62. Boycott BB, Peichl L, Wassle H: Morphological types of horizontal cell in the retina of the domestic cat. Proc Roy Soc B 203:229, 1978 63. Steinberg RH: Rod and cone contributions to S-potentials from the cat retina. Vision Res 9:1319, 1969 64. Raviola E, Gilula NB: Gap junction between photoreceptor cells in the vertebrate retina. Proc Natl Acad Sci USA 70:1677, 1973 65. Werblin FS, Dowling JE: Organization of the retina of the mudpuppy Necturus maculosus: II. Intracellular recording. J Neurophysiol 32:339, 1969 66. Nelson R, Kolb H: Synaptic patterns and response properties of bipolar and ganglion cells
in the cat retina. Vision Res. 23: 1183–1195, 1983 67. Dacheux RF, Raviola E: The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine
cell. J Neurosci 6:331, 1986 68. Dacey D, Packer OS, Diller L et al: Center surround receptive field structure of cone bipolar cells in primate
retina. Vision Res 40:1801, 2000 69. Nawy S, Jahr CE: Suppression by glutamate of cGMP-activated conductance in retina bipolar
cells. Nature 346:269, 1991 70. Marc RE, Stell WK, Bok D, Lam DM-K: GABAergic pathways in the goldfish retina. J Comp Neurol 182:221, 1978 71. Baylor DA, Fuortes MGF, O'Bryan PM: Receptive fields of cones in the retina of the turtle. J Physiol Lond 214:265, 1971 72. Schiller PH, Sandell JH, Maunsell JHR: Functions of the ON and OFF channels of the visual system. Nture 322:824, 1986 73. Slaughter MM, Miller RF: 2-Amino-4-phosphonobutyric acid: A new pharmacological tool for retina
research. Science 211:182, 1981 74. Kolb H: Organization of the outer plexiform layer of the primate retina: Electron
microscopy of Golgi-impregnated cells. Philos Trans R Soc 258:261, 1970 75. Famiglietti EV Jr, Kolb H: Structural basis for on- andoff-center responses in retinal ganglion cells. Science 194:193, 1976 76. Euler T, Wässle H: Immunocytochemical identification of cone bipolar cells in the rat retina. J Comp Neurol 361:461, 1995 77. Kolb H: Amacrine cells of the mammalian retina: neurocircuitry and functional roles. Eye 11:904, 1997 78. Jeon C-J, Strettoi E, Masland RH: The major cell populations of the mouse retina. J Neurosci 18:8936, 1998 79. MacNeil MA, Heussy JK, Dacheux RF et al: The shapes and numbers of amacrine cells: matching of photofilled with
Golgi-stained cells in the rabbit retina and comparison with other mammalian
species. J Comp Neurol 413:305, 1999 80. Dacey DM: Axon-bearing amacrine cells of the macaque monkey retina. J Comp Neurol 284:275, 1989 81. Haverkamp S, Wässle H: Immunocytochemical analysis of the mouse retina. J Comp Neurol 424:1, 2000 82. Wässle H, Boycott BB: Functional architecture of the mammalian retina. Physiol Rev 71:447, 1991 83. Ehinger B: Neurotransmitter systems in the retina. Retina 2:305, 1982 84. Ammermuller J, Kolb H: The organization of the turtle inner retina. I. ON- and OFF-center pathways. J Comp Neurol 358:1, 1995 85. Gallego A: Horizontal and amacrine cells in the mammal's retina. Vision Res (Suppl) 3:33, 1971 86. Dowling JE, Ehinger B: Synaptic organization of the amine-containing interplexiform cells of the
goldfish and Cebus monkey retina. Science 188:270, 1975 87. Versaux-Botter C, Ngyuen-Legros J, Vigny A, Raoux N: Morphology, density and distribution of tyrosinehydroxylase-like immunoreacive
cells in the retina of mice. Brain Res 301:192, 1984 88. Nakamura Y, McGuire BA, Sterling P: Interplexiform cell in cat retina: Identification by uptake of γ-[3H]aminobutyric acid and serial reconstruction. Proc Natl Acad Sci USA 77:658, 1980 89. Kolb H, Cuenca N, Wang HH, DeKorver L: The synaptic organization of the dopaminergic amacrine cell in the cat
retina. J Neurocytol 19:343, 1990 90. Dowling JE, Boycott BB: Organization of the primate retina: Electron microscopy. Proc R Soc Lond Biol 166:80, 1966 91. Kolb H, DeKorver L: Midget ganglion cells of the parafovea of the human retina: a study by
electron microscopy and serial section reconstructions. J Comp Neurol 303:617, 1991 92. Allen RA: The retinal bipolar cells and their synapses in the inner plexiform
layer. In Straatsma BR, Hall MO, Allen RA, Crescitelli F, eds: The
Retina: Morphology, Functional and Clinical Characteristics, vol. 3, pp 101–143. Los
Angeles, University of California Press, 1969 93. Yazulla S: GABAergic mechanisms in the retina. Prog Retina Res 5:1, 1986 94. Poucho R, Goebel DJ: A combined Golgi and autoradiographic study of (3H)-glycine-accumulating amacrine cells in the cat retina. J Comp Neurol 233:473, 1985 95. Kaneko A, Tachibana M: GABA mediates the negative feedback from amacrine to bipolar cells. Neurosci Res (Suppl) 6:S239, 1987 96. Tauck DL, Frosch MP, Lipton SA: Characterization of GABA- and glycine-induced currents of solitary rodent
retinal ganglion cells in culture. Neuroscience 27:193, 1988 97. Merigan WH, Maunsell JHR: How parallel are the primate visual pathways? Annu Rev Neurosci 16:369, 1993 98. Moore RY, Speh JC, Card JP: The retino-hypothalamic tract originates from a distinct subset of retinal
ganglion cells. J Comp Neurol 352:351, 1995 99. Hartline HK: The response of single optic nerve fibers of the vertebrate eye to illumination
of the retina. Am J Physiol 121:400, 1938 100. Kuffler SW: Discharge patterns and functional organization of mammalian retina. J Neurophysiol 16:37, 1953 101. Nelson R, Famiglietti EV Jr, Kolb H: Intracellular staining reveals different levels of stratification for on- and
off-center ganglion cells in cat retina. J Neurophysiol 41:472, 1978 102. Naka K: Functional organization of catfish retina. J Neurophysiol 40:26, 1977 103. Enroth-Cugell C, Robson JG: The contrast sensitivity of retinal ganglion cells of the cat. J Physiol 187:517, 1966 104. Stone J, Hoffman K-P: Very slow conduction ganglion cells in the cat's retina: A major new
functional type? Brain Res 43:610, 1972 105. Boycott BB, Wässle H: The morphological types of ganglion cells of the domestic cat's retina. J Physiol Lond 240:397, 1974 106. Levick WR: Receptive fields of retinal ganglion cells. In Fuortes MGF, ed: Handbook
of Sensory Physiology, vol Vll/2, pp 531–566. Berlin, Springer-Verlag, 1972 107. Nathans J, Thomas D, Hogness DS: Molecular genetics of human color vision: the genes encoding blue, green
and red pigments. Science 232:193, 1986 108. Baylor DA, Nunn BJ, Schnapf JL: Spectral sensitivity of cones of the monkey Macaca fascicularis. J Physiol Lond 390:145, 1987 109. Bowmaker JK, Dartnall HJA, Mollon JD: Microscopectrophotometric demonstration of four classes of photoreceptors
in an old world primate, Macaca fascicularis. J Physiol Lond 298:131, 1980 110. Cicerone CM, Nerger JL: The relative numbers of long-wavelength-sensitive to middle-wavelength-sensitive
cones in the human fovea centralis. Vision Res 29:115, 1989 111. Packer OS, Williams DR, Bensinger DG: Photopigment transmittance imaging of the primate photoreceptor mosaic. J Neurosci 16:2251, 1996 112. Marc RE, Sperling H: Chromatic organization of primate cones. Science 196:454, 1977 113. Marc RE: Chromatic pattern of cone photoreceptors. Am J Optom Physiol Opt 54:212. 1977 114. Svaetichin G, MacNichol EF: Retinal mechanisms for chromatic and achromaic vision. Ann NY Acad Sci 74:385, 1958 115. Dacey DM, Lee BB, Stafford DK et al: Horizontal cells of the primate retina: cone specificity without spectral
opponency. Science 271:656, 1996 116. Gouras P: Identification of cone mechanisms in monkey ganglion cells. J Physiol Lond 199:533, 1968 117. Gouras P. Color vision. Prog Retina Res 3:227, 1984 118. Dacey DM, Lee BB: The blue-ON opponent pathway in primate retina originates from a distinct
bistratified ganglion cell type. Nature 367:731, 1994 119. Wyatt HJ, Daw NW: Directionally sensitive ganglion cells in the rabbit: Specificity for stimulus
direction, size and speed. J Neurophysiol 28:613, 1975 120. Famiglietti EV: “Starburst” amacrine cells and cholinergic neurons: mirror-symmetric
ON and OFF amacrine cells of rabbit retina. Brain Res 261:138, 1983 121. Grzywacz NM, Amthor FR, Merwine DK: Necessity of acetylcholine for retinal directionally selective responses
to drifting gratings in rabbit. J Physiol Lond 512:575, 1998 122. Eliasof S, Barnes S, Werblin F: The interaction of ionic currents mediating single spike activity in retinal
amacrine cells of the tiger salamander. J Neurosci 7:3512, 1987 123. Lukasiewicz P, Werblin F: A slowly inactivating potassium current truncates spike activity in ganglion
cells of the tiger salamander retina. J Neurosci 8:4470, 1988 124. Jacoby R, Stafford D, Kouyama N, Marshak D: Synaptic inputs to ON parasol ganglion cells in the primate retina. J Neurosci 16:8041, 1996 125. Awatramani GB, Slaughter MM: Origin of transient and sustained responses in ganglion cells of the retina. J Neurosci 20:7087, 2000 126. Mittman S, Taylor WR, Copenhagen DR: Concomitant activation of two types of glutamate receptor mediates excitation
of salamander retinal ganglion cells. J Physiol 428:275, 1990 127. Schneeweis DM, Schnapf JL: Photovoltage of rods and cones in the macaque retina. Science 268:1053, 1995 128. Kolb H, Famiglietti EV: Rod and cone pathways in the inner plexiform layer of ct retina. Science 186:47, 1974 129. Nelson R, Kolb H: A17: a broad-field amacrine cell of the rod system in the retina of the
cat. J Neurophysiol 54:592, 1989 130. Kolb H, Nelson R: Off-alpha and off-beta ganglion cells in the cat retina. II. Neural circuitry
as revealed by electron microscopy of HRP stains. J Comp Neurol 329:85, 1993 131. Famiglietti EV, Kolb H: A bistratified amacrine cell and synaptic circuitry in the inner plexiform
layer of the retina. Brain Res 84:293, 1975 132. Voigt T, Wässle H: Dopaminergic innervation of AII amacrine cells in mammalian retina. J Neurosci 7:4115, 1987 133. Kaczmarek LK, Levitan I: Neuromodulation. The Biochemical Control of Neuronal
Excitability. New York, Oxford University Press, 1987 134. Thoreson WB, Witkovsky P: Glutamate receptors andcircuits in the vertebrate retina. Prog Retina Eye Res 18:765, 1999 135. Koulen P, Kuhn R, Wassle H, Brandstatter JH: Modulation of the intracellular calcium concentration in photoreceptor
terminals by a presynaptic metabotropic glutamate receptor. Proc Natl Acad Sci USA 96:9909, 1999 136. Witkovsky P, Schutte M: The organization of doaminergic neurons in vertebrate retinas. Vis Neurosci 7:113, 1991 137. Witkovsky P, Dearry A: Functional roles of dopamine in the vertebrate retina. Prog Retina Res 11:247, 1991 138. Witkovsky P, Nicholson C, Rice ME et al: Extracellular dopamine concentration in the retina of the clawed frog, Xenopus laevis. Proc Natl Acad Sci USA 90:5667, 1993 139. Bjelke B, Goldstein M, Tinner B et al: Dopaminergic transmission in the rat retina: evidence for volume transmission. J Chem Neuroanat 12:37, 1996 140. Iuvone PM: Regulation of retinal dopamine biosynthesis and tyrosine hydroxylase activity
by light. Fed Proc 43:2709, 1984 141. Haycock JW, Haycock DA: Tyrosine hydroxylase in rat dopaminergic nerve terminals: multiple phosphorylation
in vivo and in synaptosomes. J Biol Chem 266:5650, 1991 142. Witkovsky P, Gabriel R, Haycock JW, Meller E: Ifluence of light and neural circuitry on tyrosine hydroxylase phosphorylation
in the rat retina. J Chem Neuroanat 19:105, 2000 143. Gustincich S, Feigenspan A, Wu DK et al: Control of dopamine release in the retina: a transgenic approach to neural
networks. Neuron 18:723, 1997 144. Iuvone PM, Galli CL, Garrison-Gund CK, Neff NH: Light stimulates tyrosine hydroxylase activity and dopamine synthesis in
retinal amacrine neurons. Science 202:901, 1978 145. Knapp AG, Dowling JE: Dopamine enhances excitatory amino acid-gated conductances in cultured
retinal horizontal cells. Nature 325:437, 1987 146. Liu Y, Lasater EM: Calcium currents in turtle retinal ganglion cells II. Dopamine modulation
via a cyclic AMP-dependent mechanism. J Neurophysiol 71:743, 1994 147. Cahill GM, Besharse JC: Resetting the circadian clock in cultured Xenopus eyecups: regulation of
retinal melatonin rhythms by light and D2 dopamine receptors. J Neurosci 11:2959, 1991 148. Besharse JC, Dunis DA. Methoxyindoles and photoreceptor metabolism: activation of rod shedding. Science 219:1341, 1983 149. Stone RA, Lin T, Laties AM, Iuvone PM: Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci USA 86:704, 1989 150. Vaney DI: Patterns of neuronal coupling in the retina. Prog Retina Eye Res 13:301, 1994 151. Mills SL, Massey SC: Differential properties of two gap junctional pathways made by AII amacrine
cells. Nature 377:734, 1995 152. Lasater EM, Dowling JE: Dopamine decreases conductance of the electrical junctions between cultured
horizontal cells. Proc Natl Acad Sci USA 82:3025, 1985 153. Piccolino M, Neyton J, Gerschenfeld HM: Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3':5' monophosphate in horizontal cells of turtle
retina. J Neurosci 4:2477, 1984 154. deVries SH, Schwartz EA: Modulation of an electrical synapse between solitary pairs of catfish horizontal
cells by dopamine and second messengers. J Physiol 414:351, 1989 155. Lu C, McMahon DG: Moulation of retinal gap junction channel gating by nitric oxide. J Physiol Lond 499:689, 1997 156. Krizaj D, Gabriel R, Owen WG, Witkovsky P: Dopamine D2 receptor-mediated modulation of rod-cone coupling in the Xenopus retina. J Comp Neurol 398:529, 1998 157. Weiler R, He S, Vaney DI: Retinoic acid modulates gap junctional permeability between horizontal
cellsof the mammalian retina. Eur J Neurosci 11:3346, 1999 158. Newman EA: Inward-rectifying potassium channels in retinal glial (Muller) cells. J Neurosci 13:3333, 1993 159. Barbour B, Brew H, Attwell D: Electrogenic uptake of glutamate and asparte into glial cells isolated
from the salamander (Ambystoma) retina. J Physiol Lond 436:169, 1991 160. Riepe RE, Norenberg MD: Muller cell localization of glutamine synthetase in rat retina. Nature 268:654, 1977 161. Puro DG, Uyan JP, Sucher NJ: Activation of NMDA receptor-channels in human retinal Muller glial cells
inhib-its inward-rectifying potassium currents. Vis Neurosci 13:319, 1996 162. Puro DG: Growth factors and Muller cells. Prog Retina Eye Res 15:89, 1995 163. Newman EA: High potassium conductance in astrocyteendfeet. Science 233:453, 1986 164. Newman EA, Zahs KR: Modulation of neuronal activity by glial cells in the retina. J Neurosci 18:4022, 1998 165. Tosini G, Menaker M: Circadian rhythms in cultured mammalian retina. Science 272:419, 1996 166. Cahill GM, Grace MS, Besharse JC: Rhythmic regulation of retinal melatonin: metabolic pathways, neurochemical
mechanism, and the ocular circadian clock. Cell Mol Neurobiol 11:529, 1991 |