1. Sprafka JM, Fritsche TL, Baker R et al: Prevalence of undiagnosed eye disease in high-risk diabetic individuals. Arch Intern Med 150:857, 1990 2. Javitt JC, Canner JK, Frank RG et al: Detecting and treating retinopathy in patients with type I diabetes mellitus: a
health policy model. Ophthalmology 97:483, 1990 3. Harris MI, Hadden WC, Knowler WC et al: Prevalence of diabetes and impaired glucose tolerance and plasma glucose
levels in U.S. population aged 20–74 yr. Diabetes 36:523, 1987 4. Diabetic Retinopathy Study Research Group: Photocoagulation treatment of
proliferative diabetic retinopathy: Clinical application of Diabetic
Retinopathy Study (DRS) findings, DRS report number 8. Ophthalmology 88:583, 1981 5. Marshall G, Garg SK, Jackson WE et al: Factors influencing the onset and progression of diabetic retinopathy in
subjects with insulin-dependent diabetes mellitus. Ophthalmology 100:1133, 1993 6. Kostraba JN, Klein R, Dorman JS et al: The epidemiology of diabetes complications study: IV. Correlates of diabetic
background and proliferative retinopathy. Am J Epidemiol 133:381, 1991 7. Frank RN: On the pathogenesis of diabetic retinopathy: a 1990 update. Ophthalmology 98:586, 1991 8. Kinoshita JH: Aldose reductase in the diabetic eye: XLIII Edward Jackson Memorial Lecture. Am J Ophthalmol 102:685, 1986 9. Sorbinil Retinopathy Trial Research Group: A randomized trial of sorbinil, an
aldose reductase inhibitor, in diabetic retinopathy. Arch Ophthalmol 108:1234, 1990 10. Goldstein DE, Blinder KJ, Ide CH et al: Glycemic control and development of retinopathy in youth-onset insulin-dependent
diabetes mellitus: results of a 12-year longitudinal study. Ophthalmology 100:1125, 1993 11. Azar DT, Spurr-Michaud SJ, Tisdale AS et al: Altered epithelial-basement membrane interactions in diabetic corneas. Arch Ophthalmol 110:537, 1992 12. Schultz RO, Peters MA, Sobocinski K et al: Diabetic keratopathy as a manifestation of peripheral neuropathy. Am J Ophthalmol 96:368, 1983 13. Hyndiuk RA, Kazarian EL, Schultz RO et al: Neurotrophic corneal ulcers in diabetes mellitus. Arch Ophthalmol 95:2193, 1977 14. Ishida N, Rao GN, del Cerro M et al: Corneal nerve alterations in diabetes mellitus. Arch Ophthalmol 102:1380, 1984 15. Tsubota K, Yamada M: The effect of aldose reductase inhibitor on the corneal epithelium. Cornea 12:161, 1993 16. Henkind P, Wise GN: Descemet's wrinkles in diabetes. Am J Ophthalmol 51:371, 1961 17. Eva PR, Pascoe PT, Vaughn DG: Refractive change in hyperglycemia: hyperopia, not myopia. Br J Ophthalmol 66:500, 1982 18. Yanoff M: Ocular pathology of diabetes mellitus. Am J Ophthalmol 67:21, 1969 19. Smith ME, Glickman P: Diabetic vacuolation of the iris pigment epithelium. Am J Ophthalmol 79:875, 1975 20. Kincaid MC, Green WR, Fine SL et al: An ocular clinicopathologic correlative study of six patients from the
Diabetic Retinopathy Study. Retina 3:218, 1983 21. Gartner S, Henkind P: Neovascularization of the iris (rubeosis iridis). Surv Ophthalmol 22:291, 1978 22. Blinder KJ, Friedman SM, Mames RN: Diabetic iris neovascularization. Am J Ophthalmol 120:393, 1995 23. John T, Sassani JW, Eagle RC Jr: The myofibroblastic component of rubeosis iridis. Ophthalmology 90:721, 1983 24. Hidayat AA, Fine BS: Diabetic choroidopathy: light and electron microscopic observations of
seven cases. Ophthalmology 92:512, 1985 25. D'Amore PA: Mechanisms of retinal and choroidal neovascularization. Invest Ophthalmol Vis Sci 35:3974, 1994 26. Little HL: Alterations in blood elements in the pathogenesis of diabetic retinopathy. Ophthalmology 88:647, 1981 27. Engerman RL, Kern TS: Progression of incipient diabetic retinopathy during good glycemic control. Diabetes 36:808, 1987 28. Petersen MR, Vine AK, Committee T: Progression of diabetic retinopathy after pancreas transplantation. Ophthalmology 97:496, 1990 29. Friberg TR, Tzakis AG, Carroll PB et al: Visual improvement after long-term success of pancreatic transplantation. Am J Ophthalmol 110:564, 1990 30. The Diabetes Control and Complications Trial Research Group: Progression
of retinopathy with intensive versus conventional treatment in the Diabetes
Control and Complications Trial. Ophthalmology 102:647, 1995 31. Klein R, Klein BEK, Moss SE et al: The Wisconsin Epidemiologic Study of Diabetic Retinopathy: II. Prevalence
and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol 102:520, 1984 32. Klein R, Klein BEK, Moss SE et al: The Wisconsin Epidemiologic Study of Diabetic Retinopathy: III. Prevalence
and risk of diabetic retinopathy when age at diagnosis is 30 or more
years. Arch Ophthalmol 102:527, 1984 33. Murphy RP, Nanda M, Plotnick L et al: The relationship of puberty to diabetic retinopathy. Arch Ophthalmol 108:215, 1990 34. Faria de Abreu JR, Silva R, Cunha-Vaz JG: The blood-retinal barrier in diabetes during puberty. Arch Ophthalmol 112:1334, 1994 35. Bresnick GH, Davis MD, Myers FL et al: Clinicopathologic correlations in diabetic retinopathy: II. Clinical and
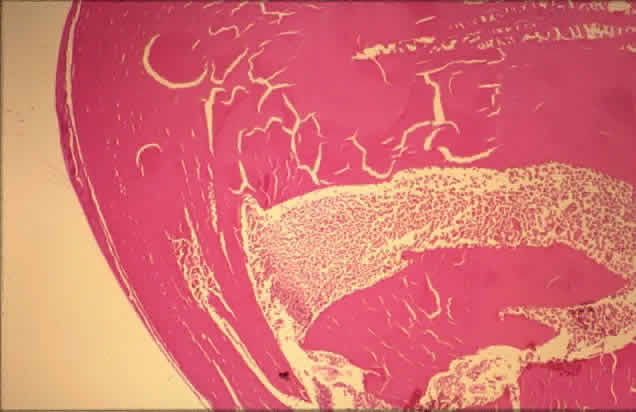
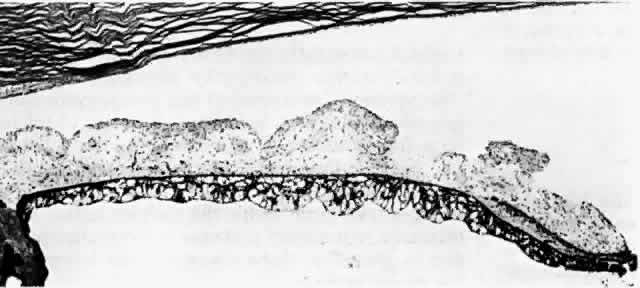
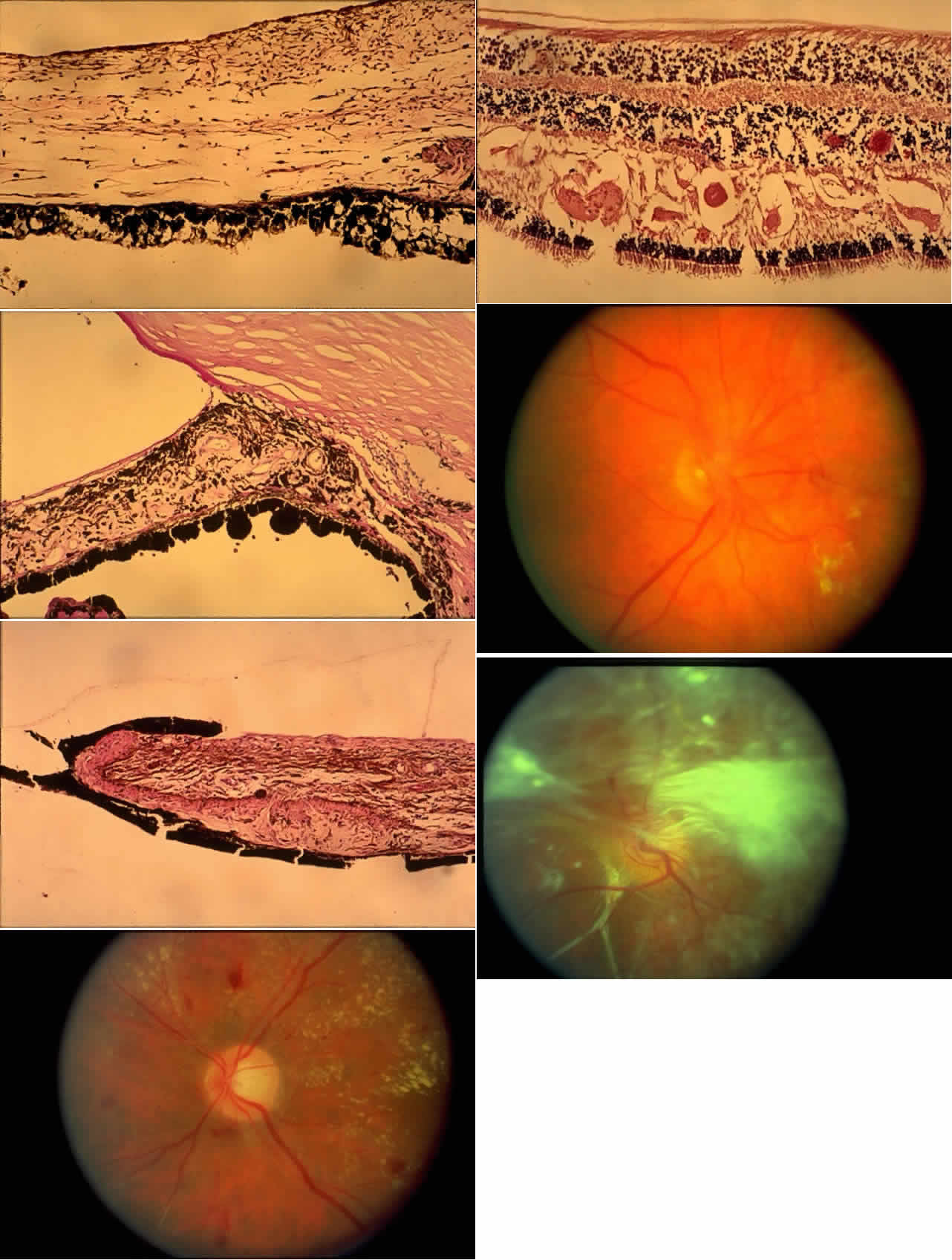
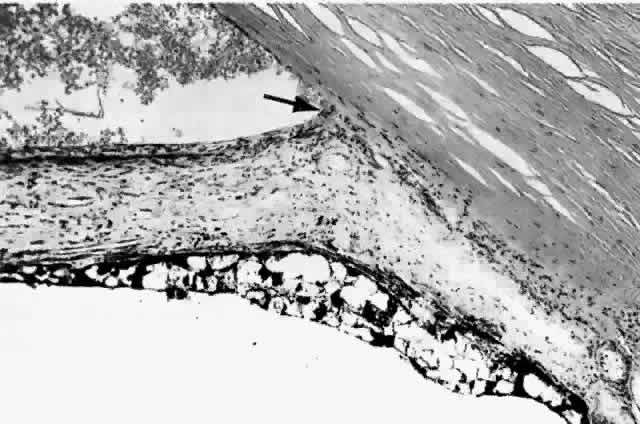
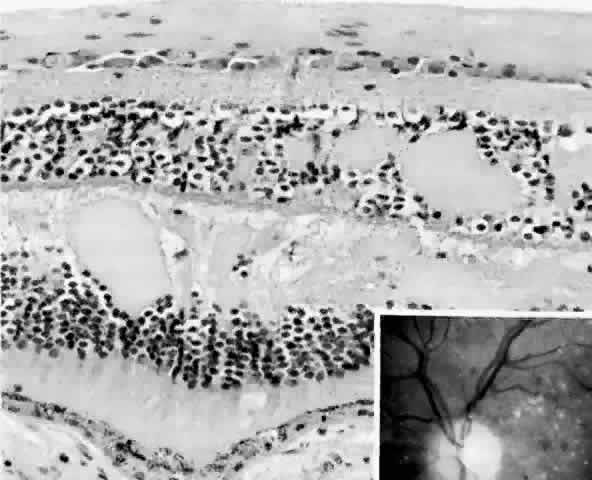
histologic appearances of retinal capillary microaneurysms. Arch Ophthalmol 95:1215, 1977 36. de Venecia G, Davis M, Engerman R: Clinicopathologic correlations in diabetic retinopathy: I. Histology and
fluorescein angiography of microaneurysms. Arch Ophthalmol 94:1766, 1976 37. Akagi Y, Kador PF, Kuwabara T et al: Aldose reductase localization in human retinal mural cells. Invest Ophthalmol Vis Sci 24:1516, 1983 38. Cunha-Vaz J: The blood-ocular barriers. Surv Ophthalmol 23:279, 1979 39. Wallow IHL, Geldner PS: Endothelial fenestrae in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci 19:1176, 1980 40. Reeser F, Fleischman J, Williams GA et al: Efficacy of argon laser photocoagulation in the treatment of circinate
diabetic retinopathy. Am J Ophthalmol 92:762, 1981 41. Fine BS, Brucker AJ: Macular edema and cystoid macular edema. Am J Ophthalmol 92:466, 1981 42. Frangieh GT, Green WR, Engel HM: A histopathologic study of macular cysts and holes. Retina 1:311, 1981 43. Bresnick GH, Engerman R, Davis MD et al: Patterns of ischemia in diabetic
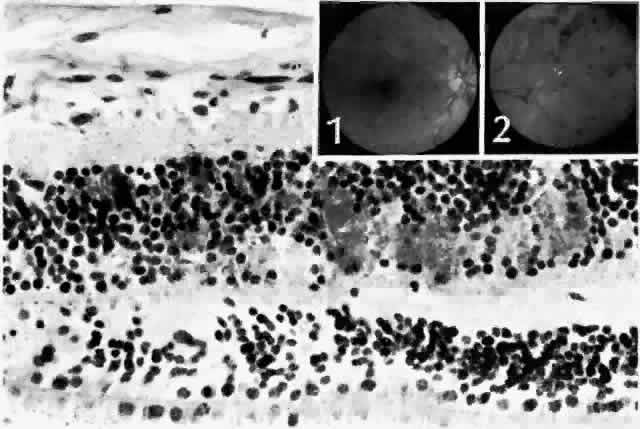
retinopathy. Trans Am Acad Ophthalmol Otolaryngol 81:OP-694, 1976 44. Wolter JR: Axonal enlargements in the nerve-fiber layer of the human retina. Am J Ophthalmol 65:1, 1968 45. Mansour AM, Schachat A, Bodiford G et al: Foveal avascular zone in diabetes mellitus. Retina 13:125, 1993 46. Shimizu K, Kobayashi Y, Muraoka K: Midperipheral fundus involvement in diabetic retinopathy. Ophthalmology 88:601, 1981 47. Bowers DK, Finkelstein D, Wolff SM et al: Branch retinal vein occlusion: A clinicopathologic case report. Retina 7:252, 1987 48. O'Hare JA: Proliferative retinopathy and nephropathy at presentation in young insulin
dependent diabetics. Br Med J 300:579, 1990 49. Wiedemann P: Growth factors in retinal diseases: Proliferative vitreoretinopathy, proliferative
diabetic retinopathy, and retinal degeneration. Surv Ophthalmol 36:373, 1992 50. Malecaze F, Clamens S, Simorre-Pinatel V et al: Detection of vascular endothelial growth factor messenger RNA and vascular
endothelial growth factor-like activity in proliferative diabetic
retinopathy. Arch Ophthalmol 112:1476, 1994 51. Adamis AP, Miller JW, Bernal M-T et al: Increased vascular endothelial growth factor levels in the vitreous of
eyes with proliferative diabetic retinopathy. Am J Ophthalmol 118:445, 1994 52. Jampol LM, Goldbaum MH: Peripheral proliferative retinopathies. Surv Ophthalmol 25:1, 1980 53. Williams JM Sr, de Juan E Jr, Machemer R: Ultrastructural characteristics of new vessels in proliferative diabetic
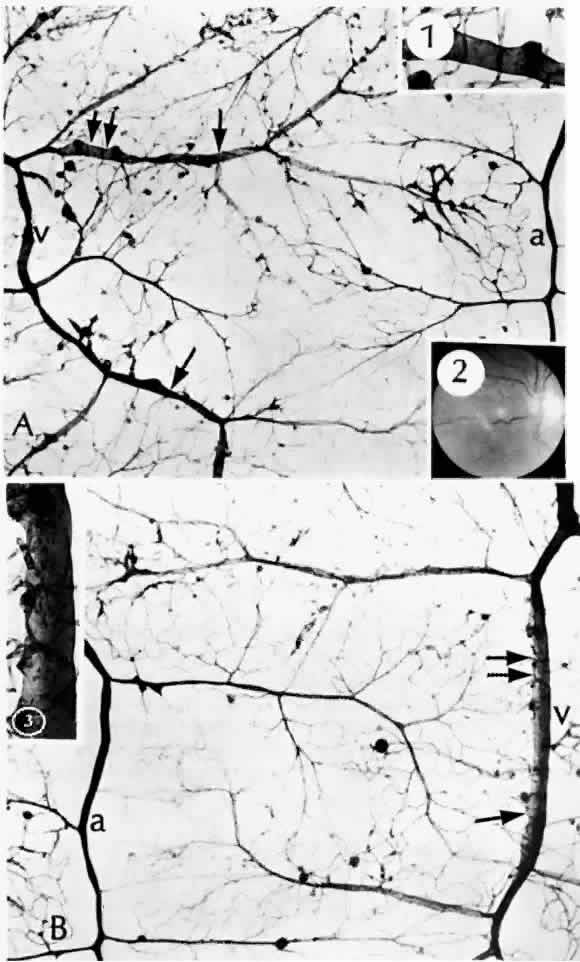
retinopathy. Am J Ophthalmol 105:491, 1988 54. Wallow IHL, Greaser ML, Stevens TS: Actin filaments in diabetic fibrovascular preretinal membrane. Arch Ophthalmol 99:2175, 1981 55. Hersh PS, Green WR, Thomas JV: Tractional venous loops in diabetic retinopathy. Am J Ophthalmol 92:661, 1981 56. Michael JC, de Venecia G, Bresnick GH: Macular heterotopia in proliferative diabetic retinopathy. Arch Ophthalmol 112:1455, 1994 57. Nork TM, Wallow IHL, Sramek SJ et al: Müller's cell involvement in proliferative diabetic retinopathy. Arch Ophthalmol 105:1424, 1987 58. Hamilton CW, Chandler D, Klintworth GK et al: A transmission and scanning electron microscopic study of surgically excised
preretinal membrane proliferations in diabetes mellitus. Am J Ophthalmol 94:473, 1982 59. The Diabetic Retinopathy Study Research Group: Photocoagulation treatment
of proliferative diabetic retinopathy: the second report of Diabetic
Retinopathy Study findings. Ophthalmology 85:82, 1978 60. Blankenship GW: Fifteen-year argon laser and xenon photocoagulation results of Bascom Palmer
Eye Institute's patients participating in the Diabetic Retinopathy
Study. Ophthalmology 98:125, 1991 61. Swartz M: Histology of macular photocoagulation. Ophthalmology 93:959, 1986 62. Wilson DJ, Green WR: Argon laser panretinal photocoagulation for diabetic retinopathy: scanning
electron microscopy of human choroidal vascular casts. Arch Ophthalmol 105:239, 1987 63. Landers MB, III, Stefansson E, Wolbarsht ML: Panretinal photocoagulation and retinal oxygenation. Retina 2:167, 1982 64. Stefánsson E, Machemer R, de Juan E Jr et al: Retinal oxygenation and laser treatment in patients with diabetic retinopathy. Am J Ophthalmol 113:36, 1992 65. Thomas EL, Apple DJ, Swartz M et al: Histopathology and ultrastructure of krypton and argon laser lesions in
a human retina-choroid. Retina 4:22, 1984 66. Smiddy WE, Fine SL, Green WR et al: Clinicopathologic correlation of krypton red, argon blue-green, and argon
green laser photocoagulation in the human fundus. Retina 4:15, 1984 67. Wilson DJ, Finkelstein D, Quigley HA et al: Macular grid photocoagulation: an experimental study on the primate retina. Arch Ophthalmol 106:100, 1988 68. Ho AC, Maguire AM, Yannuzzi LA et al: Rapidly progressive optic disk neovascularization after diabetic papillopathy. Am J Ophthalmol 120:673, 1995 69. Quigley HA, Miller NR, Green WR: The pattern of optic nerve fiber loss in anterior ischemic optic neuropathy. Am Ophthalmol 100:769, 1985 70. Jabs DA, Miller NR, Green WR: Ischaemic optic neuropathy with painful ophthalmoplegia in diabetes mellitus. Br J Ophthalmol 65:673, 1981 71. Foos RY, Kreiger AE, Forsythe AB et al: Posterior vitreous detachment in diabetic subjects. Ophthalmology 87:122, 1980 72. Foos RY, Kreiger AE, Nofsinger K: Pathologic study following vitrectomy for proliferative diabetic retinopathy. Retina 5:101, 1985 73. Ulbig MRW, Hykin PG, Foss AJE et al: Anterior hyaloidal fibrovascular proliferation after extracapsular cataract
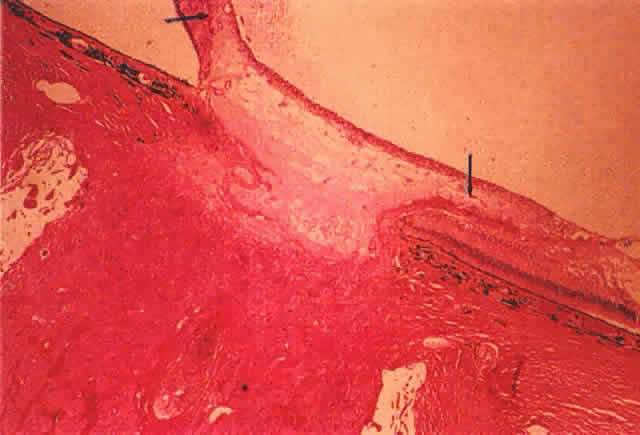
extraction in diabetic eyes. Am J Ophthalmol 115:321, 1993 74. Lewis H, Abrams GW, Foos RY: Clinicopathologic findings in anterior hyaloidal fibrovascular proliferation
after diabetic vitrectomy. Am J Ophthalmol 104:614, 1987 75. Streeten BW: Vitreous asteroid bodies: ultrastructural characteristics and composition. Arch Ophthalmol 100:969, 1982 76. Wasano T, Hirokawa H, Tagawa H et al: Asteroid hyalosis: posterior vitreous detachment and diabetic retinopathy. Ann Ophthalmol 19:255, 1987 |