CT revolutionized the fields of neuro-ophthalmology and orbital diseases by its ability to show many of the lesions responsible for various clinical symptoms and signs. Magnetic resonance imaging (MRI) has surpassed CT in terms of clinical utility in most neuro-ophthalmic applications such as the evaluation of cranial nerve palsies and optic nerve lesions, especially if intracranial extension is suspected. CT remains a quick, clinically effective, and cost-effective method for evaluating most orbital problems. Most orbital lesions are adequately visualized, which is helpful with differential diagnosis or planning a surgical approach to minimize morbidity. The effects on adjacent bone are better appreciated with CT. The strength of being able to evaluate bone also is one of the weaknesses in that visualization of the orbital apex is compromised by the dense bone. Although one may be able to imply certain characteristics of tissue type with CT, MRI is better. Finally, there are certain instances in which CT and MRI are complementary such as with a patient with an ethmoid sinus tumor who also has an opacified frontal sinus. Bony changes around the ethmoid sinus are seen with the CT, but MRI is valuable in differentiating tumor from benign inflammatory sinus disease.
CT traces its beginnings to research initiated by Hounsfield in Great Britain in 1969. Scanning was accomplished by inserting a patient's head intoa latex cap that projected into a plastic water-containing box. The head was scanned by a slitx-ray beam, and the emergent photons were detected by two crystal photomultiplier detectors that moved parallel to and in accurate alignment with the x-ray tube across the patient's head. Data were displayed on an 80 × 80-matrix cathode ray tube in which the brightness of each point was proportional to its absorption coefficient. Initial slices (axial only) were 13-mm thick, although 8-mm slices could be produced. Scanning times ranged from 5 to 10 minutes per slice. Iodinated contrast material was not used.
CT of the head was introduced into clinical practice in the United States in June 1973. Two reports detailing early experience quickly followed.2,3 Orbital applications of this new technique were subsequently developed in 1974.4,5
The early CT scanners (EMI scanners) were specifically designed for scanning the head, and technical difficulties were encountered in obtaining tomographic slices low enough to visualize the entire orbit adequately. Evolutionary changes in technology now permit a 512 × 512 matrix, overlapping slices 1.5 to 3 mm thick, computer software that can reformat images in three dimensions and in other planes, a gantry that angulates, faster data acquisition, and intravenous contrast material. In addition, dynamic scans every 2 to 4 seconds can be taken while intravenous contrast material is being given to assess vascularity of a lesion while ultrafast scanning (five scans per second) permits detailed views of the globe and optic nerve in motion.
Optimal visualization of the orbit requires imaging from at least two planes. Axial slices should be oriented parallel to the optic nerve (-10 degreesto the orbitomeatal line) and no thicker than 3 mm. Axial views, because of volume averaging, may miss lesions located along the floor or roof. Additional views, typically coronal, can be obtained by reformatting data obtained during axial imaging or by direct coronal scanning.
Direct coronal views usually are preferable because of better resolution.6,7 They can be obtained by having patients lie either prone or supine, extending their neck, and angulating the gantry sufficiently to provide coronal imaging while avoiding artifacts from the teeth. Direct coronal scans also should be no thicker than 3 mm.
Coronal views may need to be reformatted from axial scans if a patient has extensive dental fillings, is anesthetized, or cannot extend the neck sufficiently for direct coronal scanning. The resolution on these images can be improved if data are collected from 1.5-mm contiguous axial slides.6 Spiral CT has resulted in improved multiplanar reformation with thin section (1 to 1.5 mm) axial images. High-resolution images necessary for leukocoria or foreign body imaging are obtained with 1-mm axial slices at 1:1 pitch at 1-mm intervals. Ideally, most screening orbit studies are performed at 3-mm direct axial and direct coronal images.
After the orbit has been visualized adequately, it also has been our practice to obtain 10-mm axial slices through the remaining portion of the head to complete the study. Intravenous contrast material usually is given, although the low-density orbital fat produces an inherent high level of contrast for most orbital CT studies. Intravenous administration of iodinated contrast medium is most helpful in detecting intracranial extension of an orbital process or identifying a pathologic process involving the optic nerve/sheath, most notably optic nerve sheath meningioma. Specific contraindications for contrast material include allergy or renal failure.
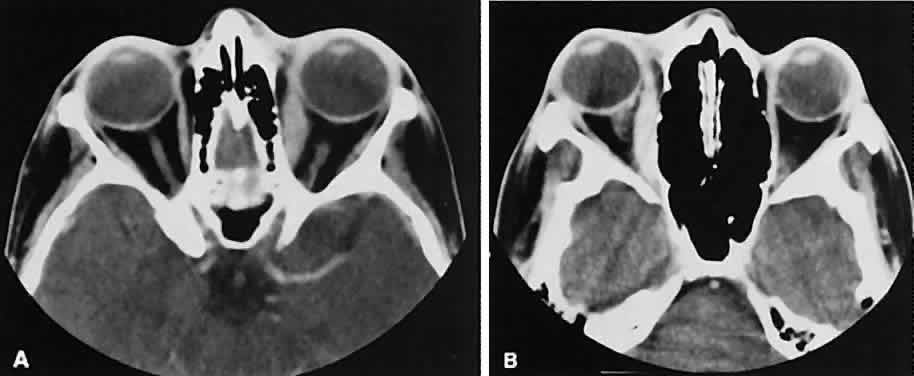
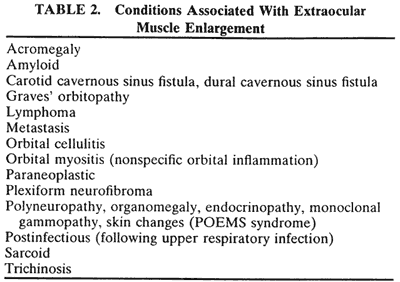
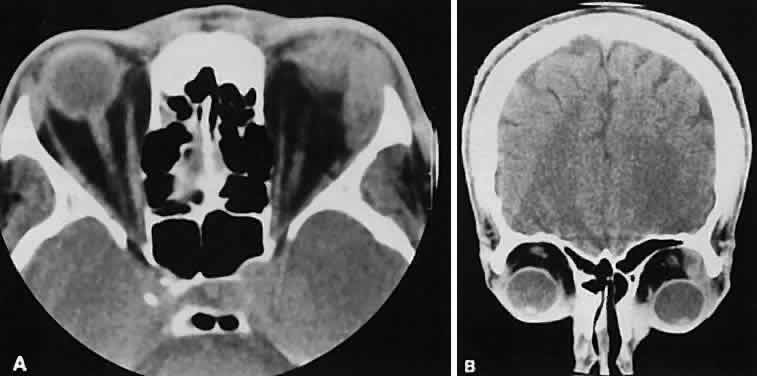
If a patient with severe Graves' orbitopathy, in whom the diagnosis is not in question, requires orbital CT scanning, it generally is performed without intravenous contrast.8 The administered iodine load would interfere with therapy directed at the thyroid gland and generally is not necessary to evaluate muscle enlargement or other features of Graves' orbitopathy.
For proper interpretation, it is not necessary to understand all of the technical aspects of CT scanning, just as it is not necessary to completely understand the inner workings of a personal computer to benefit from it. Familiarity with certain terms and concepts, however, facilitates a more fundamental appreciation for some of the limits of CT scanning.
The CT image consists of a matrix (or grid) of small squares known as pixels. Resolution is increased by increasing the numbers of pixels that are used to create the image. Total pixel number is limited by image contrast necessary to differentiate the various tissue densities in the image. A higher numbered matrix is composed of smaller pixels. A voxel is the 3D counterpart that includes the added dimension of slice thickness.
By altering window setting and level of attenuation on the console, subtle differences in tissuecomposition or bone changes can be appreciated.Window setting refers to the amount of the pixel sampling used by the computer to determine image contrast. Level refers to the scale of density used by the computer to create the image. Both the window setting and level of attenuation are extremely important, for example, when evaluating lesions in the fossa of the lacrimal gland, when the surrounding bone must be examined carefully for either pressure thinning or tumor erosion.
The display console also has the ability to move a cursor to any area on the screen. The computer can then examine this area to determine its den-sity. By convention, these density units are calledHounsfield units. Air and fat have the lowest values, whereas bone has the highest.
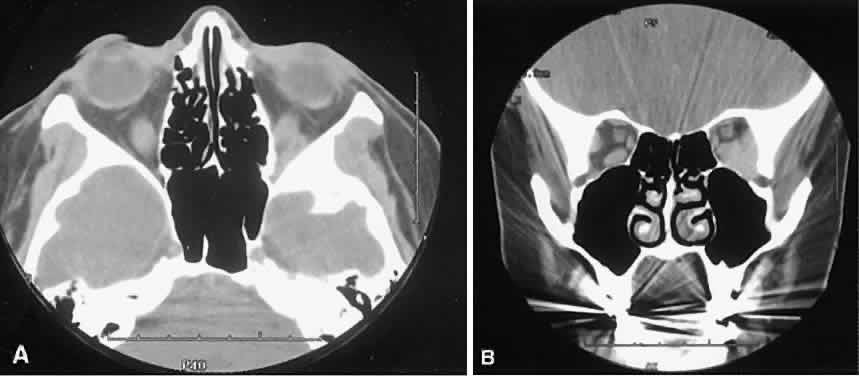
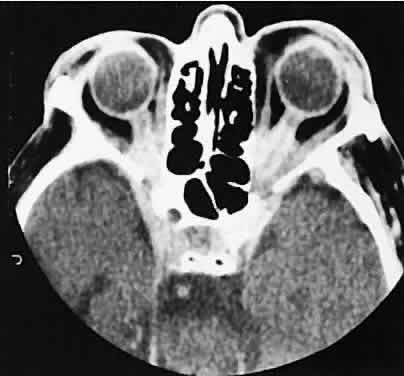
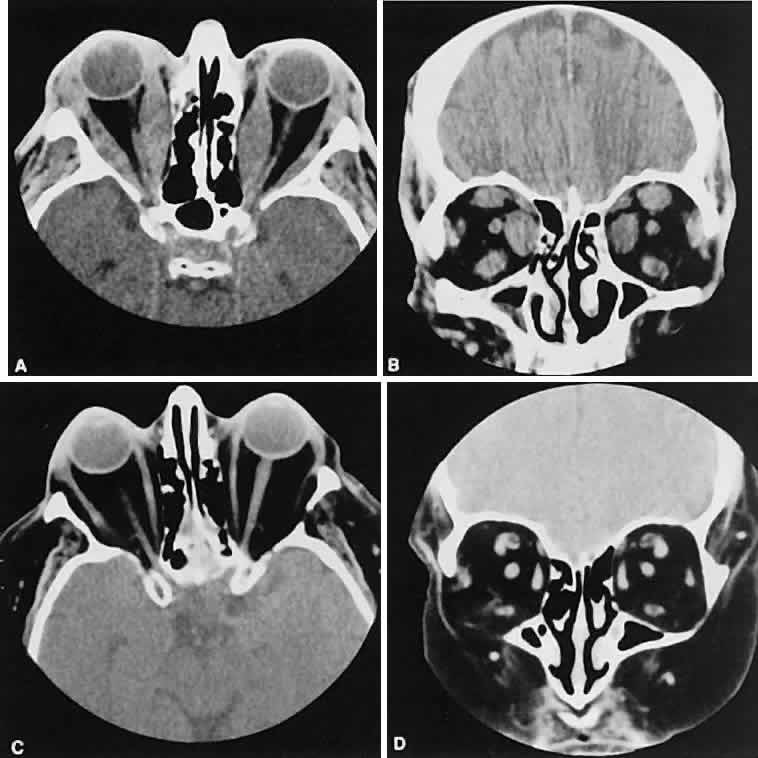
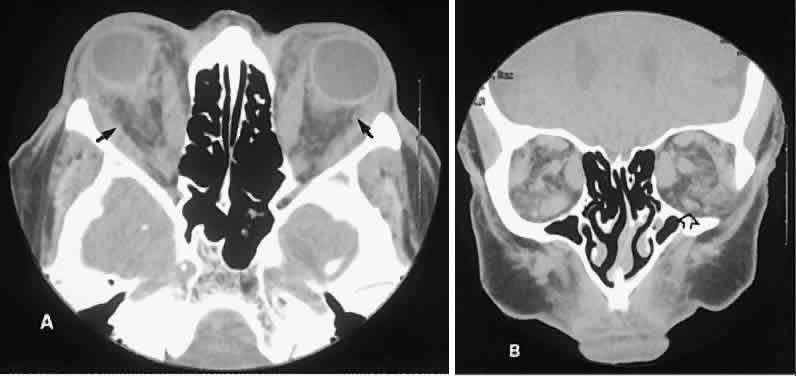
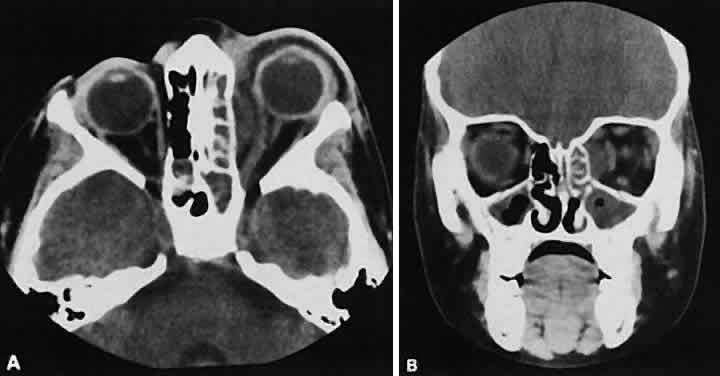
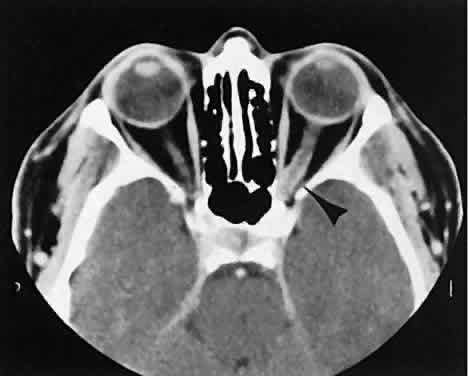
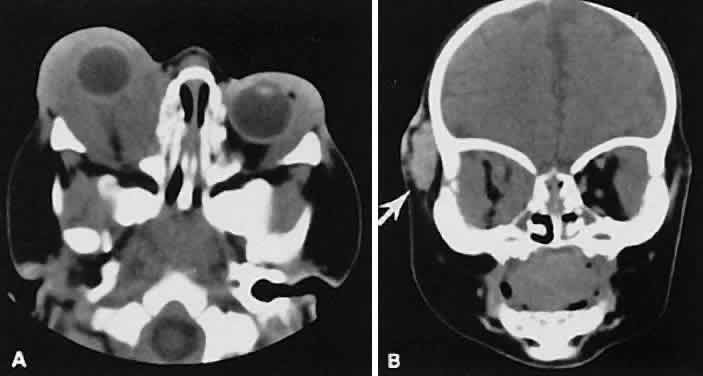
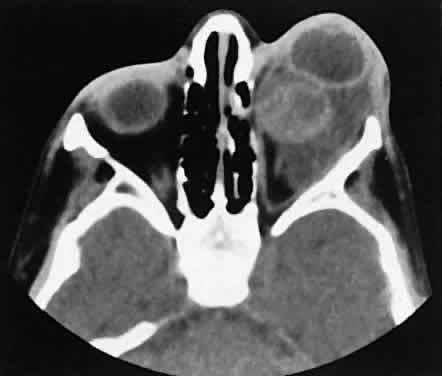
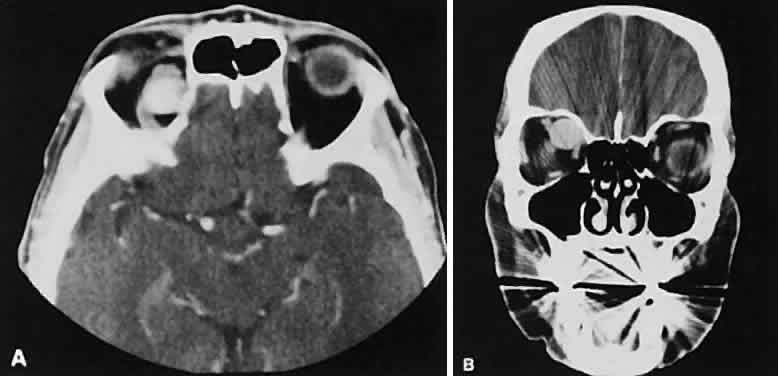
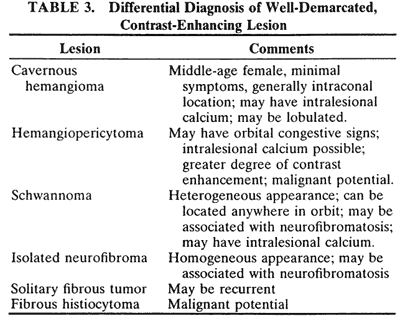
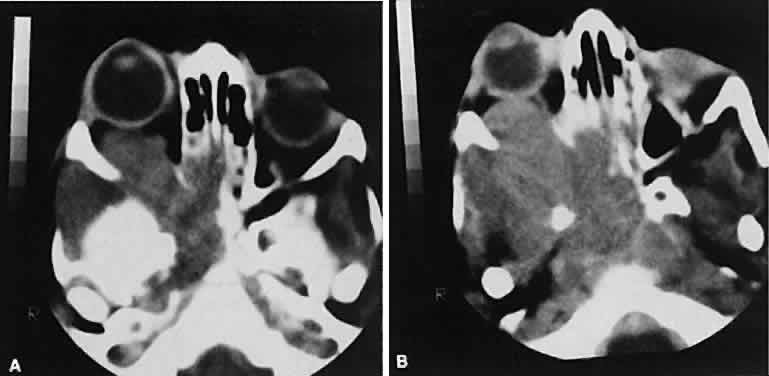
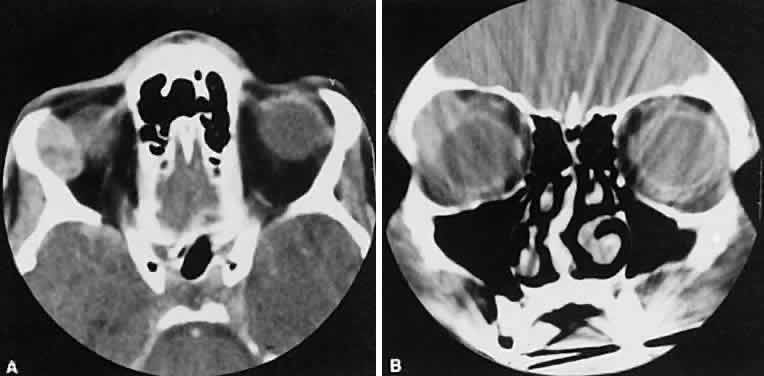
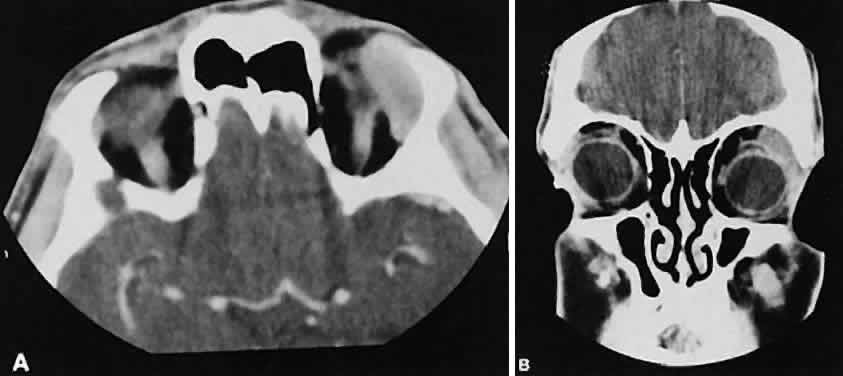
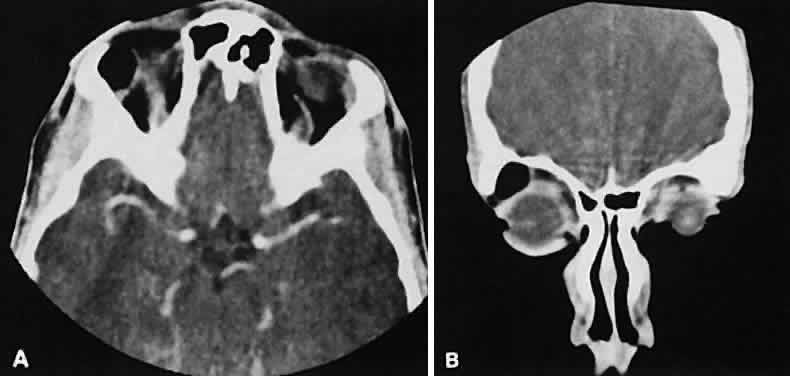
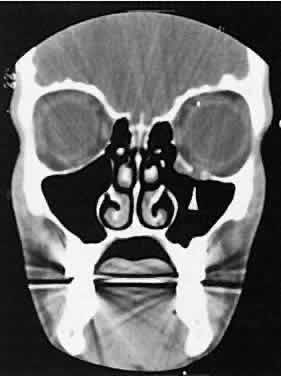
An appreciation for the various pathologic processes that affect the orbit is facilitated by an understanding of the normal orbital anatomy (Fig. 1). The orbit is a pyramid-shaped bony structure bounded inferiorly by the maxillary sinus, medially by the ethmoidal sinus, and superiorly by the frontal sinus. The sphenoidal sinus is situated posteriorly along the medial orbital wall and has a common wall with the optic canal. The lacrimal gland lies within its fossa located in the superior temporal aspect of the orbit and can be seen on both axial and coronal views.
The extraocular muscles (EOMs), with the exception of the inferior oblique, originate from the anulus of Zinn in the orbital apex. The inferior oblique takes its origin from the frontal process of the maxilla and is seen occasionally on CT imaging. The superior oblique, after originating from the anulus, courses along the superior nasal orbital wall just above the medial rectus muscle before passing through the trochlea. The rectus muscles conveniently form a muscle cone, which is sometimes helpful in terms of differential diagnosis. Before thinner axial slices and multiplanar imaging were available, an enlarged inferior rectus muscle often was imaged as an apical mass, especially if dysthy-roid optic neuropathy was present. The importance of imaging from two different planes cannot be overemphasized in this situation.
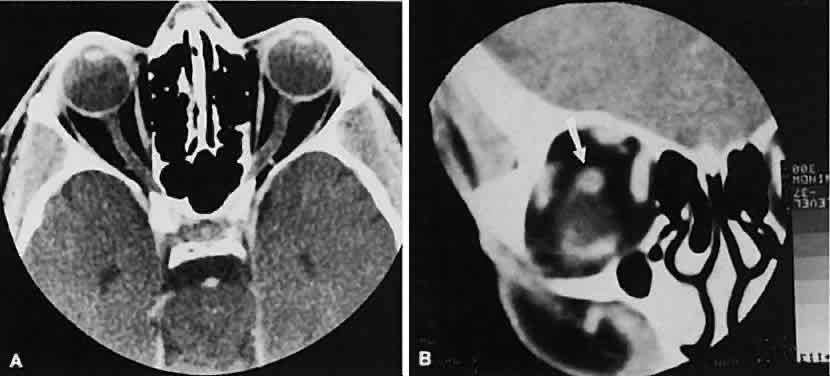
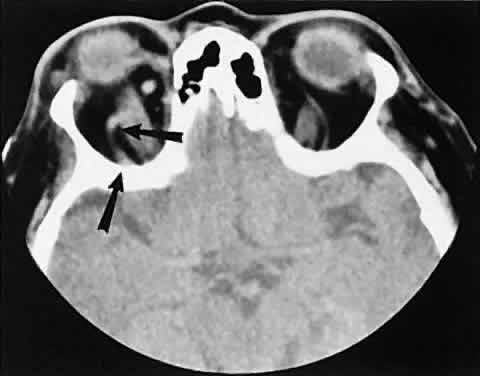
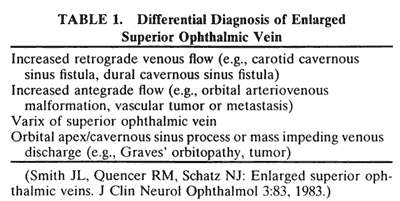
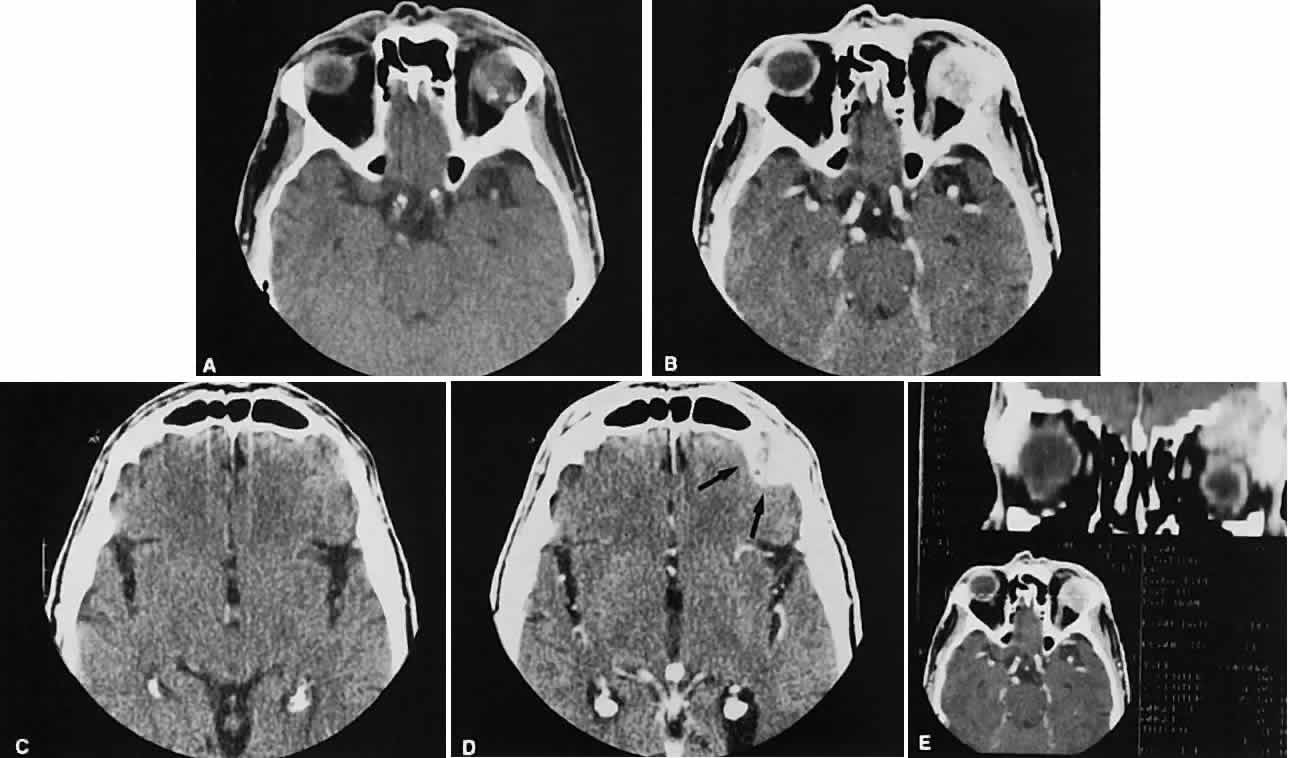
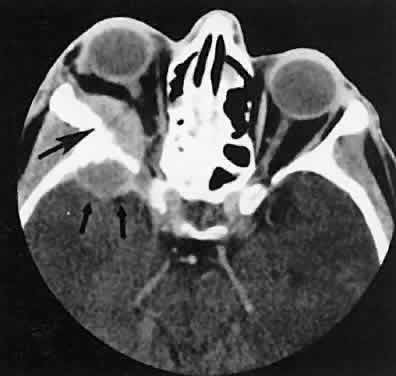
The superior ophthalmic vein (SOV) is an important vascular structure to recognize. It begins in the superior nasal quadrant near the trochlea before coursing posteriorly and laterally beneath the superior rectus muscle, exiting the orbit through the superior orbital fissure. Drainage is into the cavernous sinus. Asymmetric enlargement, especially in the presence of an ipsilateral cavernous sinus enlargement, suggests a vascular anomaly, which may require selective carotid angiography for further definition. Enlargement of one or multiple EOMs in this setting is likely. The SOV also may be enlarged as a result of any process impeding drainage from the orbital apex, such as dysthyroid orbitopathy or metastatic disease.
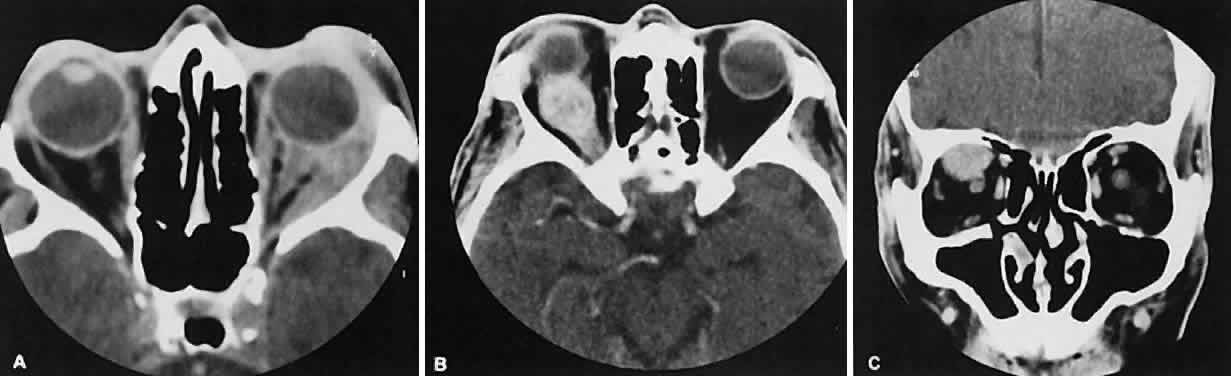
The optic nerve occupies the central intraconal space. By necessity, the nerve has a certain amount of slack, which is necessary to permit movement of the globe. In the axial plane, the optic nerve has an undulating course and thus may appear thicker or thinner as a result of partial volume averaging as it passes in and out of the axial plane. It is imperative to recognize this normal pattern for proper interpretation of axial images.
The optic nerve itself is invested by the same meningeal layers that cover the brain, and the intracranial space may extend along the course of the optic nerve to the back of the globe. Enlargement of this space may be recognized as pseudomeningoceles of the optic nerve sheath. It is sometimes possible to tell whether the nerve, the sheath, or both are enlarged by CT scanning, although MRI affords the better view.