PATHOLOGY
Occlusion of the central retinal vein is probably a result of both local and systemic causes. The actual mechanisms producing the clinical picture of central retinal vein occlusion may be roughly divided into those conditions that produce a physical blockage at the level of the lamina cribrosa, and those conditions in which hemodynamic factors result in an obstruction to the flow of blood. These mechanisms probably coexist in many patients with central retinal vein occlusion.
The pathogenesis of this condition and the underlying histopathology have remained controversial ever since Michel1 first correlated the clinical appearance with the histopathology. The fact that relatively few eyes have been histopathologically examined during the freshly obstructed stage has contributed to the problem. Many of the reported cases have involved eyes that were enucleated because of long-standing neovascular glaucoma; secondary changes that did not play a role in the original occlusion may have occurred in these eyes.
Histopathologic evaluation of eyes removed because of a central retinal vein occlusion demonstrates an occlusion at or just behind the level of the lamina cribrosa.2–7 At this location, certain anatomic factors predispose the central retinal vein to occlusion. First, the lumina of the central retinal artery and central retinal vein are narrower than they are in the orbital optic nerve, and the vessels are bound by a common adventitial sheath.8 Second, the lamina cribrosa is a sievelike, bisecting structure of connective tissue that not only provides support to the optic nerve, but also limits expansion and displacement of the optic nerve and the vessels within it.
In 1878, Michel1 found a thrombus in one patient studied. Later, both Coats2 and Harms9 believed, based on their histopathologic findings, that a primary thrombus within the intraluminal portion of the central retinal vein was the most common cause of occlusion. Verhoeff,3 however, was an early advocate of the concept that endothelial cell proliferation was the primary obstructing mechanism, and he believed that thrombosis within the vein did not occur except in patients with sepsis. He believed that most cases diagnosed as thrombosis were actually dissecting aneurysms because he found the intimal lining forced away from the venous wall by the backup of blood in a tributary vein.
Klein,5,6 who has done extensive clinical and pathologic studies of central retinal vein occlusion, believes that although primary thrombosis may occur, it is rare. She believes that thrombosis may occur more frequently as an end-stage phenomenon, complicating other initiating mechanisms in the obstructive process.
Green and co-workers7 felt that the interval between occlusion and the time of histopathologic study must be considered when interpreting the histopathology of vein occlusion. They studied 29 eyes that were enucleated 6 hours to 10 years after occlusion. As a result of this study, they hypothesized that the flow of blood through the central retinal vein becomes increasingly turbulent as the vein progressively narrows at the lamina cribrosa, where it also may be further impinged on by arteriosclerosis of the adjacent central retinal artery. This turbulence damages the endothelium in the retrolaminar vein, which exposes collagen and initiates platelet aggregation and thrombosis.7,10 Their studies show the evolution of this thrombus. Initially, the thrombus adheres where the endothelium has been severely damaged. Endothelial cell proliferation and recanalization of the vein often occur as a reparative event. Inflammation manifesting itself as phlebitis, periphlebitis, or obliterating endophlebitis is a secondary late-onset factor. Years later, a thick-walled vein with a single channel may occur (phlebosclerosis).7
In some eyes an adjacent, partially obstructed, or narrowed central retinal artery has been observed. This observation is consistent with the prevailing clinical impression that the principal condition associated with retinal vein occlusion is arteriosclerosis. Because the central retinal artery is a true artery, it may be involved in the patchy disease of larger arteries (i.e., atherosclerosis). There is an increased incidence of generalized atheromatous disease in patients who have a central retinal vein occlusion.11,12 As part of this atheromatous change, sclerosis occurs in the common adventitia, which encircles both vessels within the rather rigid support structure of the lamina cribrosa. Compression or constriction of the vein lumen and changes within the vein wall, described as phlebosclerosis, occur. As mentioned, occlusion of the central retinal vein is also influenced by the anatomic confinement of the vein and the artery within the optic nerve, as well as the compactness of the lamina cribrosa and its surrounding connective tissue.
Hayreh and co-workers13–16 have investigated the role of occlusion of the central retinal vein and central retinal artery in an animal model. They attempted to produce central retinal vein occlusion in healthy young monkeys by diathermy of the central retinal vessels in the orbit near their entry into the optic nerve sheath. Their study showed that occlusion in the orbit of the central retinal vein alone produced mildly engorged and tortuous vessels and a few retinal hemorrhages; all these conditions returned to normal in approximately 2 weeks. However, when both the central retinal vein and the central retinal artery in the orbit were obstructed simultaneously, a fundus appearance was produced that was “entirely characteristic” of central retinal vein occlusion.13 Later, histopathologic examination of these eyes showed a hemorrhagic infarct of the inner retinal layer. Hayreh and co-workers15 concluded from these experiments that concomitant arterial occlusion is essential in the production of an ischemic central retinal vein occlusion, although its occurrence is possibly only transient,16 and that the site of occlusion is important in determining both the severity and type of occlusion.16 However, this model of occlusion in the orbit of healthy young monkeys may not be comparable to the situation in the aging human, where the occlusion is located at or just posterior to the lamina cribrosa.379
Because fluorescein angiography does not typically show prolongation of arterial filling in central retinal vein occlusion, Fujino and associates17 investigated the role of arterial occlusion by producing central retinal vein occlusion in monkeys using an intravenous injection of neoprene. They were able to show that a primary and complete occlusion of the central retinal vein at the disc produces a secondary artery insufficiency. The ophthalmoscopic appearance produced in monkeys, however, is not identical to the appearance of central retinal vein occlusion in humans. This may be because this technique obstructs all the branch retinal vessels in the peripapillary region, which, in turn, may preclude collateralization.16
McLeod18 noted that in eyes with both a central retinal vein occlusion and a cilioretinal artery occlusion, there was a lack of retinal hemorrhages within the area of retina that was infarcted. He presented this an argument against the combined artery and vein occlusion hypothesis of Hayreh and colleagues.13–16 If an artery occlusion as well as a vein occlusion (combined occlusion) is necessary to produce the typical ophthalmoscopic picture of a central retinal vein occlusion, the retina should exhibit increased hemorrhage in the area supplied by the occluded cilioretinal artery.
The histopathologic picture in venous occlusion is now considerably clearer as a result of a series of experiments on branch retinal vein occlusion in the monkey.19–21 This work shows that capillary nonperfusion (ischemia) can result after isolated venous outflow occlusion without the occurrence of primary arterial inflow occlusion (ischemic capillaropathy).22 Although these experiments were performed on branch retinal vein occlusions, there is every reason to believe that the ischemia of the retina seen in central retinal vein occlusion can result from venous outflow disease alone.23
Doppler ultrasound imaging has been used to examine the blood flow in the orbit, including the optic nerve head,24,25 and has been used to examine patients with central retinal vein occlusion.26–28,256,257 As might be expected, the venous velocity in the eye of a patient with central retinal vein occlusion is markedly reduced compared either with the unaffected eye or to control eyes.24,25 There is evidence, however, that the central retinal artery blood flow is also impaired in eyes with acute central retinal vein occlusion.28 In addition, vascular resistance is slightly higher in the ophthalmic artery and short posterior ciliary arteries of both the involved and the clinically healthy fellow eye of patients with central retinal vein occlusion compared with control eyes.28 There is also a trend toward higher vascular resistance of the central retinal artery in the clinically healthy eyes of patients with central retinal vein occlusion compared with control eyes.28
The retinal pathology in an ischemic central retinal vein occlusion consists of a hemorrhagic infarction of the retina that affects primarily the inner retinal layers.29 Neovascularization of the iris and anterior chamber angle can develop; less frequently, retinal neovascularization can also occur.10 This neovascularization is likely related to the unregulated expression of vascular endothelial growth factor (VEGF) in the cells of the neurosensory retinal when affected by the hypoxia in central retinal vein occlusion.329 Later changes include thickening of the retina and reactive gliosis.30
ETIOLOGY
The precise etiology of central retinal vein occlusion is not entirely clear. There are now some clues as to the conditions associated with this condition. Many published articles have reported on the association between central retinal vein occlusion and some other condition, whether systemic or ocular. Although some of these associated conditions probably are, in some cases, related to central retinal vein occlusion, there is no way to determine in most cases whether the association is only coincidental on the basis of single-case reports.
Any study that attempts to determine either the etiology of or the features associated with central retinal vein occlusion must be a large enough prospective study that it takes into account age- and sex-matched controls and includes a comprehensive, systemic evaluation. Some reports in the literature have been retrospective,31–33 others have had no control group,33–37,258,259,260 and some have not performed a prospective, systemic evaluation.31–34
We are aware of only one prospective, large study of risk factors for central retinal vein occlusion that includes an appropriate age- and sex-matched control group and a standardized, prospective, systemic evaluation.38 The Eye Disease Case-Control Study Group examined 258 cases of central retinal vein occlusion as well as 1,142 controls. An increased risk of central retinal vein occlusion was found in patients with systemic hypertension, diabetes mellitus, and open-angle glaucoma; the risk of central vein occlusion was decreased for patients with increasing levels of physical activity and increasing levels of alcohol consumption (Table 1). For women, the risk decreased with the use of postmenopausal estrogen and increased with a higher erythrocyte sedimentation rate. The authors did attempt to divide the cases into ischemic and nonischemic central retinal vein occlusion. The following conditions all showed a significant association with ischemic cases only: cardiovascular disease, electrocardiographic abnormalities, albumin-globulin ratio, α1-globulin, history of treatment for diabetes mellitus, and blood glucose level. Both systolic and diastolic blood pressure showed significant associations with both types of central retinal vein occlusion, but the odds ratio is greater for the central retinal vein occlusion. Overall, a stronger cardiovascular risk profile was shown for the ischemic type of central retinal vein occlusion.
TABLE 1. Risk Factors for Central Retinal Vein Occlusion
| Increased Risk | Decreased Risk |
| Systemic hypertension | Increasing levels of physical activity |
| Diabetes mellitus | |
| Increased erythrocyte sedimentation rate (women only) | Increasing levels of alcohol consumption |
| History of glaucoma | Exogenous estrogens (women only) |
(Data from The Eye Disease Case-Control Study Group: Risk factors for central retinal vein occlusion. Arch Ophthalmol 114:545, 1996)
A number of studies have been done to identify both genetic and acquired risk factors for large vessel venous thromboembolism. The term thrombophilia has been used to apply to those cases in which a risk factor has been identified for spontaneously occurring large-vessel venous occlusion.293,295 In a European population the three most common markers of thrombophilia are the factor V Leiden variant, hyperhomocysteinemia, and protein C deficiency.293
A number of studies have reported various hematological abnormalities in patients with a central retinal vein occlusion.39,40,41,45,260,261,262,265,295,390 These reports are difficult to interpret for a number of reasons. Many of them are single-case reports (not sited here) where the association may only be coincidental, some do not provide an appropriate control group,260 some do not separate central retinal vein occlusions from branch retinal vein occlusions,268,296 the sample size may not be large enough to pick up a significant difference,277 better and more specific tests are now available for some abnormalities that invalidate earlier results,277 and in some the parameters measured are probably the result of the occlusion and not the cause.
In some reports patients were studied at varying intervals after the occlusion, often many months after the acute event.295 The timing of laboratory investigation is important for some parameters. Williamson and co-workers showed that relative elevated blood viscosities examined within 1 month of the acute event fell significantly 1 year later.42 Iijima and co-workers found that elevated thrombin-antithrombin III complex levels measured soon after venous occlusion were not maintained, but fell markedly over several months.262
The Eye Disease Case-Control Study Group studied only a few parameters of hematologic function in their large study; these included hematocrit, erythrocyte sedimentation rate, fibrinogen, and antithrombin III.38 There was an increasing risk of central retinal vein occlusion with a calculated odds ratio for antithrombin III level and an elevated erythrocyte sedimentation rate associated with central retinal vein occlusion in women but not in men. In the multivariant logistic regression, however, the antithrombin III level did not attain significance, but for women the erythrocyte sedimentation rate was still significantly correlated.
In a number of studies comparing patients with central retinal vein occlusion to appropriately matched controls,42,43,266–276 activated protein C resistance has been found to be significantly associated with central retinal vein occlusion. This association is true both for patients younger than 50 years of age43 and for those older than 64.42 Other studies have not shown a significant association between protein C resistance and central retinal vein occlusion.288–291 A case has been reported of a 63-year-old woman who presented with a central retinal vein occlusion in one eye and a small branch retinal vein occlusion in the opposite eye; evaluation found that she was heterozygous for the factor V Leiden mutation.308 Another patient with a bilateral central retinal vein occlusion and the factor V Leiden mutation has been reported; she was also pregnant and diabetic.313
Factor V is an inactive procofactor that, when activated by thrombin producing factor Va, serves as the cofactor for factor Xa in the conversion of prothrombin to thrombin. Factor Va is inactivated by a proteolytic cleavage by activated protein C. Protein C is activated by thrombin binding to the vascular endothelium into activated protein C. Resistance to the cleavage by activated protein C is a cause for venous thrombosis.294 In almost all patients this resistance is caused by a mutation in the factor V gene (FVR506Q), which is called factor V Leiden and is present in approximately 5% of the Caucasian population. Although some patients with a central retinal vein occlusion have activated protein C resistance, a statistically significant association between the two appears not to have been proven from the available literature, and no evidence has been presented that it is a cause of central retinal vein occlusion.265,277,293,294 The fact that there is a high prevalence of factor V Leiden in the population means that any study of this factor and central retinal vein occlusion requires a large sample size to detect a significant association between the two.277,294
Another marker of thrombophilia is the presence of autoimmune antibodies reactive against cellular components of phospholipids; these include the anticardiolipin antibody or the lupus anticoagulant.46,293 The antiphospholipid–antibody syndrome often occurs in patients with systemic lupus erythematosis, but when recurrent thrombosis and antiphospholipid antibodies occur in patients without lupus it is called the primary antiphospholipid syndrome.46 Several studies have suggested an association between these antibodies and central retinal vein occlusion.277–299,301,302,384,319 Others have failed to show an association.47,381
A case have been reported of a 40-year-old woman with the primary antiphospholipid syndrome who presented with a central retinal artery occlusion in one eye and 6 years later presented with a central retinal vein occlusion in the opposite eye,301 as well as a bilateral central retinal vein occlusion associated with anticardiolipin antibodies and leukemia.316
Elevated levels of homocysteinemia are also a risk factor for vascular disease, possibly because of endothelial cell damage.305 It can be acquired in a number of ways, including smoking, increased age, insufficient intake of folic acid, renal failure, some chronic diseases, postmenopausal hormone replacement, and certain medications.293,294,305,390 It can also be inherited due to homozygous mutations on genes that encode two enzymes, methylenetetrahydrofolate reductase (MTHFR) and cystation-β-syntase (CBS), which interferes with remethylation of homocysteine.293,294
Several studies have shown an association between hyperhomocysteinemia and central retinal vein occlusion.302–307,310 However, Larsson et al. studied 116 patients with a central retinal vein occlusion who were tested for the MTHFR C677T mutation and found no statistically significant association compared to a control group.278 Some of the studies that did show an association reported on small numbers of patients,303,305,307 and another did not use a matched control group and did not take the blood samples fasting.310 Two patients have been reported with bilateral central retinal vein occlusion and elevated serum homocysteine levels311,312 and one with bilateral central retinal vein occlusion and the MTHFR 677CT mutation, although the blood homocysteine levels were not measured.317
There have been a few cases of elevated blood viscosity producing a central retinal vein occlusion. Two patients with Waldenström's macroglobulinemia presented with what appears to have been bilateral nonischemic central retinal vein occlusion, which resolved with plasmapheresis.48 A 60-year-old woman with Eisenmenger syndrome presented with a bilateral retinal vein occlusion and a secondary polycythemia.314 Multiple, bilateral retinal vein occlusions were reported in a patient with essential thrombocythemia.327
Cahill and associates have performed a meta-analysis of the published literature on total plasma homocysteine levels, serum folate and vitamin B12 levels, and homozygosity for thermolabile methylenetetrahydrofolate reductase genotype as risk factors for retinal vascular disease.390 They found that the studies in the published literature showed plasma total homocysteine levels were elevated in retinal vascular occlusion, including patients with a central retinal occlusion.390 They also found that there was a significantly low serum folate level in patients with a retinal vascular occlusion, although a separate analysis of central retinal vein occlusion was not performed.390 For those patients with a retina vascular occlusion and elevated plasma total homocysteine and a low serum folate, they recommend folate supplementation in conjunction with the patient's primary care physician.390
Except for those rare patients with bilateral retinal vein occlusion due to hyperviscosity, it is unlikely that there is a hematological cause alone for central retinal vein occlusion. Greaves has postulated that retinal vein thrombosis is a “multiple hit” phenomena in which several adverse influences affecting the composition of the blood, the vessel wall, and the blood flow produce a thrombotic event.315 Because the incidence of retinal vein occlusion increases with age, it is likely that with age there is an increased likelihood of the accumulation of a number of adverse events, only one of which may be an inherited or acquired blood disorder, that cause this occlusion.315
There is no evidence that extracranial carotid artery occlusive disease is associated with central retinal vein obstruction. Using digital subtraction angiography, Brown and associates49 studied 37 patients with central retinal vein occlusion; they found that significant ipsilateral stenosis (greater than 50%) was not higher in these patients compared with historically matched controls. They did find, however, that patients with ischemic central retinal vein occlusion had a higher incidence of overall carotid atherosclerotic obstruction (ipsilateral and contralateral) than patients with nonischemic central retinal vein occlusion.49
There appears to be no relationship between optic disc size50 and cup-to-disc ratio in central retinal vein occlusion.38,50,51 Two studies found that the axial length of patients with central retinal vein occlusion is slightly shorter than that of controls,52,318 and one did not find an association.319
CLASSIFICATION
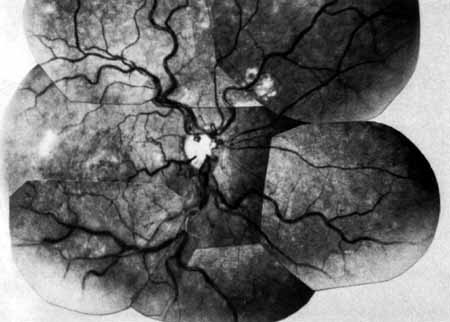
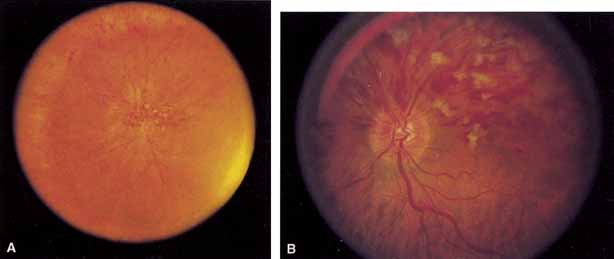
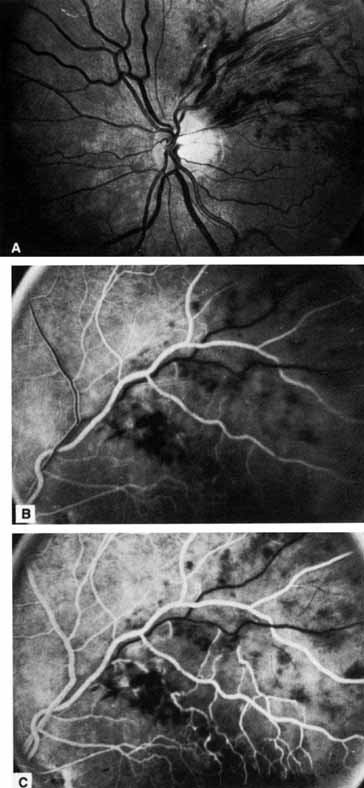
Coats55 may have been the first to suggest that patients with central retinal vein occlusion fall into two groups: one with a dramatic, “blood and thunder” ophthalmoscopic appearance, loss of vision, and a poor prognosis (see Fig. 1); and the other with mild ophthalmoscopic changes, generally good visual acuity, and a relatively good prognosis (Fig. 2). Other investigators have commented on the difference in severity among central retinal vein occlusions, relying principally on the fluorescein angiogram to assess the severity of occlusion.56–59
Hayreh60–64 also divided central retinal vein occlusion into two categories: a nonischemic type, which he called “venous stasis retinopathy,” and an ischemic type, which he called “hemorrhagic retinopathy.” Magargal and colleagues65 may have been the first to divide central retinal vein occlusion into three categories: nonperfused, which they called “hyperpermeable”; ischemic or nonperfused; and a category in which eyes could not be classified on fluorescein angiography, which they termed “indeterminate.” Hayreh and co-workers66 recently also subdivided each of these eyes into mild, moderate, and marked retinopathy based on the maximum capillary nonperfusion on the fluorescein angiogram.
Laatikainen and co-workers67 and Kohner and Shilling68 also divided central retinal vein occlusion into two groups that are similar to Hayreh's original groups.60 Magargal and associates believe that venous occlusion is a spectrum of disease with capillary nonperfusion ranging from little if any ischemia to marked ischemia, and that the amount or extent of ischemia is roughly correlated with the development of neovascular complications.65,69,70
Capillary nonperfusion following central retinal vein occlusion does appear to be a spectrum rather than one or two distinct entities that can be categorized easily. However, the recognition of the severity of capillary occlusion is clinically useful, primarily in predicting the clinical outcome. For the category with minimal to moderate capillary nonperfusion (less than 50%), nonischemic (or perfused) is a better term than venous stasis retinopathy because of the widespread (but not entirely accurate) use of the latter to refer to the retinopathy associated with chronic hypoperfusion caused by extracranial carotid artery occlusive disease.71,72
Similarly, for the category with significant capillary nonperfusion (greater than 50%), ischemic retinal vein occlusion is a better term than hemorrhagic retinopathy because neovascularization, the major complication of central retinal vein occlusion, is correlated with the degree of capillary nonperfusion69,70,73 and not with retinal hemorrhages, which change with the duration of the disease.
The amount of nonperfusion or ischemia is determined by inspecting the fluorescein angiogram. The photographer inspects not only the central 30° or 45°, but also as much of the peripheral retina as possible. The angiographer should be instructed to take photographs during the angiogram of as much of the periphery as possible (a peripheral sweep). Another method has been to classify eyes with less than 10 disc diameters of perfusion on fluorescein angiography as perfused or nonischemic, and eyes with 10 or more areas of nonperfusion as nonperfused or ischemic,74 although this method may not be accurate.320,321
It is impossible to categorize some eyes as either ischemic or nonischemic on the initial evaluation because retinal hemorrhages preclude adequate visualization of the capillary bed on fluorescein angiography.321 These unclassifiable eyes can be placed into a third category, indeterminate,65,74,75 and patients can be reevaluated when the hemorrhages begin to resolve during follow-up. Most of the eyes in this category, however, will develop ischemia (83% in the Central Vein Occlusion Study) on follow-up, and for the purposes of further evaluation, these eyes should probably be considered ischemic.74
Hayreh and associates,76 however, believe it is necessary to perform six clinical tests in order to differentiate eyes with nonischemic central retinal vein occlusion from ischemic central vein occlusion. According to them, the most reliable tests, in order, are: the relative afferent pupil defect (in unilateral central retinal vein occlusion with a normal fellow eye), the electroretinogram, perimetry, visual acuity, intravenous fluorescein angiography, and ophthalmoscopy.76 They believe that the intravenous fluorescein angiogram either provides no information at all on capillary nonperfusion or sometimes provides misleading information.76
CLINICAL CHARACTERISTICS
Nonischemic Central Retinal Vein Occlusion
Nonischemic central retinal vein occlusion is a much milder and more variable disease in appearance, symptoms, and course compared with ischemic central retinal vein occlusion. Patients with nonischemic central retinal vein occlusion are an average of 5 years younger (average age, 63 years) than those with ischemic vein occlusion.66 Complaints vary from none (i.e., condition is discovered on a routine examination) to blurred vision, which is often transient.66 The visual acuity may range from normal to counting fingers, but the majority of patients have an initial visual acuity of 20/50 or better.62
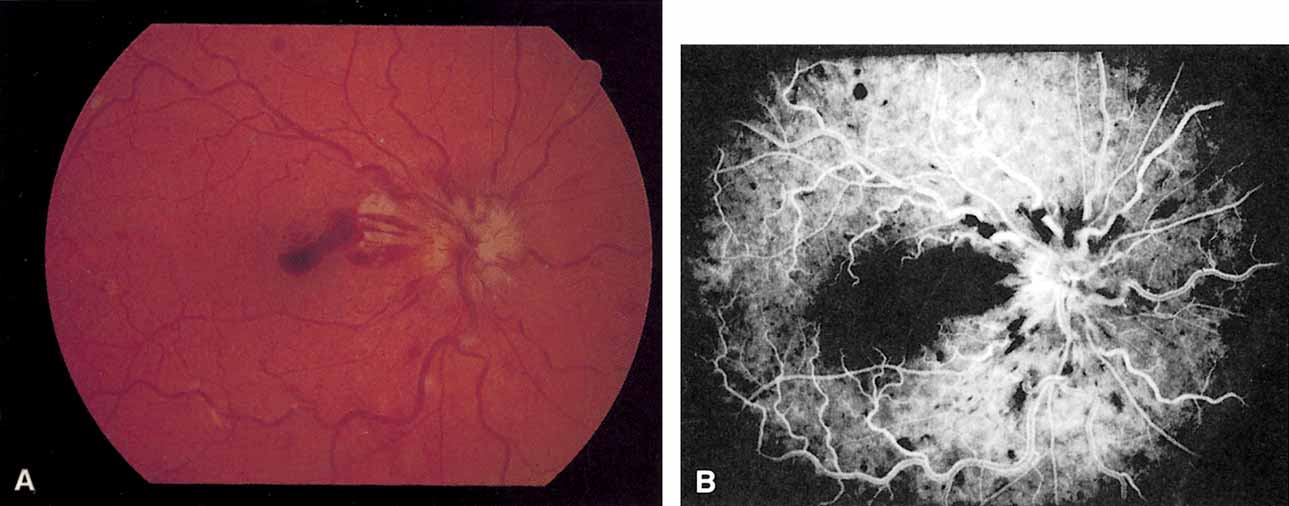
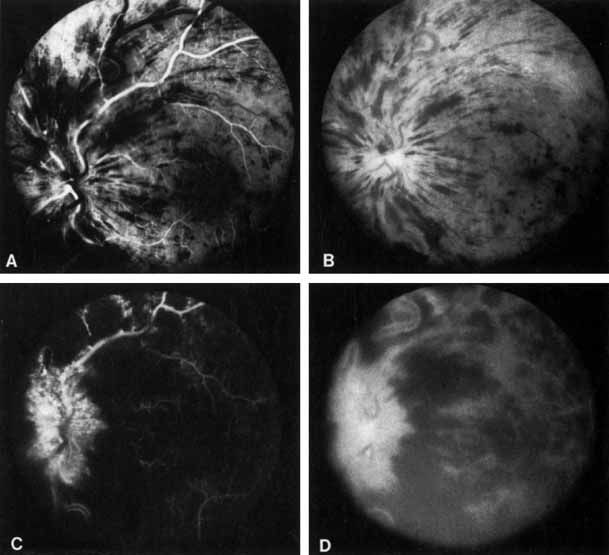
The ophthalmoscopic features of nonischemic central retinal vein occlusion are similar to those of ischemic central retinal vein occlusion, but are much less extensive (see Fig. 2; Fig. 3A and 3B). Engorgement of the venous tree (including the capillaries) is prominent; there is increased tortuosity and dilation and a darker appearance of the blood column. Retinal hemorrhages vary markedly. Sometimes they occur only peripherally; at other times, they may be rather prominent in the posterior pole.60 Cotton-wool spots are rare. Vision may be decreased because of macular edema or macular hemorrhage.
Hvarfner and Larsson have studied 74 patients with both a nonischemic and ischemic central retinal vein occlusion, 48 of whom had optic disc swelling.381 They found that optic nerve swelling was of no prognostic value in predicting neovascular complications and visual acuity 1 year after the acute event.381 Beaumont and Kang divided central retinal vein occlusion into two distinct groups based on the presence or absence of optic nerve head swelling.379 Those with optic nerve head swelling were postulated to have a venous occlusion in the retrocribrosal space and were of younger age, had less severe vascular nonperfusion, and had a better visual acuity than those without swelling who were postulated to have an occlusion at the lamina cribrosa.379
Beaumont and Kang have also identified the clinical characteristics with different sites of occlusion and proposed a new classification.382 These sites are the arteriovenous crossing, optic cup, or optic nerve; those in the nerve were further subdivided into the presence or absence of optic nerve head swelling.382 Among their findings are that primary open-angle glaucoma is significantly more common in patients with an occlusion at the optic cup and a history of smoking and hypertension is more common with occlusions at the arteriovenous crossing.383
Patchy ischemic retinal whitening located in a perivenular distribution near the macula is a transient abnormality in patients with a nonischemic central retinal vein occlusion and is associated with a generally good visual outcome.324,386 The cause is unknown.
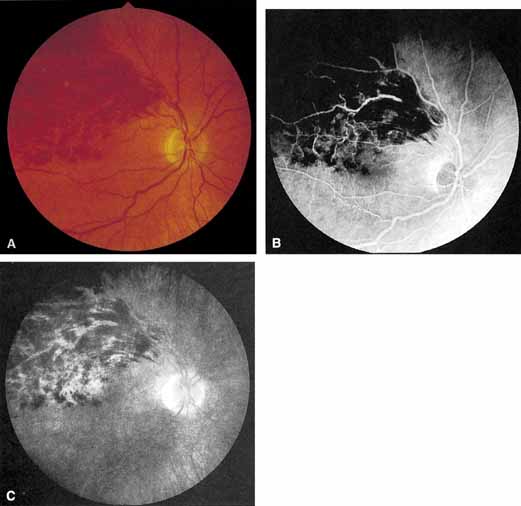
The angiographic pattern may show little except occasionally a prolonged venous transit time. Dilation of the retinal venous circulation, mild staining of the walls of veins, and varying degrees of disc and macular edema may be present (including cystoid macular edema). Capillary nonperfusion is not a prominent feature, nor is its sequela, neovascularization. The electroretinogram is nearly normal, confirming the lack of ischemia.77 The intraocular pressure is frequently lower on the side of the occlusion.78 Synonyms for this type of central retinal vein occlusion have included partial, incomplete, imminent, threatened, incipient, or impending central retinal vein occlusion.60,76 How many central retinal vein occlusions in this category are actually incomplete or partial occlusions that then progress to a more complete occlusion is unknown. It does appear that some eyes with nonischemic central retinal vein occlusion go on to develop a more ischemic type of central retinal vein occlusion (see Fig. 3); whether this represents a progression of the vein occlusion62 or simply progressive retinal capillary nonperfusion is unknown. In series in which the incidence of conversion for the nonischemic occlusion to the ischemic type has been studied, the incidence ranges from approximately 5% to 22%, depending on the duration of follow-up, and is higher for older patients.63,74,79–81
The natural course of nonischemic central retinal vein occlusion is relatively benign, except in those who go on to develop additional ischemia. The hemorrhages, vascular congestion, and engorgement gradually resolve over several months. Some patients are left with permanent cystoid macular edema, macular cystic changes, pigmentary changes, or residual microvascular abnormalities.82 Neovascularization does not generally occur, and morbidity is generally limited to a persistent, mild decrease in visual acuity with a relative central scotoma. The majority of patients will have a final visual acuity of 20/40 or better.79
Ischemic Central Retinal Vein Occlusion
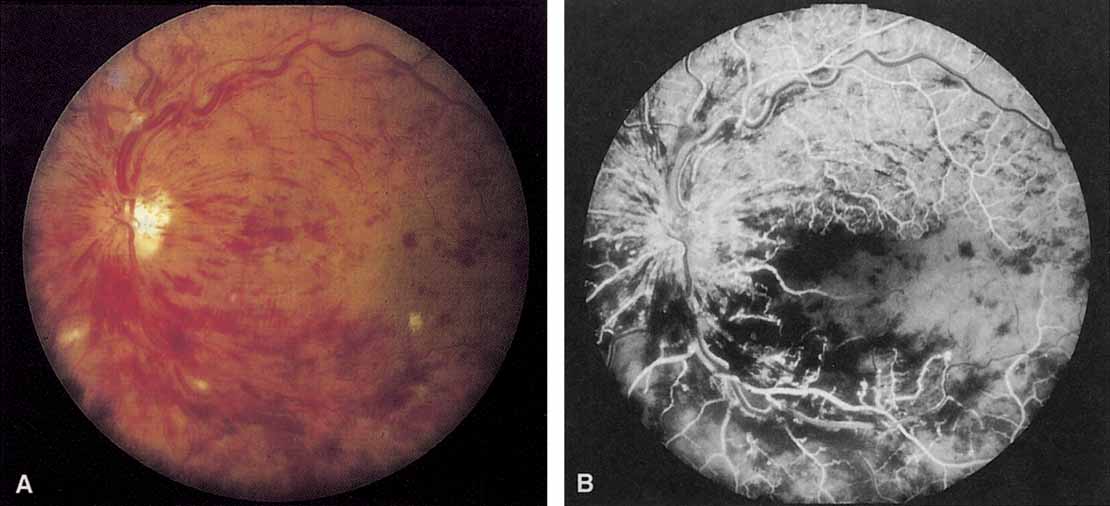
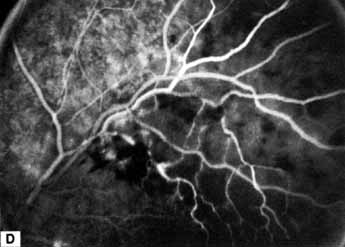
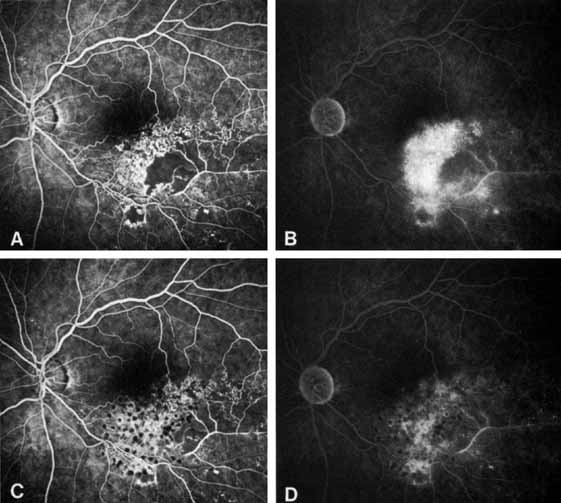
Patients with an ischemic pattern are usually aware of a sudden, painless decrease in visual acuity. Vision ranges from 20/400 to hand movements. The onset, however, is generally not as rapid or the visual loss as extensive as in central retinal artery occlusion. Exceptional cases have been noted in which patients with an acute onset had reasonably good vision and yet demonstrated a picture of ischemic central retinal vein occlusion. Patients with ischemic occlusion have an average age of 68.5 years.66 Confluent hemorrhages are the most prominent ophthalmoscopic feature of an acute ischemic central retinal vein occlusion (see Fig. 3C and 3D). These hemorrhages occur in a wide variety of shapes and sizes; they are usually concentrated in the posterior pole, but may be seen throughout the retina. Hemorrhages in the superficial retina may be so prominent about the posterior pole that the underlying retina is obscured. Many hemorrhages are flame shaped, reflecting the orientation of the nerve fibers. Dot and punctate hemorrhages are interspersed and indicate involvement of the deeper retinal layers. Bleeding may be extensive, erupting through the internal limiting membrane to form a preretinal hemorrhage or extending into the vitreous. Small dot hemorrhages may be seen either isolated or clustered around small venules. The entire venous tree is tortuous, engorged, dilated, and dark. The retina is edematous, particularly in the posterior pole; some of this edema may obscure portions of the retinal vessels. Cotton-wool patches (soft exudates) are often present.
The disc margin is blurred or obscured, and the precapillary arterioles appear engorged. Splinter hemorrhages and edema are present on the disc surface and extend into the surrounding retina. The physiologic cup is filled, and the venous pulse is absent. The arterioles, often overlooked because of the other more striking pathologic features, are frequently narrowed. Sometimes in central retinal vein occlusion of acute onset, the fundus picture is less dramatic, and all the findings previously discussed may be present, but to a lesser degree. Vision depends primarily on the extent of macular involvement.
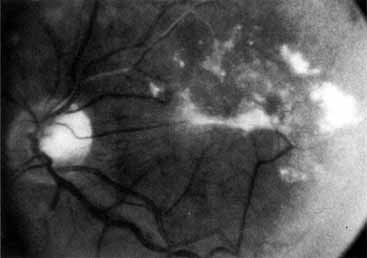
The intravenous fluorescein angiogram pattern of an ischemic central retinal vein occlusion is usually characterized by a delayed filling time of the venous tree of the retina, capillary and venous dilation, and extensive leaking of fluorescein into the retina, particularly in the macular area and in the area adjacent to the larger venous trunks and capillary nonperfusion (see Fig. 3C and 3D; Figs. 4 and 5). Microaneurysms may not be noted at the time of initial occlusion, but are usually manifest shortly thereafter. Late-phase photographs show patchy extravascular areas of fluorescence and staining of the retinal veins. Fluorescence in the macula indicates capillary leakage and edema; this not only may account for much of the initial visual loss in the acute phase, but may also eventually result in permanent structural changes. Intravenous indocyanine green videoangiography may also be helpful in showing the arterial and venous flow alterations in this condition.322
|
The prognosis for ischemic central retinal vein occlusion is generally poor because of decreased visual acuity and neovascularization. Visual loss occurs because of macular edema, capillary nonperfusion, overlying hemorrhage (either retinal or vitreal), or a combination of all these. Retinal edema usually gradually subsides except in the macula, where it may persist for many months or years. Macular holes or cysts may form.83,84 Pigment clumping or fine pigment stippling and pigment atrophy are not uncommon, and persistent macular hemorrhage, even years after the occlusion, has been noted.83 Hard exudates often form an irregular circinate configuration around the macula and become more prominent months later. Occasionally an epiretinal membrane may form.
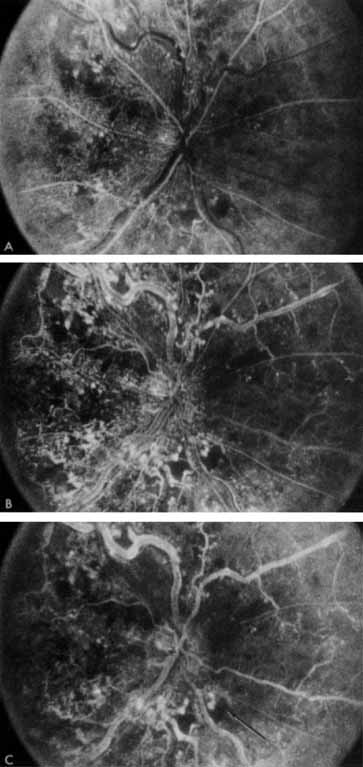
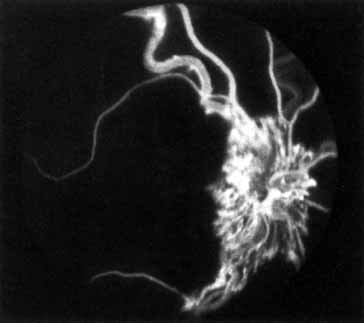
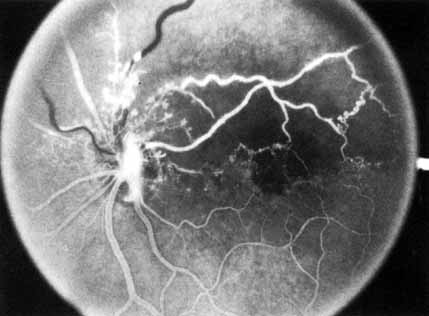
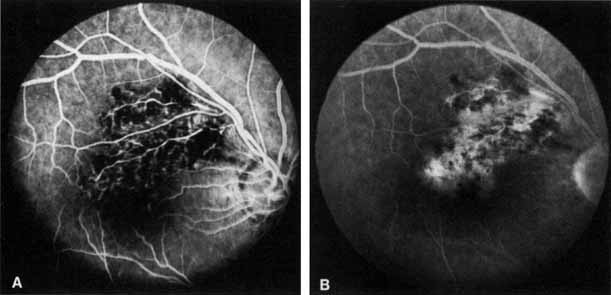
In the chronic phase, most hemorrhages gradually disappear over many months; however, scattered, flame-shaped hemorrhages and dot hemorrhages, particularly in the periphery, may be seen for years. Cotton-wool patches and microaneurysms likewise tend to disappear after several months, although in some cases the latter may persist. The venous tree becomes less tortuous and dilated. Prominent venous loops, which are collateral communications, may be observed on the surface of the disc (Fig. 6).85 These loops develop within 3 to 14 months after occlusion from the existing retinal vasculature and are collateral vessels between the obstructed disc capillaries and the unobstructed choroidal or pial capillaries.323 These retinochoroidal collateral veins, if they develop, may protect against anterior segment neovascularization,328 but may not be associated with a better visual prognosis.105 Collaterals between the central retinal vein within the globe and the patent central retinal vein behind the occlusion have not been observed.86 The extent and speed of retinal recovery probably depends to some degree on how quickly collateral vessels form, how rapidly recanalization occurs, and how adequately these compensatory mechanisms restore normal outflow. However, the exact nature and course of the collateral vessels are disputed. Anastomotic channels may develop within the retinal vasculature if pressure differentials develop between its major venous trunks. Changes in the retinal arterioles include both segmental and generalized narrowing as well as sclerosis, which is evidenced by both sheathing and widening of the light reflex. Sheathing of the veins is also common. The disc may appear nearly normal except for sheathing of the vessels in and around the papilla, and some blurring of the margins may persist. Sometimes optic atrophy is present.
The fluorescein angiographic appearance varies greatly, depending on the extent of recovery. All the findings in the acute phase, consisting of venous and capillary engorgement, microaneurysms, staining of the veins, patchy extravascular fluorescence, and capillary nonperfusion, may persist indefinitely. In most instances, these findings eventually diminish so that few significant features are present on the angiogram; collateral vessels, if present, may be the only pathognomonic feature.
The most serious complication of central retinal vein occlusion is neovascularization (Table 2). Neovascularization elsewhere (NVE) occurs less frequently than neovascularization of the iris (NVI), and usually only in ischemic occlusions.66 The low incidence of retinal surface neovascularization in ischemic central retinal vein occlusion is thought to be due to the destruction of endothelial cells, which provide the source for endothelial proliferation and neovascularization.87
TABLE 2. Percentage of Ocular Neovascularization in Venous Occlusion*
| Overall Incidence of Ocular Neovascularization | ||||
| Type of Vein Occlusion | NVD | NVE | NVI | NVG |
| CRVO | 1.1%66 | 1.6%66 | 13%66 | 8.3–25%66,73,88 |
| Hemi-CRVO66 | 9.3% | 13.4% | 3.2% | 1% |
| HRVO75 | 11% | 9% | 9% | 3% |
| Quadrantic BRVO180 | 10.6% | 20.7% | 1.6% | 0.8% |
| Macular BRVO182 | 0.0% | 0.0% | 0.0% | 0.0% |
*This table is for comparison of neovascularization between the types of vein occlusion. Most of the neovascularization occurs in patients with the ischemic form of the listed occlusion. CRVO, central retinal vein occlusion; HRVO, hemispheric retinal vein occlusion; BRVO, branch retinal vein occlusion; NVD, neovascularization of the disc; NVE, neovascularization elsewhere; NVI, neovascularization of the iris; NVG, neovascular glaucoma.
Neovascularization of the iris and frequently neovascular glaucoma occurs in approximately 8%62 to 25%65,66,73,88 of all central retinal vein occlusions and generally only in those eyes that exhibit an ischemic pattern of occlusion.63,65,66,68,89 Magargal and co-workers70 have shown that the incidence of neovascularization increases dramatically above approximately 50% capillary nonperfusion. The incidence of anterior segment neovascularization in nonischemic central retinal vein occlusion is approximately 1%, compared with approximately 35% to 45% for ischemic central retinal vein occlusion.63,69,70,89 Neovascularization of the iris or angle is significantly correlated with the extent of capillary nonperfusion on the fluorescein angiogram.73,89 In the series of Sinclair and Gragoudas,73 rubeosis developed in 80% to 86% of the eyes with severe nonperfusion of three to four quadrants of the posterior pole or the periphery, but in only 3% to 9% of those with less capillary nonperfusion. Abnormalities on fluorescein angiography of the iris appear not to be correlated with the development of secondary glaucoma in ischemic central retinal vein occlusion.325
Neovascularization of the iris may develop as early as 2 weeks after central retinal vein occlusion or as late as 2½years.2,65,89 Neovascularization of the iris, when it does occur, will develop in almost all patients within the first year, but usually in the first 3 months.89 Symptomatically, patients complain of tearing, irritation, pain, and further blurring of vision as the intraocular pressure in the affected eye begins to rise. The pain may become excruciating. The cornea is hazy and the pupil dilated, and a network of fine vessels is seen over the surface of the iris (rubeosis iridis) on slit-lamp examination. By the time gonioscopy reveals extension of this neovascular membrane into the trabecular network and throughout the angle, the intraocular pressure is usually markedly elevated. The angle is initially open, but later in the disease, peripheral anterior synechiae develop and the angle may become irreversibly closed, resulting in neovascular glaucoma. Large, extremely irritating bullae may form on the surface of the cornea and then break down. Dense cataracts eventually form, obscuring the fundus.
Nonperfusion in central retinal vein occlusion is also correlated with a relative afferent pupil defect.76,90,91 Servais and colleagues90 found that 100% of a group of patients with unilateral ischemic central retinal vein occlusion had a relative afferent pupil defect, whereas only 31% of patients with nonischemic occlusion had such a defect. Hayreh and associates76 believe that relative afferent pupil defect is the most reliable test for ischemic central vein occlusion in patients with unilateral disease (i.e., fellow eye is normal).
Hikichi and co-workers examined eyes with a central retinal vein occlusion and the presence or absence of a posterior vitreous detachment.350 They found that a complete posterior vitreous detachment appears to protect against retinal or disc neovascularization, although not iris neovascularization, and that vitreomacular attachment contributed to persistent macular edema in nonischemic central retinal vein occlusion.350
Besides having the complications already discussed, patients with central retinal vein occlusion are also at risk for vascular occlusion in the contralateral eye. Approximately 6% to 17% of patients can be expected to have a bilateral nonsimultaneous central retinal vein occlusion.92–95 Mieler and Blumenkranz95 followed 79 patients with central retinal vein occlusion for a minimum of 5 years. In 25% of these patients, the contralateral eye developed a vascular occlusion within 5 years; of these, 50% were branch retinal vein occlusions, 30% were central retinal vein occlusions, 10% were central retinal artery occlusions, and 10% were branch retinal artery occlusions. There is some doubt as to whether the life expectancy of patients with a central vein occlusion is shortened96 or not97 compared with an age-matched population.
Occasionally a patient will have a simultaneous occlusion of both the central retinal vein and the central retinal artery.98,99 Unlike patients with only a central retinal vein occlusion, these patients often have some retrobulbar pain, and vision may be decreased to no light perception. The retina appears pale, with a cherry-red spot in the macula. Disc edema and retinal hemorrhage may be present. Fluorescein angiography will demonstrate occlusion of both the central retinal vein and the central retinal artery. In the recovery phase, optic atrophy develops, and extreme narrowing of the retinal arterioles occurs.98
Although rare, an occlusion of the cilioretinal artery may also be seen in conjunction with central retinal vein occlusion.18,100–103 Patients with this occurrence have a pallor typical of ischemia in the distribution of the cilioretinal artery; fluorescein angiography will confirm occlusion of the cilioretinal artery. Occlusion of the cilioretinal artery may be due to a relative hypoperfusion caused by the increased venous pressure.103 The visual acuity, in general, is no worse than in a pure central retinal vein occlusion.99 Simultaneous occlusion of the central retinal vein and a branch retinal artery has been reported in a few patients.104
The electroretinogram is abnormal in patients with central retinal vein occlusion, and one or more of the electroretinographic parameters can be used to group patients in terms of having either an ischemic or a nonischemic vein occlusion.77,106 Some investigators believe that the initial electroretinogram can be used as a predictor of anterior segment neovascularization in patients with central retinal vein occlusion,107–110 whereas others do not consider it useful in this regard.106 In a large study of patients with unilateral central retinal vein occlusion and an apparently normal contralateral eye, 36% of patients had an abnormal electroretinogram in the uninvolved fellow eye,111 which is suggestive of a retinal circulatory disturbance in the clinically normal eye. The multifocal electroretinogram has also been studied in patients with a central retinal vein occlusion and compared to standard electroretinography.378
The electrooculogram is also abnormal in most patients with central retinal vein occlusion.112,113 It is correlated with the degree of ischemia112 and may serve as a predictor of the development of rubeosis iridis.113