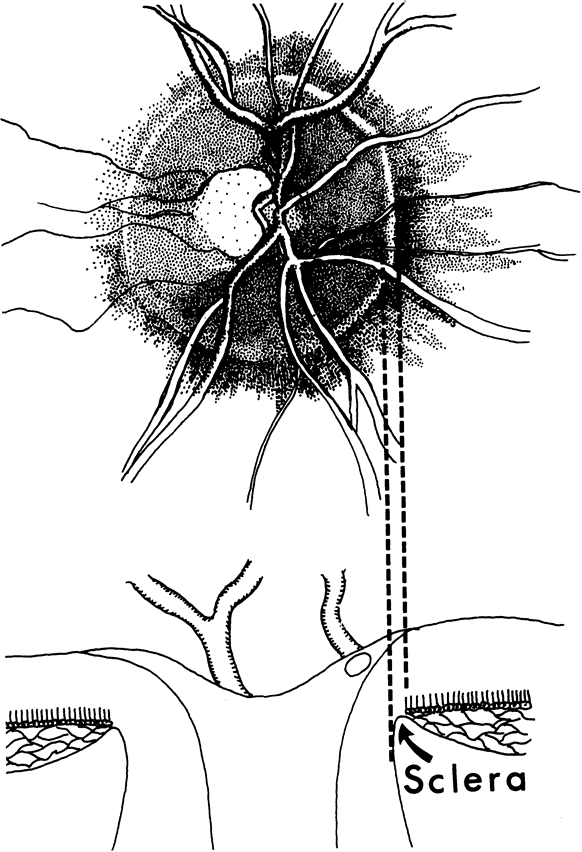
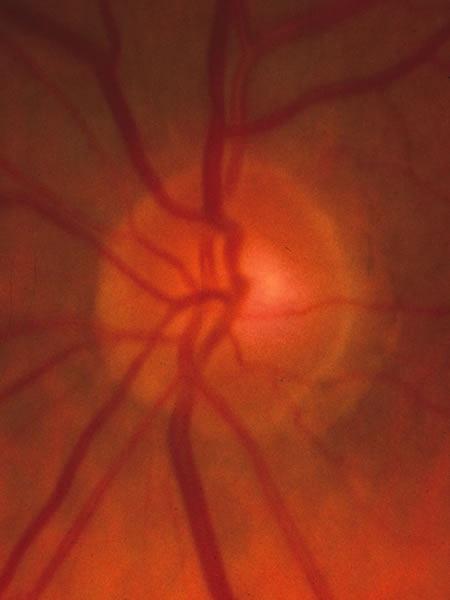
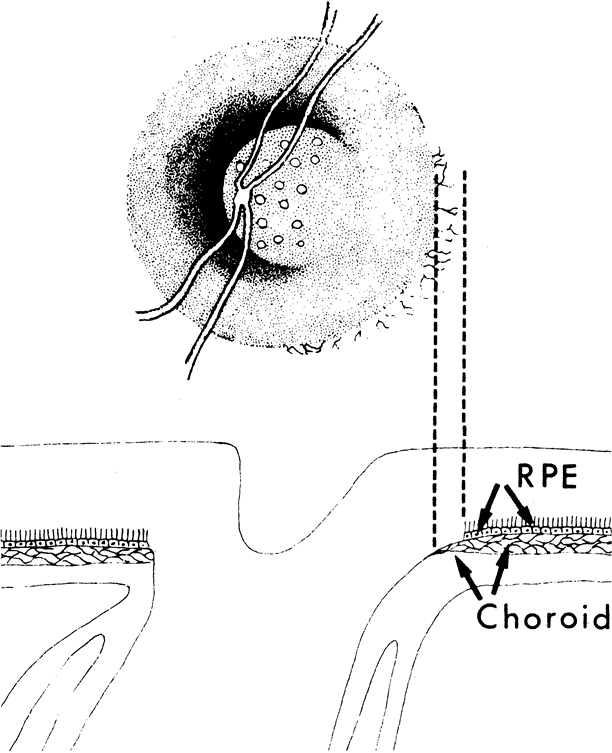
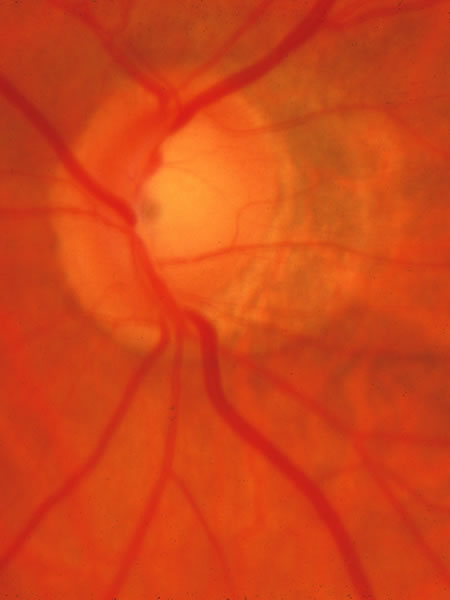
1. Radius RL, Anderson DR: The histology of retinal nerve fiber layer bundles and bundle defects. Arch Ophthalmol 97:948, 1979 2. Radius RL, Anderson DR: The course of axons through the retina and optic nerve head. Arch Ophthalmol 97:1154, 1979 3. Minckler DS: The organization of nerve fiber bundles in the primate optic nerve head. Arch Ophthalmol 98:1630, 1980 4. Hoyt WF, Frisén L, Newman NM: Funduscopy of nerve fiber layer defects in glaucoma. Invest Ophthalmol 12:814, 1973 5. Radius RL: Thickness of the retinal nerve fiber layer in primate eyes. Arch Ophthalmol 98:1625, 1980 6. Quigley HA, Addicks EM: Quantitative studies of retinal nerve fiber layer defects. Arch Ophthalmol 100:807, 1982 7. Hoyt WF, Schlicke B, Eckelhoff RJ: Funduscopic appearance of a nerve-fiber-bundle defect. Br J Ophthalmol 56:577, 1972 8. Sommer A, Miller NR, Pollack I, et al: The nerve fiber layer in the diagnosis of glaucoma. Arch Ophthalmol 95:2149, 1977 9. Quigley HA, Miller NR, George T: Clinical evaluation of nerve fiber layer atrophy as an indicator of glaucomatous
optic nerve damage. Arch Ophthalmol 98:1564, 1980 10. Airaksinen PJ, Nieminen H, Mustonen E: Retinal nerve fibre layer photography with a wide angle fundus camera. Acta Ophthalmol (Copenh) 60:362, 1982 11. Huang XR, Knighton RW: Linear birefringence of the retinal nerve fiber layer measured in vitro
with a multispectral imaging micropolarimeter. J Biomed Opt 7:199, 2002 12. Weinreb RN, Dreher AW, Coleman A, et al: Histopathologic validation of Fourier-ellipsometry measurements
of retinal nerve fiber layer thickness. Arch Ophthalmol 108:557, 1990 13. Knighton RW, Huang XR: Directional and spectral reflectance of the rat retinal nerve fiber layer. Invest Ophthalmol Vis Sci 40:639, 1999 14. Knighton RW, Huang X, Zhou Q: Microtubule contribution to the reflectance of the retinal nerve fiber
layer. Invest Ophthalmol Vis Sci 39:189, 1998 15. Ogden TE: The nerve-fiber layer of the primate retina: Aan autoradiographic
study. Invest Ophthalmol 13:95, 1974 16. Harrington DO: Differential diagnosis of the arcuate scotoma. Invest Ophthalmol 8:96, 1969 17. Hoyt WF, Luis O: Visual fiber anatomy in the infrageniculate pathway of the primate. Arch Ophthalmol 68:94, 1962 18. Hoyt WF, Luis O: The primate chiasm. Arch Ophthalmol 70:69, 1963 19. Hoyt WF: Anatomic considerations of arcuate scotomas associated with lesions of
the optic nerve and chiasm: A nauta axon degeneration study in the monkey. Bull Johns Hopkins Hosp 111:57, 1962 20. Teal PK, Morin JD, McCulloch C: Assessment of the normal disc. Trans Am Ophthalmol Soc 70:164, 1972 21. Bengtsson B: The variation and covariation of cup and disc diameters. Acta Ophthalmol (Copenh) 54:804, 1976 22. Kirsch RE, Anderson DR: Clinical recognition of glaucomatous cupping. Am J Ophthalmol 75:442, 1973 23. Jonas JB, Gusek GC, Nnaumann GOH: Die parapapilläre Region in Normal- und Glaukomaugen I. Planimetrische
Werte von 312 Glaukom- und 125 Normalaugen. Klin Monastbl Augenheilkd 193:52, 1988 24. Guist G: Coincident Ophthalmoscopy and Histology of the Optic Nerve. Vienna, Guist, 1934 25. Elschnig A: Das Colobom am Sehnerveneintritte und der Conus nach unten. Albrecht von Graefes Arch Ophthalmol 51:391, 1900 26. Anderson DR, Hoyt WF: Ultrastructure of intraorbital portion of human and monkey optic nerve. Arch Ophthalmol 82:506, 1969 27. Anderson DR: Correlation of the peripapillary anatomy with the disc damage and full
abnormalities in glaucoma. Doc Ophthalmol Proc Ser 35:1, 1983 28. Armaly MF, Krueger DE, Maunder L, et al: Biostatistical analysis of the collaborative glaucoma study: I. Summary report of the risk factors for glaucomatous visual-field
defects. Arch Ophthalmol 98:2163, 1980 29. Armaly MF: Lessons to be learned from the collaborative glaucoma study. Surv Ophthalmol 25:139, 1980 30. Anderson DR: The management of elevated intraocular pressure with normal optic discs
and visual fields: I. Therapeutic approach based on high risk factors. Surv Ophthalmol 21:479, 1977 31. Anderson DR: Glaucoma: The damage caused by pressure. Am J Ophthalmol 108:485, 1989 32. Kronfeld PC, McGarry HI: Five year follow-up of glaucoma. JAMA 136:957, 1948 33. Heijl A, Leske MC, Bengtsson B, et al: Reduction of intraocular pressure and glaucoma progression. Arch Ophthalmol 120:1268, 2000 34. Anderson DR, Drance SM, Schulzer M: Writing Committee for Collaborative Normal-tension Glaucoma Study
Group. The effectiveness of intraocular pressure reduction in the treatment
of normal-tension glaucoma. Am J Ophthalmol 126:498, 1998 35. Kass MA, Heuer DK, Higginbotham EJ, et al: The Ocular Hypertension Study: A randomized trial determines that topical
ocular hypotensive medication delays or prevents the onset of primary
open-angle glaucoma. Arch Ophthalmol 120:701, 2002 36. Anderson DR, Quigley HA: The optic nerve. In Moses RA, Hart WM Jr (eds): Adler's Physiology of the Eye, 9th ed., pp. 616–640. St. Louis, CV Mosby, 1992 37. Anderson DR: Glaucoma, capillaries, and pericytes. 1. Blood flow regulation. Ophthalmologica 210:257, 1996 38. Flammer J, Orgul S, Costa VP, et al: The impact of ocular blood flow in glaucoma. Prog Retin Eye Res 21:359, 2002 39. Pillunat LE, Anderson DR, Knighton RW, et al: Autoregulation in human optic nerve head circulation in response to increased
intraocular pressure. Exp Eye Res 64:737, 1997 40. Matsugi T, Chen Q, Anderson DR: Suppression of CO2-induced relaxation of bovine retinal pericytes by Angiotensin II. Invest Ophthalmol Visual Sci 38:652, 1997 41. Liu B, Neufeld AH: Nitric oxide synthase-2 in human optic nerve head astrocytes induced
by elevated pressure in vitro. Arch Ophthalmol 119:2405, 2001 42. Anderson DR, Hendrickson A: Effect of intraocular pressure on rapid axoplasmic transport in monkey
optic nerve. Invest Ophthalmol Vis Sci 13:771, 1974 43. Radius RL, Anderson DR: Reversibility of optic nerve damage in primate eyes subjected to intraocular
pressure above systolic blood pressure. Br J Ophthalmol 65:661, 1981 44. Kerrigan LA, Zack DJ, Quigley HA, et al: TUNEL-positive ganglion cells in human primary open-angle
glaucoma. Arch Ophthalmol 115:1031, 1997 45. Vorwerk CK, Gorla MS, Dreyer EB: An experimental basis for implicating excitotoxicity in glaucomatous optic
neuropathy. Surv Ophthalmol 43(Suppl 1):S142, 1999 46. Gordon MO, Beiser JA, Brandt JD, et al: The ocular hypertension treatment study: Baseline factors that predict
the onset of primary open-angle glaucoma. Arch Ophthalmol 120:714, 2000 47. Schulzer M, Drance SM, Carter CJ, et al: Biostatistical evidence for two distinct populations with chronic open
angle glaucoma. Br J Ophthalmol 74:196, 1990 48. Kass MA, Kolker AE, Becker B: Prognostic factors in glaucomatous visual field loss. Arch Ophthalmol 94:1274, 1976 49. Cartwright MJ, Anderson DR: Correlation of asymmetric damage with asymmetric intraocular pressure in
normal-tension glaucoma (low-tension glaucoma). Arch Ophthalmol 106:898, 1988 50. Crichton A, Drance SM, Douglas GR, et al: Unequal intraocular pressure and its relation to asymmetric visual field
defects in low-tension glaucoma. Ophthalmology 96:1312, 1989 51. Drance SM, Morgan RW, Sweeney VP: Shock-induced optic neuropathy: Cause of nonprogressive glaucoma. N Engl J Med 288:392, 1973 52. Drance SM, Begg IS: Sector haemorrhage: A probable acute ischemic disc change in chronic simple
glaucoma. Can J Ophthalmol 5:137, 1970 53. Drance SM, Fairclough M, Butler DM, et al: The importance of disc hemorrhage in the prognosis of chronic open angle
glaucoma. Arch Ophthalmol 95:226, 1977 54. Susanna R, Drance SM, Douglas GR: Disc hemorrhages in patients with elevated intraocular pressure: Occurrence
with and without field changes. Arch Ophthalmol 97:284, 1979 55. Airaksinen PJ, Mustonen E, Alanko HI: Optic disc hemorrhages: Analysis of stereophotographs and clinical data
of 112 patients. Arch Ophthalmol 99:1795, 1981 56. Bengtsson B, Holmin C, Krakau CET: Disc haemorrhage and glaucoma. Acta Ophthalmol (Copenh) 59:1, 1981 57. Airaksinen PJ, Mustonen E, Alanko HI: Optic disc haemorrhages precede retinal nerve fibre layer defects in ocular
hypertension. Acta Ophthalmol (Copenh) 59:627, 1981 58. Pederson JE, Anderson DR: The mode of progressive disc cupping in ocular hypertension and glaucoma. Arch Ophthalmol 98:490, 1980 59. Anderson DR: What happens to the optic disc and retina in glaucoma? Ophthalmology 90:766, 1983 60. Drance SM, Lakowski R, Schulzer M, et al: Acquired color vision changes in glaucoma: Use of 100-hue test and
Pickford anomaloscope as predictors of glaucomatous field change. Arch Ophthalmol 99:829, 1981 61. Austin DJ: Acquired colour vision defects in patients suffering from chronic simple
glaucoma. Trans Ophthalmol Soc UK 94:880, 1974 62. Poinoosawmy D, Nagasubramanian S, Gloster J: Colour vision in patients with chronic simple glaucoma and ocular hypertension. Br J Ophthalmol 64:852, 1980 63. Motolko M, Drance SM, Douglas GR: The early psychophysical disturbances in chronic open-angle glaucoma: A
study of visual functions with asymmetric disc cupping. Arch Ophthalmol 100:1632, 1982 64. Atkin A, Bodis-Wollner I, Wolkstein M, et al: Abnormalities of central contrast sensitivity in glaucoma. Am J Ophthalmol 88:205, 1979. 65. Atkin A, Wolkstein M, Bodis-Wollner I, et al: Interocular comparison of contrast sensitivities in glaucoma patients and
suspects. Br J Ophthalmol 64:858, 1980 66. Stamper RL, Hsu-Winges C, Sopher M: Arden contrast sensitivity testing in glaucoma. Arch Ophthalmol 100:947, 1982 67. Phelps CD, Remijan PW, Blondeau P: Acuity perimetry. Doc Ophthalmol Proc Ser 26:111, 1981 68. Anctil JL, Anderson DR: Early foveal involvement and generalized depression of the visual field
in glaucoma. Arch Ophthalmol 102:363, 1984 69. Morin JD: Changes in the visual fields in glaucoma: Static and kinetic perimetry
in 2,000 patients. Trans Am Ophthalmol Soc 77:622, 1979 70. Drance SM: The glaucomatous visual field. Br J Ophthalmol 56:186, 1972 71. Aulhorn E, Harms H: Early visual field defects in glaucoma. In Leydhecker W (ed): Glaucoma: Tutzing Symposium, 1966, p. 151. Basel, S Karger, 1967 72. Harrington DO: The Bjerrum scotoma. Am J Ophthalmol 59:646, 1965 73. Drance SM, Fairclough M, Thomas B, et al: The early visual field defect in glaucoma and the significance of nasal
steps. Doc Ophthalmol Proc Ser 19:119, 1979 74. Brais P, Drance SM: The temporal field in chronic simple glaucoma. Arch Ophthalmol 88:518, 1972 75. Werner EB, Beraskow J: Temporal visual field defects in glaucoma. Can J Ophthalmol 15:13, 1980 76. Quigley HA, Addicks EM, Green WR: Optic nerve damage in human glaucoma: III. Quantitative correlation of
nerve fiber loss and visual field defect in glaucoma, ischemic neuropathy, papilledema, and
toxic neuropathy. Arch Ophthalmol 100:135, 1982 77. Armaly ME: Ocular pressure and visual fields: A ten-year follow-up study. Arch Ophthalmol 81:25, 1969 78. Lichter PR, Standardi CL: Early glaucomatous visual field defects and their significance to clinical
ophthalmology. Doc Ophthalmol Proc Ser 19:111, 1979 79. Hart WM Jr , Yablonski M, Kass MA, et al: Quantitative visual field and optic disc correlates early in glaucoma. Arch Ophthalmol 96:2209, 1978 80. Anderson DR: Automated Static Perimetry. St. Louis, Mosby Year Book, 1992 81. Chandler PA, Grant WM: Lectures on Glaucoma, 2nd ed, p. 57. Philadelphia, Lea & Febiger, 1979 82. Sommer A, Pollack I, Maumenee AE: Optic disc parameters and onset of glaucomatous field loss: I. Methods
and progressive changes in disc morphology. Arch Ophthalmol 97:1444, 1979 83. Feuer WJ, Parrish RK II, Schiffman JC, et al: The Ocular Hypertension Treatment Study: Reproducibility of cup/disk ratio
measurements over time at an optic disc reading center. Am J Ophthalmol 133:19, 2002 84. Schwartz B: Cupping and pallor of the optic disc. Arch Ophthalmol 89:272, 1973 85. Iwata K: Retinal nerve fiber layer, optic cupping, and visual field changes in glaucoma. In Bellows JG (ed): Glaucoma: Contemporary International Concepts, p. 139. New York, Masson, 1979 86. Shiose Y, Ohmi Y, Kawase Y, et al: Glaucoma and the optic disc: I. Studies on cup and pallor in the optic
disc. Jpn J Clin Ophthalmol 32:51, 1978 87. Chan WC, Morin JD, McCulloch C: Optic disc observations in glaucoma. Can J Ophthalmol 11:134, 1976 88. Armaly MF: Optic cup in normal and glaucomatous eyes. Invest Ophthalmol 9:425, 1970 89. Armaly MF: The correlation between appearance of the optic cup and visual function. Trans Am Acad Ophthalmol Otolaryngol 73:898, 1969 90. Fishman RS: Optic disc asymmetry: A sign of ocular hypertension. Arch Ophthalmol 84:590, 1970 91. Pickard R: Variations in the size of the physiological cup and their relation to glaucoma. Proc R Soc Med 14:31, 1921 92. Snydacker D: The normal optic disc: Ophthalmoscopic and photographic studies. Am J Ophthalmol 58:958, 1964 93. Armaly MF: Genetic determination of cup/disc ratio of the optic nerve. Arch Ophthalmol 78:35, 1967 94. Richardson KT: Optic cup symmetry in normal newborn infants. Invest Ophthalmol 7:137, 1968 95. Schwartz B, Reinstein NM, Lieberman DM: Pallor of the optic disc: Quantitative photographic evaluation. Arch Ophthalmol 89:278, 1973 96. Colenbrander MC: Measurement of the excavation. Ophthalmologica 139:491, 1960 97. Anderson DR: Clinical evaluation of the glaucomatous fundus. In: Symposium on Glaucoma: Transactions of the New Orleans Academy of Ophthalmology, p. 95. St. Louis, CV Mosby, 1975 98. Gundersen KG, Heijl A, Bengtsson B. Optic nerve head sector analysis recognizes glaucoma most effectively around
disc poles. Acta Ophthalmol Scand 77:13, 1999 99. Gundersen KG, Heijl A, Bengtsson B: Comparability of three-dimensional optic disc imaging with different
techniques; a study with confocal scanning laser tomography and raster
tomography. Acta Ophthalmol Scand 78:9, 2000 100. Jonas JB, Fernandez MD, Naumann GOH: Glaucomatous optic nerve atrophy in small discs with low cup-to-disc
ratios. Ophthalmology 97:1211, 1990 101. Kronfeld PC: The optic nerve. In: Symposium on Glaucoma: Transactions of the New Orleans Academy of Ophthalmology, p. 62. St. Louis, CV Mosby, 1967 102. Begg IS, Drance SM, Goldman H: Fluorescein angiography in the evaluation of focal circulatory ischaemia
of the optic nervehead in relation to the arcuate scotoma in glaucoma. Can J Ophthalmol 7:68, 1972 103. Read RM, Spaeth GL: The practical clinical appraisal of the optic disc in glaucoma: The natural
history of cup progression and some specific disc-field correlations. Trans Am Acad Ophthalmol Otolaryngol 78:OP-255, 1974 104. Phelps CD: Recognition of glaucomatous cupping. In Blodi FC (ed): Current Concepts in Ophthalmology, Vol 4, p. 72. St. Louis, CV Mosby, 1974 105. Hitchings RA, Spaeth GL: The optic disc in glaucoma: I. Classification. Br J Ophthalmol 60:778, 1976 106. Radius RL, Maumenee AE, Green WR: Pit-like changes of the optic nerve head in open-angle glaucoma. Br J Ophthalmol 62:389, 1978 107. Spaeth GL: Morphological damage of the optic nerve. In Heilmann K, Richardson KT (eds): Glaucoma: Conceptions of a Disease; Pathogenesis, Diagnosis, Therapy, p. 138. Philadelphia, WB Saunders, 1997. 108. Herschler J, Osher RH: Baring of the circumlinear vessel: An early sign of optic nerve damage. Arch Ophthalmol 98:865, 1980 109. Jonas JB, Fernandez MC, Sturmer J: Pattern of glaucomatous neuroretinal rim loss. Ophthalmology 100:63, 1993 110. Jonas JB, Nguyen NX, Naumann GO: Non-quantitative morphologic features in normal and glaucomatous
optic discs. Acta Ophthalmol 67(4):361, 1989 111. Jonas JB, Gusek GC, Naumann GOH: Optic disc morphometry in chronic primary open-angle glaucoma. I. Morphometric
intrapapillary characteristics. Graefes Arch Clin Exp Ophthalmol 226:522, 1988 112. Jonas JB, Gusek GC, Naumann GOH: Optic disc morphometry in chronic primary open-angle glaucoma. II. Correlations
of the intrapapillary parameters to visual field indices. Graefes Arch Clin Exp Ophthalmol 226:531, 1988 113. Bayer A, Harasymowycz , Henderer JD, et al: Validity of a new disk grading scale for extimating glaucomatous damage: Correlation
with visual field damage. Am J Ophthalmol 133:758–763, 2002 114. Quigley H, Anderson DR: Cupping of the optic disc in ischemic optic neuropathy. Trans Amer Acad Ophthalmol Otolaryngol 83:755–762, 1977 115. Hayrey SS, Jonas JB: Optic disc morphology after arteritic ischemic optic neuropathy. Ophthalmology 108:1586, 2001 116. Primrose J: Clinical review of glaucomatous discs. In Cant JS (ed): The Optic Nerve; Proceedings of the Second William Mackenzie Memorial Symposium, Glasgow, September 1971, p. 311. London, Henry Kimpton, 1972 117. Primrose J: Early signs of the glaucomatous disc. Br J Ophthalmol 55:820, 1971 118. Primrose J: The incidence of the peripapillary halo glaucomatosus. Trans Ophthalmol Soc UK 89:585, 1969 119. Wilensky JT, Koller AE: Peripapillary changes in glaucoma. Am J Ophthalmol 81:341, 1976 120. Laatikainen L: Fluorescein angiographic studies of the peripapillary and perilimbal regions
in simple, capsular and low-tension glaucoma. Acta Ophthalmol Suppl 111, 1971 121. Rockwood EJ, Anderson DR: Acquired peripapillary changes and progression in glaucoma. Graefes Arch Clin Exp Ophthalmol 226:510, 1988 122. Buus DR, Anderson DR: Peripapillary crescents and halos in normal-tension glaucoma and
ocular hypertension. Ophthalmology 96:16, 1989 123. Jonas JB, Xu L: Parapapillary chorioretinal atrophy in normal-pressure glaucoma. Am J Ophthalmol 115:501, 1993 124. Jonas JB, Fernandez MC, Naumann GO: Glaucomatous parapapillary atrophy. Occurrence and correlations. Arch Ophthalmol 110(2):214, 1992 125. Jonas JB, Gusek GC, Naumann GOH: Die parapapilläre region in normal- and glaukomaugen. I. Planimetrische
werte von 312 glaukom- und 125 normalaugen. Klin Monatsbl Augenheilkd 193:52, 1988 126. Jonas JB, Naumann GO: Parapapillary chorioretinal atrophy in normal and glaucoma eyes. II. Correlations. Invest Ophthalmol Vis Sci 30:919, 1989 127. Jonas JB, Nguyen NX, Gusek GC, Naumann GO: Parapapillary chorioretinal atrophy in normal and glaucoma eyes. I. Morphometric
data. Invest Ophthalmol Vis Sci 30:908, 1989 128. Jonas JB, Xu L: Parapapillary chorioretinal atrophy in normal-pressure glaucoma. Am J Ophthalmol 115:501, 1993 129. Kasner O, Feuer WJ, Anderson DR: Possibly reduced prevalence of peripapillary crescents in ocular hypertension. Can J Ophthalmol 24):211, 1989 130. Tezel G, Kolker AE, Wax MB, et al: Parapapillary chorioretinal atrophy in patients with ocular hypertension. I. An
evaluation as a predictive factor for the development of glaucomatous
damage. Arch Ophthalmol 115:1503, 1997 131. Nevarez J, Rockwood EJ, Anderson DR: The configuration of peripapillary tissue in unilateral glaucoma. Arch Ophthalmol 106:901, 1988. 132. Jonas JB, Grundler AE: Optic disc morphology in juvenile primary open-angle glaucoma. Graefe's Arch Clin Exp Ophthalmol 234:750, 1995 133. Rader J, Feuer WJ, Anderson, DR: Peripapillary vasoconstriction in the glaucomas and the anterior ischemic
optic neuropathies. Am J Ophthal 117:72, 1994 134. Nicolela M, Drance SM: Various glaucomatous optic disc appearances and their clinical correlations. Ophthalmology 103:640, 1996 135. Thompson AH: Physiological and glaucoma cups. Trans Ophthalmol Soc UK 40:334, 1920 136. Lister A: The prognosis in congenital glaucoma. Trans Ophthalmol Soc UK 86:5, 1966 137. Chandler PA, Grant WM: Lectures on Glaucoma, p. 327. Philadelphia, Lea & Febiger, 1965 138. Hetherington J: Discussion of paper by Richardson. Invest Ophthalmol 7:140, 1968 139. Shaffer RN, Hetherington J Jr : The glaucomatous disc in infants. A suggested hypothesis for disc cupping. Trans Am Acad Ophthalmol Otolaryngol 73:929, 1969 140. Hetherington JJr , Shaffer RN, Hoskins HDJr : The disc in congenital glaucoma. In Etienne R, Paterson GD (eds): International Glaucoma Symposium, Albi, France, 1974, p. 127. Marseille, Diffusion Generate de Librairie, 1975 141. Neumann E, Hyams SW: Intermittent glaucomatous excavation. Arch Ophthalmol 90:64, 1973 142. Kessing SV, Gregersen E: The distended disc in early stages of congenital glaucoma. Acta Ophthalmol 55:431, 1977 143. Quigley HA: The pathogenesis of reversible cupping in congenital glaucoma. Am J Ophthalmol 84:358, 1977 144. Spaeth GL: Appearances of the optic disc in glaucoma: A pathogenic classification. In Symposium on Glaucoma: Transactions of the New Orleans Academy of Ophthalmology, p. 114. St. Louis, CV Mosby, 1981 145. Quigley HA: Childhood glaucoma. Results with trabeculotomy and study of reversible cupping. Ophthalmology 89:219, 1982 146. Pederson JE, Herschler J: Reversal of glaucomatous cupping in adults. Arch Ophthalmol 100:426, 1982 147. Zimmerman LE, deVenecia G, Hamasaki DI: Pathology of the optic nerve in experimental acute glaucoma. Invest Ophthalmol 6:109, 1967 148. Zimmerman LE: Discussion. In Symposium on Glaucoma: Transactions of the New Orleans Academy of Ophthalmology, p. 192. St. Louis, CV Mosby, 1967 149. Kronfeld PC: Glaucoma and the optic nerve: A historical review. Surv Ophthalmol 19:154, 1974 150. Douglas GR, Drance SM, Schulzer M: The visual field and nerve head following acute angle closure glaucoma. Can J Ophthalmol 9:404, 1974 151. Douglas GR, Drance SM, Schulzer M: The visual field and nerve head in angle-closure glaucoma: A comparison
of the effects of acute and chronic angle closure. Arch Ophthalmol 93:409, 1975 152. Radius RL, Maumenee AE: Visual field changes following acute elevation of intraocular pressure. Trans Am Acad Ophthalmol Otolaryngol 83:OP-61, 1977 153. Hodapp E, Parrish RK, Anderson DR: Clinical Decisions in Glaucoma. St. Louis, CV Mosby, 1993 154. Behrendt T, Wilson LA: Spectral reflectance photography of the retina. Am J Ophthalmol 59:1079, 1965 155. Spaeth GL: Fluorescein angiography: Its contributions towards understanding the mechanisms
of visual loss in glaucoma. Trans Am Ophthalmol Soc 73:491, 1975 156. Fishbein SL, Schwartz B: Optic disc in glaucoma: Topography and extent of fluorescein filling defects. Arch Ophthalmol 95:1975, 1977 157. Schwartz B, Rieser JC, Fishbein SL: Fluorescein angiographic defects of the optic disc in glaucoma. Arch Ophthalmol 95:1961, 1977 158. Talusan E, Schwartz B: Specificity of fluorescein angio-graphic defects of the optic disc
in glaucoma. Arch Ophthalmol 95:2166, 1977 159. Talusan ED, Schwartz B, Wilcox LMJr : Fluorescein angiography of the optic disc: A longitudinal follow-up
study. Arch Ophthalmol 98:1579, 1980 |