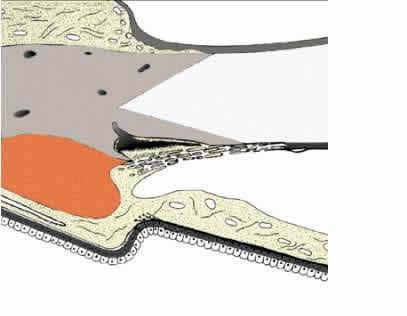
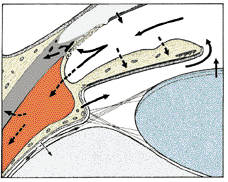
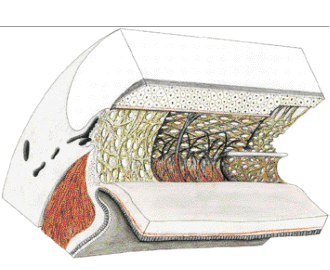
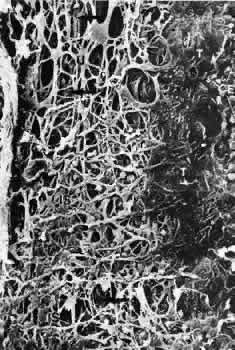
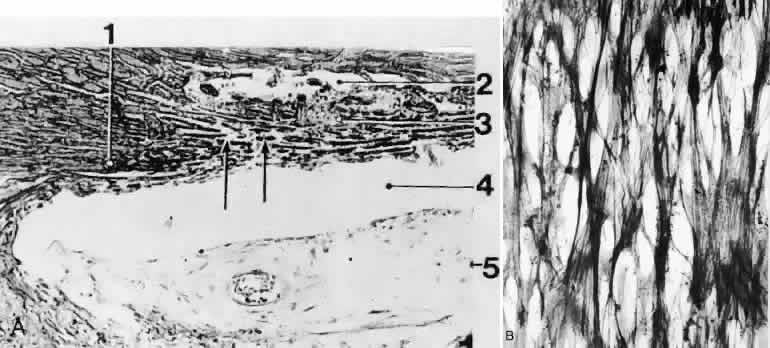
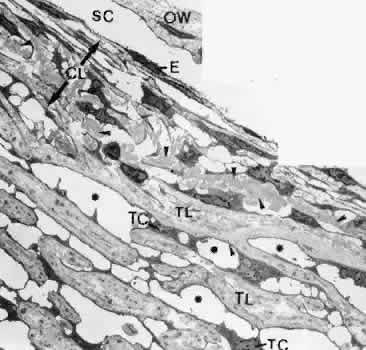
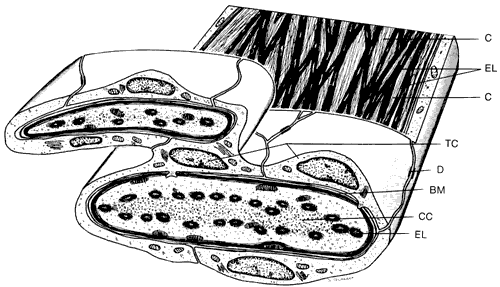
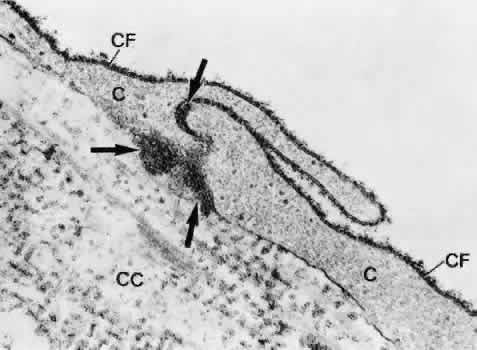
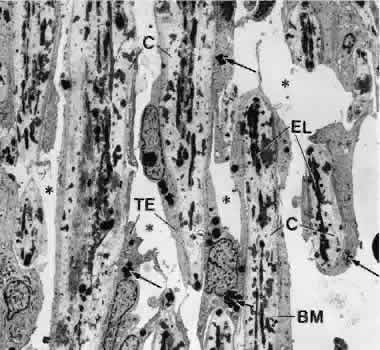
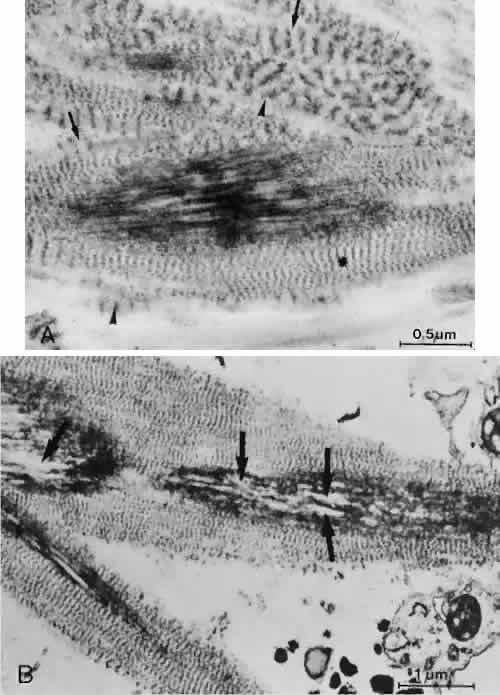
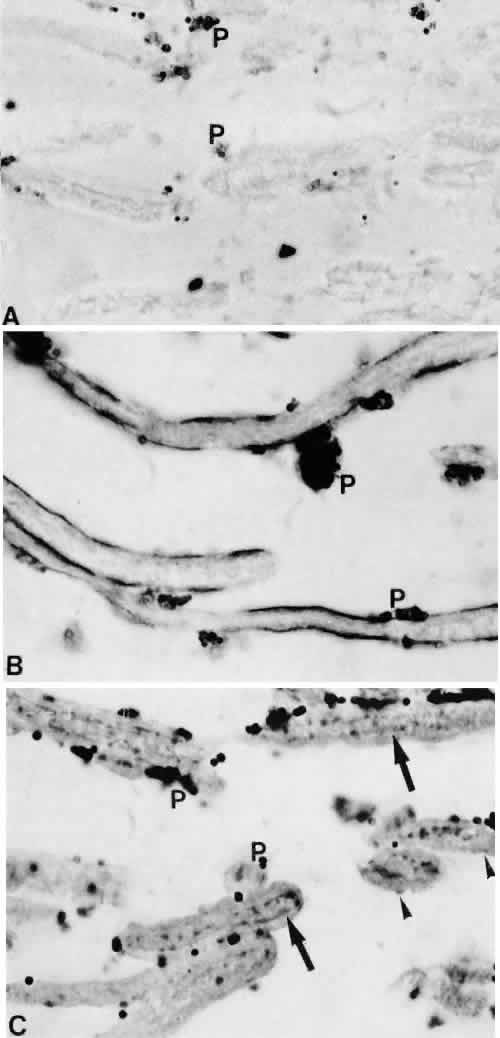
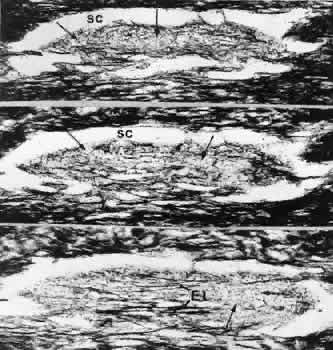
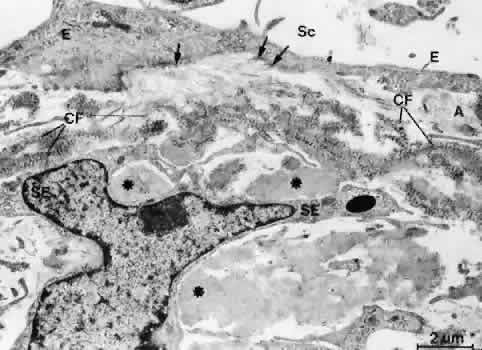
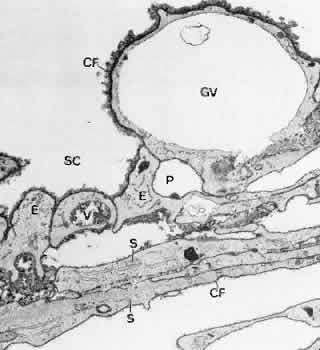
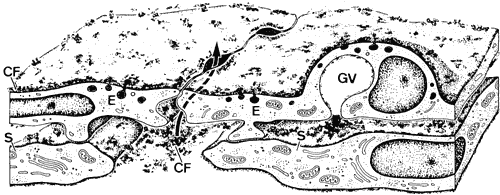
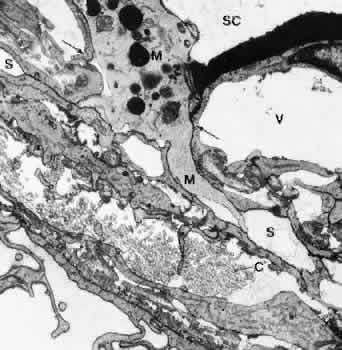
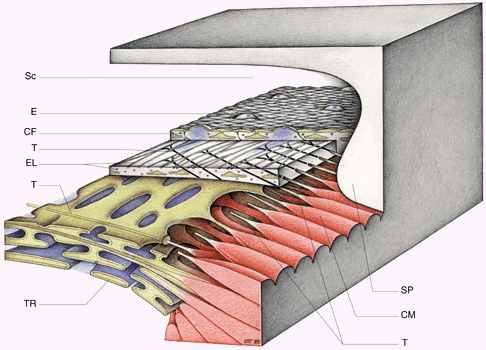
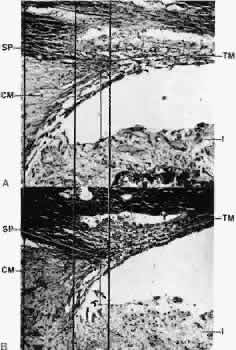
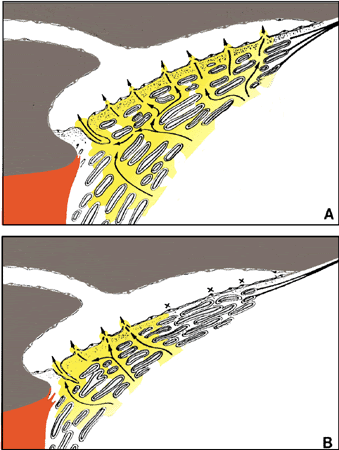
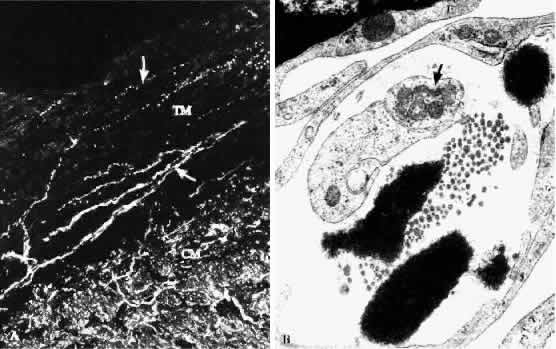
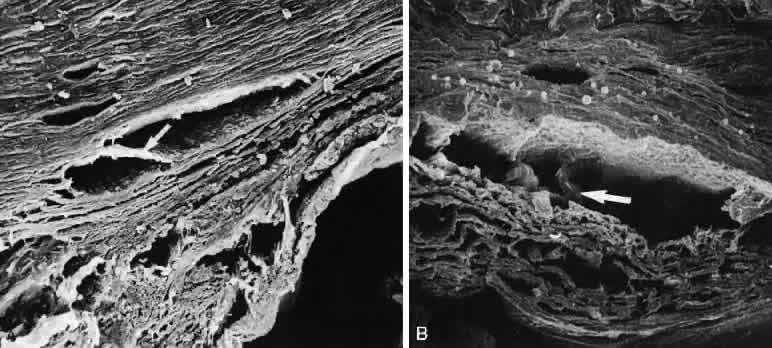
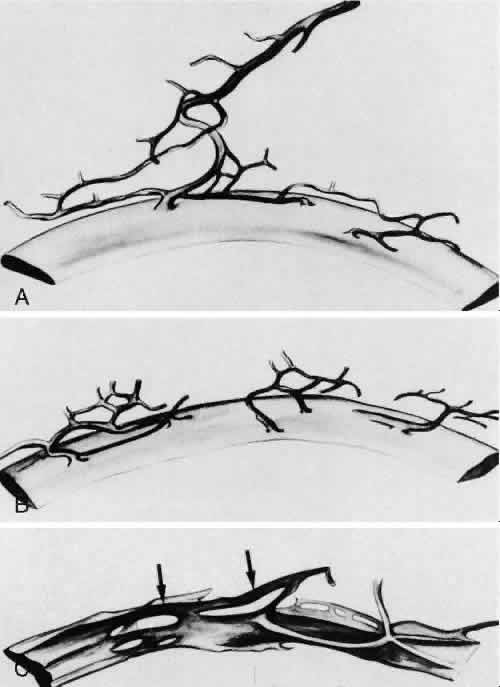
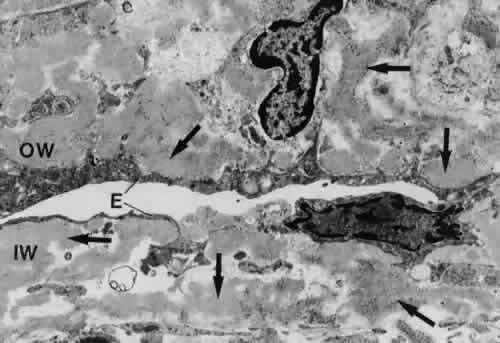
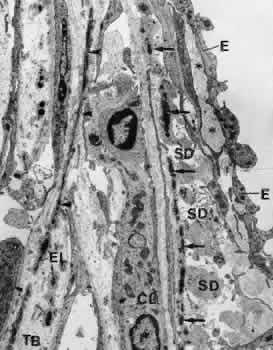
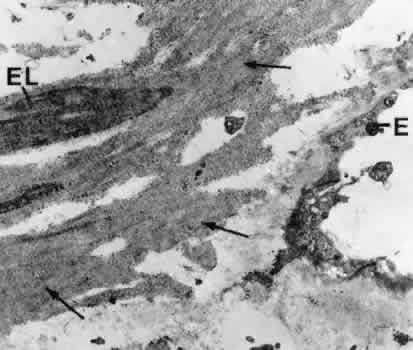
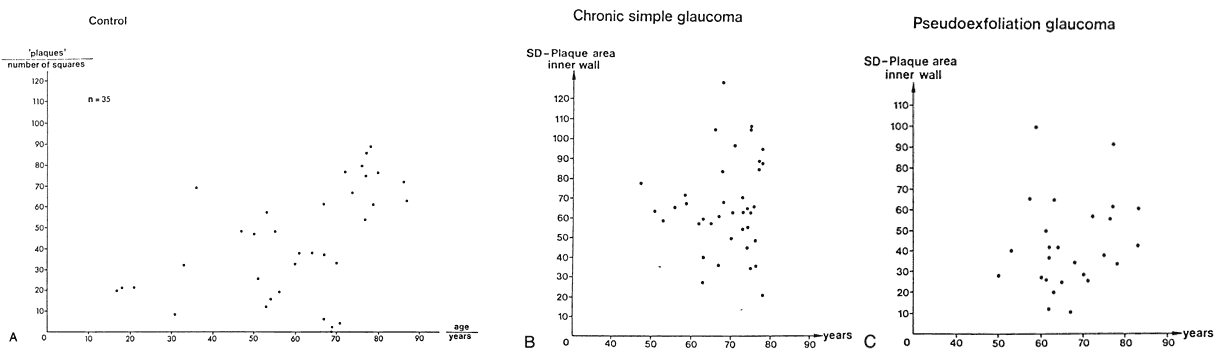
1. Ashton N: Anatomical study of Schlemm's canal and aqueous veins by means of
neoprene casts: 1. Aqueous veins. Br J Ophthalmol 35:291, 1951 2. Rohen JW, Rentsch FJ: Über den Bau des Schlemm'schen Kanals und seiner AbfluÂwege
beim Menschen. Graefes Arch Clin Exp Ophthalmol 176:309, 1968 3. Bill A: The aqueous humor drainage mechanism in the cynomolgus monkey (Macaca irus) with evidence for unconventional routes. Invest Ophthalmol 4:911, 1965 4. Bill A, Phillips C: Uveoscleral drainage of aqueous humour in human eyes. Exp Eye Res 12:275, 1971 5. Grant MW: Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol 69:783, 1963 6. Maepea O. Bill A: The pressure in the episcleral veins, Schlemm's canal and the trabecular
meshwork in monkeys: Effects of changes in intraocular pressure. Exp Eye Res 49:645, 1989 7. Lütjen-Drecoll E, Rohen JW: Morphology of aqueous outflow pathways
in normal and glaucomatous eyes. In Ritch R, Shields MB, Krupin T (eds): The
Glaucomas, pp 89–123. St. Louis, CV Mosby, 1996 8. Raviola G. Raviola E: Paracellular route of aqueous outflow in the trabecular meshwork and canal
of Schlemm. Invest Ophthalmol Vis Sci 21:52, 1981 9. Epstein DL, Rohen JW: Morphology of the trabecular meshwork and inner wall endothelium after
cationized ferritin perfusion in the monkey eye. Invest Ophthalmol Vis Sci 32:160, 1991 10. Rohen JW, Lütjen-Drecoll E: Age changes of the trabecular meshwork
in human and monkey eyes. In Bredt H, Rohen JW (eds): Aging and Development, vol 1. Stuttgart, Schattauer Verlag, 1971 11. Grierson I, Lee WR: Acid mucopolysaccharides in the outflow apparatus. Exp Eye Res 21:417, 1975 12. Grierson 1, Lee WR, Abraham S: A light microscopic study of the effects
of testicular hyaluronidase on the outflow system of the baboon (Papio cynocephalus). Invest Ophthalmol Vis Sci 18:356, 199 13. Lütjen-Drecoll E, Shimizu T, Rohrbach M, Rohen JW: Quantitative analysis of “plaque material” between ciliary
muscle tips in normal and glaucomatous eyes. Exp Eye Res 42:457, 1986 14. Lütjen-Drecoll E, Futa R. Rohen JW: Ultrahistochemical studies on tangential sections of the trabecular meshwork
in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci 21:563, 1981 15. Gong HY, Tinkaus-Randall V, Freddo TF: Ultrastructural localization of elastin in normal human trabecular meshwork. Curr Eye Res 8:1071, 1989 16. Lütjen-Drecoll E, Rittig M, Rauterberg J et al: Immunomicroscopical study of type Vl collagen in the trabecular meshwork
of normal and glaucomatous eyes. Exp Eye Res 48:139, 1989 17. Murphy, Yun AJ, Newsome DA, Alvarado JA: Localization of extracellular proteins of the human trabecular meshwork
by indirect immunofluorescence. Am J Ophthalmol 104:33, 1987 18. Rohen JW, Lütjen-Drecoll E: Biology of the trabecular meshwork. In
Lutjen-Drecoll E (ed): Basic Aspects ofGlaucoma Research, vol 1. Stuttgart, Schattauer
Verlag,1982 19. Lütjen-Drecoll E, Dietl T, Futa R, Rohen JW: Age changes of the trabecular
meshwork, a preliminary morphometric study. In Hollyfield JG (ed): The
Structure of the Eye. Amsterdam, Elsevier, 1982 20. Marshall GE, Koustas AGP, Lee WR: Immunogold ultrastructural localization of collagens in the aged human
outflow system. Ophthalmology 98:692, 1991 21. Rohen JW, Unger HH: Zur Morphologie und Pathologie der Kammerbucht des
Auges, Abhandlung der Akademie der Wissenschaften und der Literatur, Mainz, Nr. 3. Wiesbaden, Steiner Verlag, 1959 22. Rohen JW, Futa R, Lütjen-Drecoll E: The fine structure of the cribriform meshwork in normal and glaucomatous
eyes as seen in tangential sections. Invest Ophthalmol Vis Sci 21:574, 1981 23. Lütjen-Drecoll E: Structural factors influencing outflow facility and its changeability under
drugs: A study in Macaca arctoides. Inves Ophthalmol 12:280, 1973 24. Ethier CR, Kamm RD: Calculations of flow resistance in the juxtacanalicular meshwork. Invest Ophthalmol Vis Sci 27:1741, 1986 25. Seiler T, Wollensak J: The resistance of the trabecular meshwork to aqueous humor outflow. Graefes Arch Clin Exp Ophthalmol 223:88, 1985 26. Lütjen-Drecoll E, Schenholm M, Tamm E, Tengblad M: Visualization of hyaluronic acid in the anterior segment of rabbit and
monkey eyes. Exp Eye Res 51:55–63, 1990 27. Rodrigues MM, Katz S, Foidart JM, Spaeth GL: Collagen, Factor VIII antigen, and immunoglobulins in the human aqueous
drainage channels. Ophthalmology 87:337, 1980 28. Wulle KG: Electron microscopic observations of the development of Schlemm's
canal in the human eye. Trans Am Acad Ophthalmol Otolaryngol 72:765, 1968 29. Wulle KG: The development of the productive and draining system of the aqueous humor
in the human eye. Adv Ophthalmol 26:269, 1972 30. Lütjen-Drecoll E, Rohen JW: Uber die endotheliale Auskleidung des Schlemm'schen Kanals im Silberimpragnationsbild. Graefes Arch Clin Exp Ophthalmol 180:249, 1970 31. Grierson 1, Lee WR: The fine structure of the trabecu-lar meshwork at graded
levels of intraocular pressure:1. Pressure effects within the near-physiological
range(8–30 mm Hg). Exp Eye Res 20:505, 1975 32. Lee WR, Grierson I, McMenamin PG: The morphological response of the primate
outflow system to changes in pressure and flow. In Lutjen-Drecoll
E (ed): Basic Aspeelslaueoma Research. Stuttgart, Sehattauer Verlag, 1982 33. Holmberg A: Sehlemm's canal and the trabecular meshwork: An electron microscopy
study of the normal structure in man and monkey (Cercopithecus ethiops). Ophthalmol 19:339, 1965 34. Tripathi RC: Mechanism of the aqueous outflow across the trabecular wall of Schlemm's
canal. Exp Eye Res 11:116, 1971 35. Bill A, Svedbergh B: Scanning electron microscopic studies of the trabecular meshwork and the
canal of Schlemm:An attempt to localize the main resistance to outflow
of aqueous humor in man. Acta Ophthalmol 50:295, 1972 36. Tripathi RC: Aqueous outflow pathway in normal and glaucomatous eyes. Br J Ophthalmol 56:157, 1972 37. Grierson I, Lee WR: Changes in the monkey outflow apparatus at graded levels of intraocular
pressure. Exp Eye Res 19:21, 1974 38. Erikson A, Svedbergh B: Transcellular aqueous humor outflow: A theoretical and experimental study. Graefes Arch Clin Exp Ophthalmol 212:53, 1980 39. Inomata H. Bill A, Smelser GK: Aqueous humor pathways through the trabecular meshwork and into Schlemm's
canal in the cynomolgus monkey (Macaca irus): An electron microscopic study. Am J Ophthalmol 73:760, 1972 40. Barany EH: The immediate effect on outflow resistance of intravenous pilocarpine in
the vervet monkey (Cercopithecus ethiops). Invest Ophthalmol Vis Sei 6:373, 1967 41. Barany EH: The mode of action of miotics on outflow resistance: A study of pilocarpine
in the vervet monkey (Cercopithecus aethiops). Invest Ophthalmol 6:373, 1967 42. Rohen JW, Lütjen E, Barany EH: The reaction between the ciliary muscle and the trabecular meshwork and
its importance for the effect of miotics on aqueous outflow resistance: A
study in two contrasting monkey species, Macaca irus and Cercopithecus aethiops. Graefes Arch Clin Exp Ophthalmol 172:23, 1967 43. Ringvold A: Actin filaments in trabecular endothelial cells in eyes of the vervet monkey. Acta Ophthalmol 56:217, 1978 44. de Kater AW, Spurr-Michaud SJ, Gipson IK: Localization of smooth muscle myosin-containing cells in the aqueous outflow
pathway. Invest Ophthalmol Vis Sci 31:347, 1990 45. Flügel C, Tamm E, Lütjen-Drecoll E, Stefani FH: Age-related loss of a-smooth muscle actin in normal and glaucomatous human
trabecular meshwork of different age groups. J Glaucoma 1:165, 1992 46. Tamm ER, Flügel C, Stefani FH, Rohen JW: ontractile cells in the human scleral spur. Exp Eye Res 54:531, 1992 47. Lepple-Wienhues A, Rauch R, Clark AF et al: Electrophysiological properties of cultured human trabecular meshwork cells. Exp Eye Res 59:305, 1994 48. Gupta N, Drance SM, McAllister R et al: Localization of M3 muscarinic receptor subtype and mRNA in the human eye. Ophthalmic Res 26:207, 1994 49. Wiederholt M, Sturm A, Lepple-Wienhues A: Relaxation of trabecular meshwork and ciliary muscle by release of nitric
oxide. Invest Ophthalmol Vis Sci 35:2515, 1994 50. Wiederholt M, Bielka S, Schweig F et al: Regulation of outflow rate and resistance in the perfused anterior segment
of the bovine eye. Exp Eye Res 61:223, 1995 51. Ruskell GL: The source of nerve fibers of the trabeculae and adjacent structures in
monkey eyes. Exp Eye Res 23:449, 1976 52. Ruskell GL: Trigeminal innervation of the scleral spur in cynomolgus monkeys. J Anat 184:511, 1994 53. Stone RA: Nervous system and intraocular pressure. In Ritch R, Shields
MB, Krupin T (eds): The Glaucomas, 2d ed, p 357. St. Louis, Mosby, 1996 54. Selbach JM, Gottanka J, Wittmann M, Lütjen-Drecoll E: Efferent and afferent innervation of primate trabecular meshwork and scleral
spur. Invest Ophthalmol Vis Sci 41:2184, 2000 55. Tamm ER, Flügel C, Stefani FH, Lütjen-Drecoll E: Nerve endings with structural characteristics of mechanoreceptors in the
human scleral spur. Invest Ophthalmol Vis Sci 35:1157, 1994 56. Tamm ER, Koch TA, Mayer B et al: Innervation of myo-fibroblast-like scleral spur cells in human and monkey
eyes. Invest Ophthalmol Vis Sci 36:1633, 1995 57. Polansky J, Weinreb R, Baxter J, Alvarado J: Human trabecular cells: I. Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci 18:1043, 1979 58. Rohen JW, Sehachtschabel DO, Berghoff K: Histoauto-radiographic and biochemical studies on human and monkey trabecular
meshwork and ciliary body in short-term explat culture. Graefes Arch Clin Exp Ophthalmol 221:199, 1984 59. Acott TS, Wirtz MK: Biochemistry of aqueous outflow. In Ritch R, Shields
MB, Krupin T (eds): The Glaucomas, 2d ed, p 281. St. Louis, Mosby, 1996 60. Binninger EA, Schachtschabel DO, Rohen JW: Exogenous glycosaminoglycans stimulate hyaluronic acid synthesis by cultured
human trabecular meshwork cells. Exp Eye Res 45:169, 1987 61. Pandolfi M: Fibrinolysis and outflow resistance in the eye. Am J Ophthalmol 64:1141, 1967 62. Shuman MA, Polansky JR, Merkel C, Alvarado JA: Tissue plasminogen activator in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci 29:401, 1988 63. Rohen JW, van der Zypen E: The phagocytic activity of the trabecular meshwork endothelium: An electron
microscopic study of the vervet (Cercopithecus aethiops). Graefes Arch Clin Exp Ophthalmol 175:143, 1968 64. Barak MH, Weinreb RN, Ryder Ml: Quantitative assessment of cynomolgus monkey trabecular cell phagocytosis
and adsorption. Curr Eye Res 7:445, 1988 65. Chisholm IA, Grierson I: Particulate phagocytosis by trabecular meshwork endothelium. Can J Ophthalmol 12:293, 1977 66. Sherwood ME, Richardson TM: Phagocytosis by trabecular meshwork cells: Sequence of events in cats and
monkeys. Exp Eye Res 46:881, 1988 67. Johnson DH, Richardson TM, Epstein DL: Trabecular meshwork recovery after phagocytic challenge. Curr Eye Res 8:1121, 1989 68. Gottanka J, Flügel-Koch C, Martus P et al: Correlation of pseudoexfoliative
material and optic nerve damage in pseudoexfoliation syndrome. Invest
Ophthalmol Vis Sci 38:2435–2446 69. Flügel C, Kinne RW, Streilein JW, Lütjen-Drecoll E: Distinctive distribution of HLA class II presenting and bone marrow derived
cells in the anterior segment of human eyes. Curr Eye Res 11:1173–1183, 1992 70. Alvarado J, Murphy C, Polansky J, Juster R: Age-related changes in trabecular
meshwork cellularity. Invest Ophthalmol Vis Sci 21:714, 181 71. Grierson I, Howes RC: Age-related depletion of the cell population in the
human trabecular meshwork. Eye I:204, 1987 72. Grierson I, Wang Q, McMenamin PG, Lee WR: The effects of age and antiglaucoma drugs on the meshwork cell population. Res Clin Forums 4:69, 1982 73. Lütjen-Drecoll E, Kaufman PL: Biomechanics of echo thiophate-induced anatomic changes in monkey aqueous
outflow system. Graefes Arch Clin Exp Ophthalmol 224:564, 1986 74. Lütjen-Drecoll E, Kaufman PL: Long-term timolol and epinephrine in monkeys: 11. Morphological alterations
in trabecular meshwork and ciliary muscle. Trans Ophthalmol Soc UK 105:196, 1986 75. Acott TS, Samples JR, Bradley JM et al: Trabecular repopulation by anterior trabecular meshwork cells after laser
trabeculoplasty. Am J Ophthalmol 107:1, 1989 76. Raviola G: Schwalbe's line cells: A new cell type in the trabecular meshwork
of Macaca mulatta. Invest Ophthalmol Vis Sci 22:45, 1982 77. Stone RA, Kuwayama Y. Laties AM, Marangos PJ: Neuron-specific enolase-containing cells in the rhesus monkey trabecular
meshwork. Invest Ophthalmol Vis Sci 25:1332, 1984 78. Rittig M, Flügel C, Prehm P, Lütjen-Drecoll E: Hyaluronan synthase immunoreactivity in the anterior segment of the primate
eye. Graefes Arch Clin Exp Ophthalmol 231:313–317, 1993 79. Rohen JW, Rentsch FJ: Elektronenmikroskopische Untersuchungen uber den Bau der Auen, Bwand des
Schlemm'schen Kanals unter besonderer Berucksichtigung der Abflu, Bkanale
und Altersveranderungen. Graefes Arch Clin Exp Ophthalmol 177:1, 1969 80. Kleinert H: Die Vitalfarbung des Kammerwassers und seiner epibulbaren Abflu, Bwege
naeh Fluoreszeininjektion in die Vorderkammer. Klin Monatsbl Augenheilkd 122:665, 1953 81. Rohen JW, Witmer R: Electron microscopic studies on the trabecular meshwork in glaucoma simplex. Graefes Arch Clin Exp Ophthalmol 183:251, 1972 82. McMenamin PG, Lee WR: Age-related changes in extracellular matrials in the inner wall of Schlemm's
canal. Graefes Arch Clin Exp Ophthalmol 212:159, 1980 83. Lütjen-Drecoll E, Shimizu T. Rohrbach M, Rohen JW: Quantitative analysis of “plaque material” in the inner and
outer wall of Schlemm's canal in normal and glaucomatous eyes. Exp Eye Res 42:443, 1986 84. Arentsen JJ, Rodrigues MM, Laitsson PR: Histopathology of 150 trabeculectomy specimens in glaucoma. Invest Ophthalmol Vis Sci 16:32, 1977 85. Grierson I: Alterations in the outflow system in chronic simple glaucoma. Res Clin Forums 7:205, 1985 86. Gottanka J, Johnson DH, Martus P, Lütjen-Drecoll E: Severity of optic nerve damage in eyes with POAG is correlated with changes
in the trabecular meshwork. J Glaucoma 6:123–132, 1997 87. Lütjen-Drecoll E, May CA, Polansky JR et al: Localization of the stress protein αB-crystallin and trabecular meshwork
inducible glucocorticoid response protein in normal and glaucomatous
trabecular meshwork. Invest Ophthalmol Vis Sci 39:517–525, 1998 88. Siegner A, May CA, Welge-Lüssen U et al: αB-crystallin in the primate ciliary muscle and trabecular meshwork. Eur J Cell Biol 71:165–169, 1996 89. Welge-Lüssen U, May CA, Eichhorn M, et al: αB-crystallin in the trabecular meshwork is inducible by transforming
growth factor-ss. Invest Ophthalmol Vis Sci 40:2235–2241, 1999 90. Alvarado J, Murphy C, Juster R: Trabecular meshwork cellularity in POAG and non-glaucomatous normals. Ophthalmology 91:564, 1984 91. Ringvold A, Vegge T: Electron microscopy of the trabecular meshwork in eyes with exfoliation
syndrome (pseudoexfoliation of the lens capsule). Virchows Arch A 353:110, 1971 92. Layden WE, Shaffer RN: Exfoliation syndrome. Am J Ophthalmol 78:835, 1974 93. Sugar HS, Harding C, Barsky D: The exfoliation syndrome. Ann Ophthalmol 8:1165, 1976 94. Roth M, Epstein DL: Exfoliation syndrome. Am J Ophthalmol 89:477, 1980 95. Schlötzer-Schrehardt U, Naumann GOH: Trabecular meshwork in pseudoexfoliation syndrome with and without glaucoma. A
morphometric, ultrastructural study. Invest Ophthalmol Vis Sci 36:1750, 1995 96. Streeten BW, Gibson S, Dark AJ: Pseudoexfoliative material contains an elastic microfibrillar-associated
glycoprotein. Transact Am Ophthalmol Soc 84:304, 1986 97. Zong-Yi L, Streeten BW, Wallace RN: Association of elastin with pseudoexfoliative material: An immunoelectron
microscopic study. Current Eye Res 7:1163, 1988 98. Schlötzer-Schrehardt U, von der Mark K, Sakai LY, Naumann GO: Increased extracellular deposition of fibrillin-containing fibrils in pseudoexfoliation
syndrome. Invest Ophthalmol Vis Sci 38:970, 1997 99. Zong-Yi L, Streeten BW, Yohai N: Amyloid P in pseudoexfoliative fibrillopathy. Current Eye Res 8:217, 1989 100. Richardson TM, Hutchinson BT, Grant M: The outflow tract in pigmentary glaucoma. Arch Ophthalmol 95:1015, 1977 101. Rohen JW: Anatomy of the aqueous outflow channels. In Cairns JE (ed): Glaucoma, vol
I. London, Grune & Stratton, 1986 102. Johnson DH: Does pigmentation affect the trabecular meshwork? Arch Ophthalmol 107:250, 1989 103. Furuyoshi N, Furuyoshi M, Futa R et al: Ultrastructural changes in the trabecular meshwork of juvenile glaucoma. Ophthalmologica 211:140–146, 1997 104. Rohen JW, Linner E, Witmer R: Electron microscopic studies on the trabecular meshwork in two cases of
cortisone glaucoma. Exp Eye Res 17:19, 1973 105. Spaeth GL, Rodrigues MM, Weinreb S: Steroid-induced glaucoma: 1. Persistent elevation of intraocular pressure. II. Histopathologic
aspects. Trans Am Ophthalmol Soc 75:353, 1977 106. Johnson D, Gottanka J, Flügel C et al: Ultrastructural changes in the trabecular meshwork of human eyes treated
with corticosteroids. Arch Ophthalmol 115:375, 1997 |