| This section will review the anatomy of the orbit and its surrounding structures: the
cavernous sinus (CS), the paranasal sinuses, and the pterygopalatine
fossa. Particular attention will be paid to the orbital
apex (the superior and inferior orbital fissures, optic canal), the CS, and
the pterygopalatine fossa because these areas are often difficult
to understand but remain important in diagnosing orbital and neuro-ophthalmic
disease as well as in interpreting radiologic studies (CT and
MRI). Although anatomically the lacrimal drainage apparatus is preseptal (eyelid
structure) whereas the main secretory unit (lacrimal gland) is
partially postseptal (orbital structure), the entire lacrimal system
is combined in this section to unify its discussion. OSTEOLOGY The orbit may be considered a pear-shaped, conical space with a volume
of about 30 ml (Fig. 7A).1,2 Its maximum diameter occurs approximately 1 cm behind the arcus marginalis, an
important consideration during surgical dissection around the
orbital rim (Fig. 7B). Other essential measurements are summarized in Table 2. The orbit is composed of seven bones: the frontal, sphenoid, ethmoid, palatine, and
lacrimal, as well as the zygoma and maxilla, with each
wall containing different bones (Table 3). One mnemonic for remembering the number of bones contained within each
wall is to begin medially and travel around the orbit counterclockwise, arriving
at the sequence 4-3-2-2. Note that the orbital rim is not
a continuous ovoid, but rather a spiral that is discontinuous medially
to form the lacrimal sac fossa (see Fig. 7A). Other important facts regarding each orbital wall are noted in Table 4. 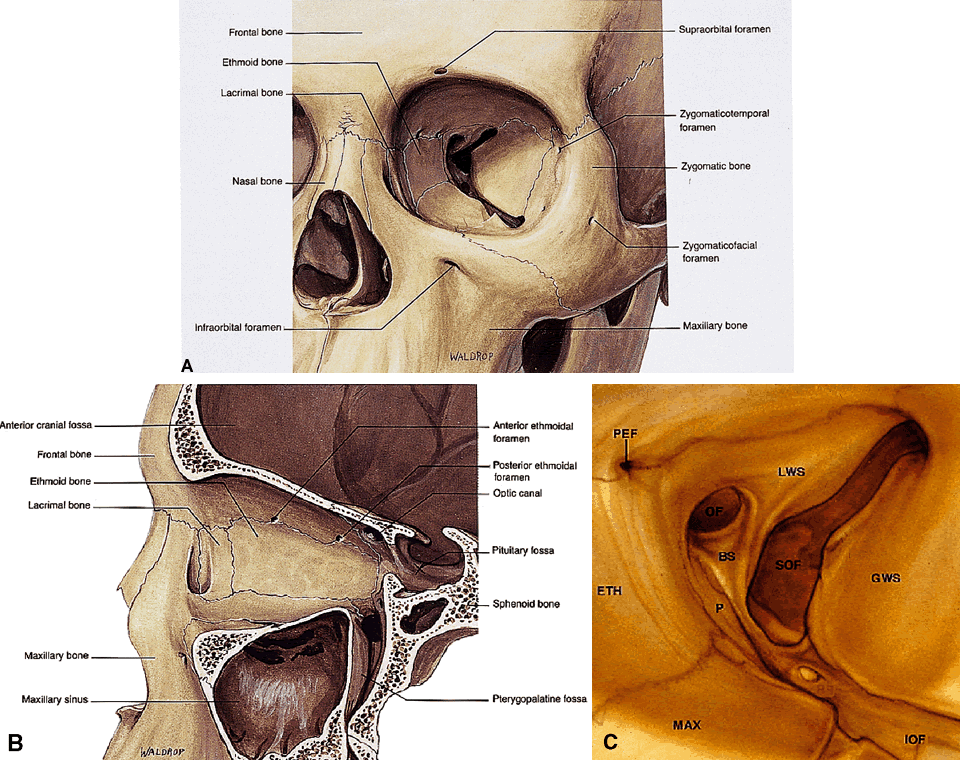 Fig. 7. Orbital osteology. A. Oblique frontal view. Note that the supraorbital foramen and infraorbital
foramen usually lie in the same vertical plane. The supraorbital foramen
may also occur as a notch. Note the discontinuity of the orbital
rim medially, forming the lacrimal sac fossa. B. Parasagittal view. The largest diameter of the orbit occurs about 1 cm
posterior to the orbital rim. Note the medial location of the optic foramen. The
superior orbital fissure and CS lie in the same plane in the
orbital apex. Note the vertical orientation of the pterygopalatine
fossa located directly behind the maxillary sinus and communicating with
the orbit through the inferior orbital fissure. C. Osteology of the orbital apex. GWS, greater wing of sphenoid; LWS, lesser
wing; BS, body of sphenoid; P, palatine bone; MAX, maxillary bone; OF, optical
foramen; SOF, superior orbital fissure; PEF, posterior ethmoidal
foramen. (A and B modified from Dutton JJ: Atlas of Clinical and Surgical Orbital Anatomy, p 8. Philadelphia, WB
Saunders, 1994. C modified from Zide BM, Jelks GW: Surgical Anatomy of the Orbit, p 8. New
York, Raven Press, 1985) Fig. 7. Orbital osteology. A. Oblique frontal view. Note that the supraorbital foramen and infraorbital
foramen usually lie in the same vertical plane. The supraorbital foramen
may also occur as a notch. Note the discontinuity of the orbital
rim medially, forming the lacrimal sac fossa. B. Parasagittal view. The largest diameter of the orbit occurs about 1 cm
posterior to the orbital rim. Note the medial location of the optic foramen. The
superior orbital fissure and CS lie in the same plane in the
orbital apex. Note the vertical orientation of the pterygopalatine
fossa located directly behind the maxillary sinus and communicating with
the orbit through the inferior orbital fissure. C. Osteology of the orbital apex. GWS, greater wing of sphenoid; LWS, lesser
wing; BS, body of sphenoid; P, palatine bone; MAX, maxillary bone; OF, optical
foramen; SOF, superior orbital fissure; PEF, posterior ethmoidal
foramen. (A and B modified from Dutton JJ: Atlas of Clinical and Surgical Orbital Anatomy, p 8. Philadelphia, WB
Saunders, 1994. C modified from Zide BM, Jelks GW: Surgical Anatomy of the Orbit, p 8. New
York, Raven Press, 1985)
|
TABLE TWO. Orbital Measurements
| Volume | 30 ml |
| Globe volume | 6.5 ml |
| Width | 45 mm |
| Height | 35 mm |
| Maximum circumference | 1 cm behind rim |
| Length: medial wall | 40–45 mm |
| Embryologic origin | Neural crest, except superotemporal orbit (mesoderm) | TABLE THREE. Bones Forming the Orbital Walls
| Orbital Wall | Bones | Borders |
| Superior (roof) | Frontal | Anterior cranial fossa |
| | Sphenoid (lesser wing) | Frontal sinus |
| Lateral | Zygoma | Temporalis fossa |
| | Sphenoid (greater wing) | Pterygopalatine fossa |
| Inferior (floor) | Maxilla (medial), zygoma (lateral), palatine (posterior) | Infraorbital canal |
| | | Maxillary sinus |
| Medial | Maxilla, lacrimal, ethmoid, sphenoid (anterior to posterior) | Ethmoid, sphenoid sinuses |
| | | Cribriform plate at level of frontoethmoidal suture | TABLE FOUR. Anatomic Findings of Each Orbital Wall
Floor
Slopes downward posterior to anterior 20°
Does not reach orbital apex (ends at pterygopalatine fossa)
Only wall without sphenoid bone contribution
Roof
Contains: Trochlea, 4 mm behind the orbital rim
Lacrimal gland fossa (postseptal)
Supraorbital notch
Medial
Medial walls parallel, 25 mm apart
“Paper thin”(lamina papyracea)
Lacrimal sac fossa (preseptal)
Lateral
Lateral walls are perpendicular to one another
Strongest wall, but offers least protection to globe (only 50% of globe
covered)
Contains lateral orbital tubercle (of Whitnall)
A clear understanding of the relation of the bony orbit to the skull and
the midface allows for a logical interpretation of the clinical and
radiographic patterns of orbital disease. The bones of the face may be
considered to hang from the skull, with attachments at the frontozygomatic
and frontoethmoidal sutures, as well as the sphenoid bone. Craniofacial
dysjunction occurs in these areas in Le Forte III fractures, and
the sites of craniofacial articulation are also the basis for the Le
Forte III osteotomies used for facial reconstruction in patients with
craniofacial synostoses. The complex shape of the sphenoid wing provides
for an intimate communication between the CS, the orbital apex, and
the pterygopalatine fossa. Radiographically, the spaces and foramina of the orbital apex may be considered
to lie in three tiers (Fig. 8). The CS is found on the same level as the orbital apex, connecting directly
with it via the superior orbital fissure (SOF) to form the middle
tier. The inferior tier is formed by the inferior orbital fissure (IOF), which
provides direct communication between the orbital apex and
the pterygopalatine fossa, a vertically oriented space directly behind
the maxillary sinus. Finally, the optic canal has no direct communication
with any of the aforementioned spaces and should be considered to
lie above the SOF and CS, exiting the orbit in a superomedial course
through the body of the sphenoid as the superior tier.26 Orbital apical lesions can therefore gain ready access to the CS and pterygopalatine
fossa (Fig. 9). Spread into the cranial vault through the optic canal is usually limited
to lesions of the optic nerve (glioma) or nerve sheath (meningioma). 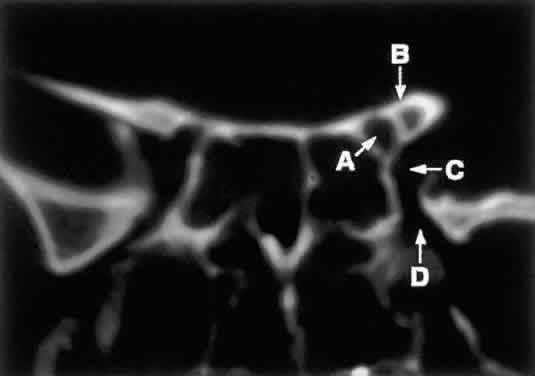 Fig. 8. Coronal CT image (bone window) of the orbital apex in a patient with facial
trauma. Note the position of the posterior orbital foramina. The
optic canal (A) is always seen in conjunction with the laterally adjacent anterior clinoid
process (B) on both axial and coronal views. Slightly lower, the superior orbital
fissure (C) communicates with the CS, found directly behind it. The inferior orbital
fissure (D) provides communication through the orbital floor with the pterygopalatine
fossa. Fig. 8. Coronal CT image (bone window) of the orbital apex in a patient with facial
trauma. Note the position of the posterior orbital foramina. The
optic canal (A) is always seen in conjunction with the laterally adjacent anterior clinoid
process (B) on both axial and coronal views. Slightly lower, the superior orbital
fissure (C) communicates with the CS, found directly behind it. The inferior orbital
fissure (D) provides communication through the orbital floor with the pterygopalatine
fossa.
|
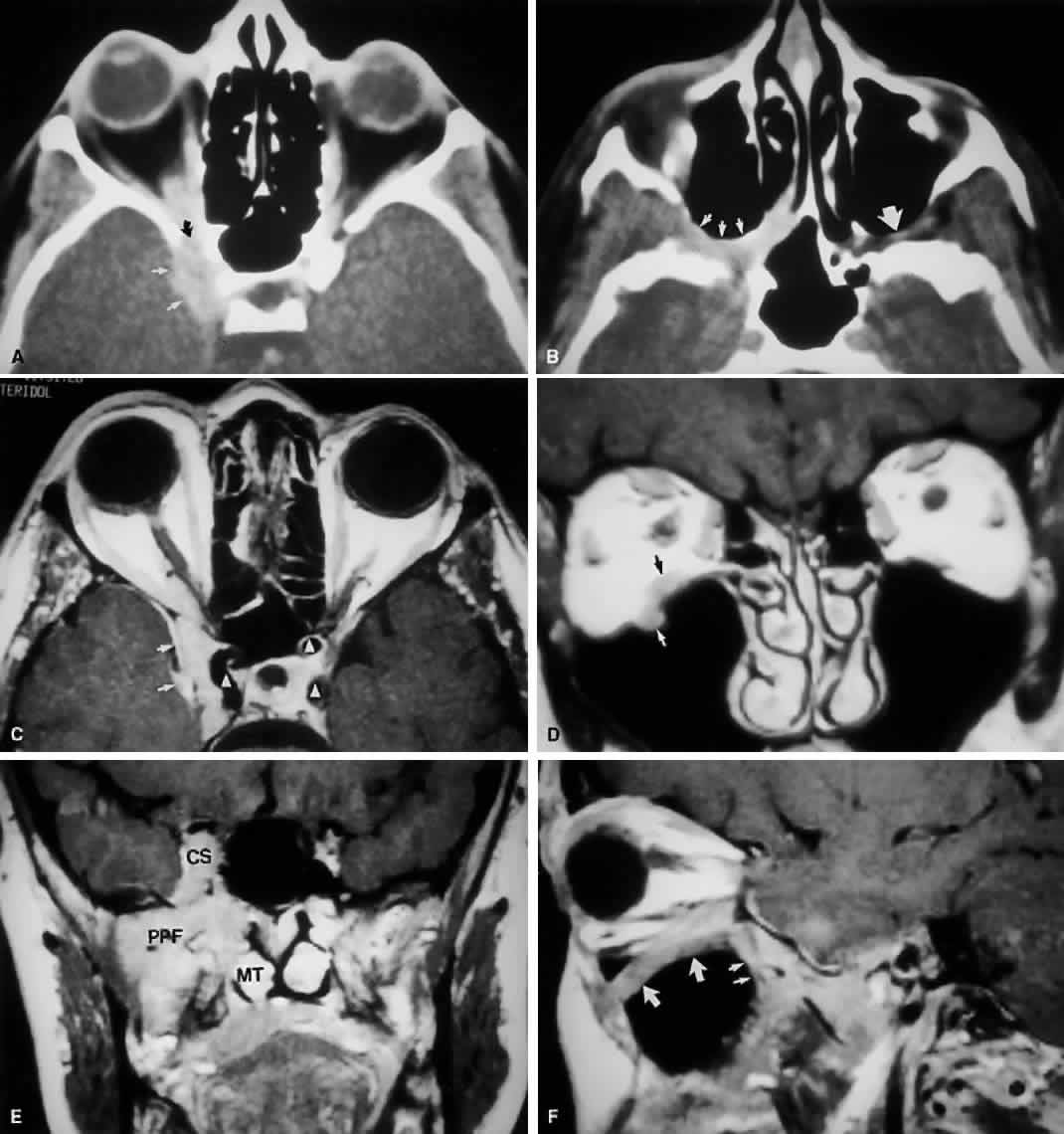 Fig. 9. An orbital lymphoma involving the skull base provides accentuation of the
apical spaces of the orbit. A. On this axial CT, the lesion infiltrates the CS, causing bulging and local
invasion of its lateral dural wall (small arrows). Invasion into the orbital apex through the superior orbital fissure (curved arrow) is seen. Note that the patient is slightly rotated in the scanner, because
the anterior clinoid and optic canal are visualized on the uninvolved
side. B. More inferiorly, the mass has invaded the pterygopalatine fossa (small arrows), located just posterior to the maxillary sinus. On the uninvolved side (large arrow), the fossa has areas of radiolucency, indicating the fat that normally
occupies this space. C. Axial MRI, T1-weighted image with gadolinium but without fat suppression. The
carotid siphon is seen within each CS as a flow void (arrowheads). Once again, note the inflamed lateral dural wall of the CS and local
invasion of the brain parenchyma (small arrows).D. Coronal T1-weighted MR image. The inferior rectus muscle is labeled with
a black arrow. The lymphoma has infiltrated the infraorbital canal (white arrow) within the orbital floor. E. Coronal MRI of the orbital apex shows infiltration from the CS to the
pterygopalatine fossa (PPF). Because there is no direct communication
between these spaces, the lesion must have spread through the superior
orbital fissure into the orbital apex, then through the inferior orbital
fissure. MT, middle turbinate. The lucency just above the CS is the
anterior clinoid process, with the optic nerve within its canal seen
as an opacity between the clinoid and the sphenoid sinus. F. Parasagittal MRI shows lymphomatous invasion of the pterygopalatine fossa
just behind the posterior wall of the maxillary sinus (small arrows). Note the thickening of the infiltrated infraorbital canal (large arrows) as it travels anteriorly to exit about 1 cm below the inferior orbital
rim. Fig. 9. An orbital lymphoma involving the skull base provides accentuation of the
apical spaces of the orbit. A. On this axial CT, the lesion infiltrates the CS, causing bulging and local
invasion of its lateral dural wall (small arrows). Invasion into the orbital apex through the superior orbital fissure (curved arrow) is seen. Note that the patient is slightly rotated in the scanner, because
the anterior clinoid and optic canal are visualized on the uninvolved
side. B. More inferiorly, the mass has invaded the pterygopalatine fossa (small arrows), located just posterior to the maxillary sinus. On the uninvolved side (large arrow), the fossa has areas of radiolucency, indicating the fat that normally
occupies this space. C. Axial MRI, T1-weighted image with gadolinium but without fat suppression. The
carotid siphon is seen within each CS as a flow void (arrowheads). Once again, note the inflamed lateral dural wall of the CS and local
invasion of the brain parenchyma (small arrows).D. Coronal T1-weighted MR image. The inferior rectus muscle is labeled with
a black arrow. The lymphoma has infiltrated the infraorbital canal (white arrow) within the orbital floor. E. Coronal MRI of the orbital apex shows infiltration from the CS to the
pterygopalatine fossa (PPF). Because there is no direct communication
between these spaces, the lesion must have spread through the superior
orbital fissure into the orbital apex, then through the inferior orbital
fissure. MT, middle turbinate. The lucency just above the CS is the
anterior clinoid process, with the optic nerve within its canal seen
as an opacity between the clinoid and the sphenoid sinus. F. Parasagittal MRI shows lymphomatous invasion of the pterygopalatine fossa
just behind the posterior wall of the maxillary sinus (small arrows). Note the thickening of the infiltrated infraorbital canal (large arrows) as it travels anteriorly to exit about 1 cm below the inferior orbital
rim.
|
PARANASAL SINUSES The orbit is surrounded on three sides by the paranasal sinuses (see Fig. 5). The ethmoid sinus runs along the medial orbital wall and is divided
into anterior, middle, and posterior air cells by a highly variable system
of septa. It is the only sinus to be fully pneumatized at birth. The
thin lamina papyracea of the medial orbital wall and the vascular
foramina for the anterior and posterior ethmoidal arteries provide scant
resistance to the extension of infections and tumors from the ethmoidal
sinus to the orbit, even in the adult (Fig. 10). 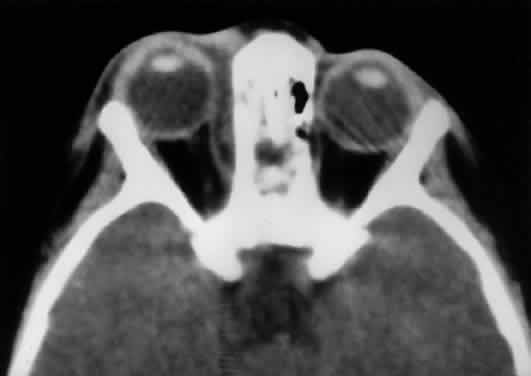 Fig. 10. The thin lamina papyracea provides little resistance to infection spread
from the adjacent paranasal sinus. In this axial CT image of a 2-year-old
child, opacification is noted within the ethmoid air cells. Note
the subperiosteal collection along the medial orbital wall. In this case, the
collection proved to be a sterile inflammatory phlegmon. Fig. 10. The thin lamina papyracea provides little resistance to infection spread
from the adjacent paranasal sinus. In this axial CT image of a 2-year-old
child, opacification is noted within the ethmoid air cells. Note
the subperiosteal collection along the medial orbital wall. In this case, the
collection proved to be a sterile inflammatory phlegmon.
|
The maxillary sinus borders the orbital floor and is fully pneumatized
by 2 to 4 years of age. Unlike the ethmoid sinus, it contains no supporting
septa. Although the lamina papyracea is the thinnest of the orbital
walls, the network of septations within the ethmoid air cells acts
as a supporting scaffolding to the medial orbital wall, much the same
way that corrugations strengthen cardboard. Thus, the orbital floor, although
not the thinnest wall, is the most frequently fractured, having
no underlying support within the maxillary sinus. The posterior wall
of the maxillary sinus forms the anterior wall of the pterygopalatine
fossa. Frontal sinus pneumatization is highly variable and may continue into the
teenage years. Because it drains into the anterior ethmoid air cells, the
frontal sinus is often concurrently involved in ethmoid sinus pathology. Supraorbital
sinuses are defined as lateral extensions of the
ethmoid sinus and span the orbital roof for variable lengths. On occasion, pneumatization
to the level of the frontozygomatic suture may occur. Finally, the sphenoid sinus abuts the orbital apex and is the last to pneumatize. Because
of the proximity of the optic canal and CS (see Fig. 8), any sphenoid sinus pathology may manifest as a parasellar syndrome (discussed
later). Pneumatization of the sphenoid sinus may extend into
the anterior clinoid process, a variation of normal anatomy often encountered
in orbital imaging studies. FORAMINA AT THE ORBITAL APEX The orbit is generally found to contain nine openings (Table 5). Only the optic foramen, SOF, and IOF will be discussed in detail (see Fig. 8). The optic foramen is located in the medial wall of the orbit in the
body and lesser wing of the sphenoid bone. The optic canal is 4 to 10 mm
long and 6.5 mm wide. On imaging studies, a 1-mm difference between
canal diameters is considered clinically significant. The optic canal
transmits the optic nerve, the ophthalmic artery, and the sympathetic
innervation to the orbit. Note that sympathetic nerves also travel with
the nasociliary nerve via the SOF. TABLE FIVE. The Nine Canals and Fissures of the Orbit*
| Orbital Opening | Features |
| Supraorbital notch (foramen) | Frontal nerve (supraorbital nerve, V-1) |
| Anterior ethmoidal foramen | 20–24 mm behind orbital rim at fronto ethmoidal suture (level of
cribriform plate) |
| Posterior ethmoidal foramen | 12 mm posterior to anterior ethmoidal foramen, 5–8 mm anterior to
optic foramen |
| Zygomatic foramina | Zygomaticofacial and zygomaticotemporal neurovascular bundles |
| Nasolacrimal duct | Beginning at the lacrimal fossa and opening intranasally in the inferior
meatus (beneath inferior turbinate) |
| Infraorbital foramen | Exits 4–10 mm below orbital rim; infraorbital neurovascular bundle (V-2) |
| Optic foramen | Optic nerve, ophthalmic artery, sympathetic fibers. Diameter  6.5 mm, length 6.5 mm, length  10 mm. 10 mm. |
| Superior orbital fissure | Length: 22 mm |
| | Between greater & lesser wings of sphenoid |
| | Below and lateral to optic foramen |
| | Split in two portions by lateral rectus |
| | Lateral: lacrimal, frontal, trochlear nerves |
| | Medial: CN III (superior and inferior divisions), nasociliary, CN VI, superior
ophthalmic vein, sympathetics/parasympathetics |
| Inferior orbital fissure | Sphenoid, maxillary, & palatine bones; V-2: infraorbital & zygomatic
nerves; inferior ophthalmic vein |
| Frontosphenoid foramina | Frontosphenoidal suture of orbital roof: anastomosis of recurrent middle
meningeal and lacrimal arteries (variable) |
*The tenth opening, the frontosphenoid foramen, is not present in all cases.
The optic nerve is tethered on both ends of the canal by the annulus of
Zinn intraorbitally and by a dural fold intracranially. The dura of the
optic nerve also has strong attachments to the periosteum within the
canal.26 Because the canal flares in diameter toward the cranial end, the tightest
attachments of dura to the optic canal are at the proximal end (annulus
of Zinn). The location of the optic canal and the tethering of the
optic nerve within it may explain the etiology of posterior indirect
traumatic optic neuropathy. Cadaver studies have shown that stress on
the frontal bone is transferred in a reproducible pattern to the body
of the sphenoid and the optic canal, potentially resulting in optic
canal fracture.27 Further, the tethering of the optic nerve at the annulus of Zinn may cause
an acute “stretch injury”at this site during deceleration: as
the rigid facial skeleton simply stops on hitting any rigid structure (e.g., the steering wheel in a motor vehicle accident), the soft tissue of the
orbit continues to moves forward until stopped by the tethered optic
nerve. Both of these mechanisms may then result in edema of the optic
nerve within the closed space of its bony canal, leading to traumatic
optic neuropathy. The SOF is 22 mm long and separates the greater and lesser wings of the
sphenoid bone. Note in Figure 8 that it lies lateral and slightly below the optic foramen in radiographic
studies. Also note that the (SOF, and not the optic foramen, is located
at the apex of the orbit (Fig. 11). The SOF is split into two compartments by the lateral rectus muscle. The
medial compartment contains the oculomotor (superior and inferior
divisions) nerve, nasociliary nerve, abducens nerve, sympathetic and
parasympathetic fibers, and superior ophthalmic vein. The lateral compartment
transmits the lacrimal, frontal, and trochlear nerves. This extraconal
location of the trochlear nerve is appreciated clinically after
retrobulbar anesthesia. Although the anesthetic block effectively causes
akinesia of the EOMs, the patient often can still intort the globe
because of the intact innervation to the superior oblique muscle. 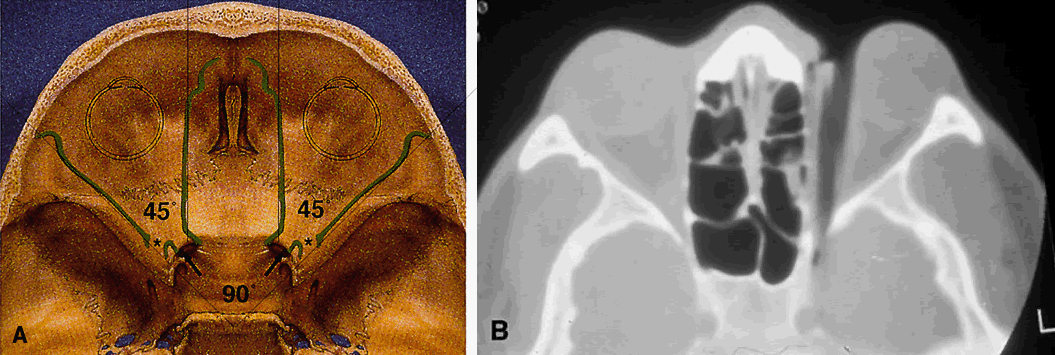 Fig. 11. Osteology. A. An axial view of the orbital roof demonstrates the parallel course of
the medial orbital walls (green). The lateral orbital walls (green) lie
at an angle of 90° from each other, or 45° from each medial
wall. Remember that the superior orbital fissure, and not the medially
placed optic canal, lies at the posterior aspect of the orbit. B. CT of a wooden foreign body within the orbit after trauma. Note that the
tip has traveled through the superior orbital fissure and lies within
the CS, not the optic canal. In this case, the greatest worry was not
the patient's vision, but the possibility of lacerating injury
of the carotid siphon, which was confirmed on subsequent arteriography (A modified from Zide BM, Jelks GW: Surgical Anatomy of the Orbit, p 9. New
York, Raven Press, 1985. B courtesy John W. Shore, MD, Austin, TX) Fig. 11. Osteology. A. An axial view of the orbital roof demonstrates the parallel course of
the medial orbital walls (green). The lateral orbital walls (green) lie
at an angle of 90° from each other, or 45° from each medial
wall. Remember that the superior orbital fissure, and not the medially
placed optic canal, lies at the posterior aspect of the orbit. B. CT of a wooden foreign body within the orbit after trauma. Note that the
tip has traveled through the superior orbital fissure and lies within
the CS, not the optic canal. In this case, the greatest worry was not
the patient's vision, but the possibility of lacerating injury
of the carotid siphon, which was confirmed on subsequent arteriography (A modified from Zide BM, Jelks GW: Surgical Anatomy of the Orbit, p 9. New
York, Raven Press, 1985. B courtesy John W. Shore, MD, Austin, TX)
|
The IOF separates the lateral and inferior orbital walls. It transmits
the maxillary nerve (V-2), zygomatic nerve, and inferior ophthalmic vein (as
well as the pterygopalatine nerve and the pterygopalatine ganglion
nerve) and directly communicates with the pterygopalatine fossa, a
vertically oriented space behind the maxillary sinus. The IOF varies
in its distance from the orbital rim but may approach it quite closely (10 mm) before
becoming the infraorbital canal. The location of the IOF
and the slope of the orbital floor are two important points to keep
in mind during surgical repair of an orbital floor fracture (see Fig. 7B). The tightly adherent periorbita at the IOF may be misinterpreted as
entrapped orbital tissue. Aggressive dissection will result in severe
bleeding from the infraorbital artery. The 15° to 20° upward slope
of the orbital floor from anterior to posterior (see Fig. 7B) causes the bone to travel out of view as more posterior dissection is
performed with the surgeon at the head of the operating table. If this
slope is not recreated during reconstruction, posttraumatic enophthalmos
may result because of an enlarged orbital space from a “flat”orbital
floor. ANNULUS OF ZINN The annulus of Zinn represents a complex, dense, fibrous band of connective
tissue with firm attachments to the periosteum of the orbital apex (Fig. 12). Medially, it is fused to the optic nerve sheath, which may account for
the pain during eye movements experienced by patients with optic neuritis. All
four rectus muscles arise directly from the annulus. Conversely, the
levator and superior oblique muscles are not attached to the
annulus, arising instead above the superior rectus muscle and the lesser
wing/body of the sphenoid, respectively (Table 6). The final EOM, the inferior oblique, begins lateral to the nasolacrimal
duct ostium in the anterior orbit. 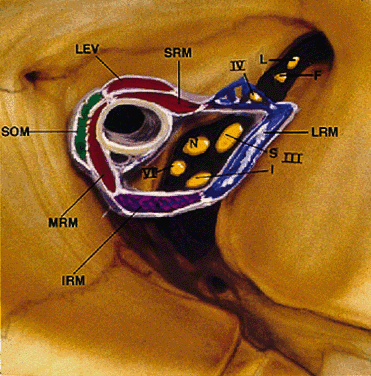 Fig. 12. Annulus of Zinn. III-S, superior division of oculomotor nerve; III-I, inferior
division of oculomotor nerve; IV, trochlear nerve; VI, abducens
nerve; L, lacrimal nerve; F, frontal nerve; N, nasociliary nerve; SRM, superior
rectus muscle; LEV, levator palpebris superioris muscle; SOM, superior
oblique muscle; MRM, medial rectus muscle; IRM, inferior
rectus muscle; LRM, lateral rectus muscle. (Modified from Zide BM, Jelks GW: Surgical Anatomy of the Orbit, p 8. New
York, Raven Press, 1985) Fig. 12. Annulus of Zinn. III-S, superior division of oculomotor nerve; III-I, inferior
division of oculomotor nerve; IV, trochlear nerve; VI, abducens
nerve; L, lacrimal nerve; F, frontal nerve; N, nasociliary nerve; SRM, superior
rectus muscle; LEV, levator palpebris superioris muscle; SOM, superior
oblique muscle; MRM, medial rectus muscle; IRM, inferior
rectus muscle; LRM, lateral rectus muscle. (Modified from Zide BM, Jelks GW: Surgical Anatomy of the Orbit, p 8. New
York, Raven Press, 1985)
|
TABLE SIX. Extraocular Muscle Pearls
Levator Palpebrae Superioris
One nucleus of CN III supplies both levators (Edinger-Westphal nucleus
also has bilateral output)
The lateral horn splits lacrimal gland into two lobes
Lateral Rectus Muscle
Only one ciliary artery, but also receives partial blood supply from lacrimal
artery
Inferior Rectus and Inferior Oblique Muscles
Partial blood supply from infraorbital artery
Inferior Oblique Muscle
Has no tendon; bleeding is often encountered during surgical disinsertion
Its nerve enters at the lateral border of the inferior rectus m. as they
cross
The condensation of the inferior rectus muscle sheath anterior to the inferior
oblique muscle forms the suspensory ligament of Lockwood
Superior Oblique Muscle
Longest tendon (26 mm)
Superior Rectus Muscle
Inserts behind ora serrata—a full-thickness bridle suture will perforate
retina
Supplied by the contralateral CN II subnucleus
CAVERNOUS SINUS The relation between the CS (Fig. 13) and the orbit is often misunderstood. Briefly, the CS is a venous space
located directly behind the SOF and serves as a conduit for most of
the motor, sensory, and autonomic nerves that supply the orbit. It is
also the major venous drainage for the orbit.3,4  Fig. 13. The CS in parasagittal views. A. Osteology. OF, optic foramen; SOF, superior orbital fissure; FR, foramen
rotundum; CC, carotid canal; Se, sella turcica; Ac, anterior clinoid
process; Pc, posterior clinoid process. Note that the optic canal runs
medial to the Ac. B. Venous plexus. The plexus consists of large-caliber fenestrated venous
spaces that may communicate with the contralateral CS through foramina
in the medial bony wall. II, optic nerve; PG, pituitary gland. C. The carotid siphon and sympathetic plexus. The ophthalmic artery is given
off as the first major intracranial branch of the internal carotid
artery just as it exits the roof of the CS. Smaller meningeal branches
are not shown. The sympathetic nerve supply travels as a neural plexus (not
shown) around the carotid artery, entering the orbit with the
ophthalmic artery and through the superior orbital fissure. ICA, internal
carotid artery (carotid siphon); OA, ophthalmic artery. D. Cranial nerve supply. All cranial nerves except the abducens travel through
the CS tightly adherent to the lateral dural wall. The abducens
nerve has an unpredictable course through the venous plexus but is usually
adherent at least in part to the carotid siphon. Note how the abducens
nerve travels vertically over the petrous ridge and becomes tethered
by Gruber's ligament just before entering the CS. This tethering
predisposes the abducens nerve to deceleration injury in head trauma. The
maxillary division is closely associated, but outside, the CS. The
parasympathetic nerve supply to the globe travels within the oculomotor
nerve. III, oculomotor nerve; IV, trochlear nerve; V-1, ophthalmic
division (trigeminal nerve); V-2, maxillary division; V-3, mandibular
division; VI, abducens nerve. E. The lateral dural wall. (Modified from Zide BM, Jelks GW: Surgical Anatomy of the Orbit, p 8. New
York, Raven Press, 1985) Fig. 13. The CS in parasagittal views. A. Osteology. OF, optic foramen; SOF, superior orbital fissure; FR, foramen
rotundum; CC, carotid canal; Se, sella turcica; Ac, anterior clinoid
process; Pc, posterior clinoid process. Note that the optic canal runs
medial to the Ac. B. Venous plexus. The plexus consists of large-caliber fenestrated venous
spaces that may communicate with the contralateral CS through foramina
in the medial bony wall. II, optic nerve; PG, pituitary gland. C. The carotid siphon and sympathetic plexus. The ophthalmic artery is given
off as the first major intracranial branch of the internal carotid
artery just as it exits the roof of the CS. Smaller meningeal branches
are not shown. The sympathetic nerve supply travels as a neural plexus (not
shown) around the carotid artery, entering the orbit with the
ophthalmic artery and through the superior orbital fissure. ICA, internal
carotid artery (carotid siphon); OA, ophthalmic artery. D. Cranial nerve supply. All cranial nerves except the abducens travel through
the CS tightly adherent to the lateral dural wall. The abducens
nerve has an unpredictable course through the venous plexus but is usually
adherent at least in part to the carotid siphon. Note how the abducens
nerve travels vertically over the petrous ridge and becomes tethered
by Gruber's ligament just before entering the CS. This tethering
predisposes the abducens nerve to deceleration injury in head trauma. The
maxillary division is closely associated, but outside, the CS. The
parasympathetic nerve supply to the globe travels within the oculomotor
nerve. III, oculomotor nerve; IV, trochlear nerve; V-1, ophthalmic
division (trigeminal nerve); V-2, maxillary division; V-3, mandibular
division; VI, abducens nerve. E. The lateral dural wall. (Modified from Zide BM, Jelks GW: Surgical Anatomy of the Orbit, p 8. New
York, Raven Press, 1985)
|
The CS contains the carotid siphon (the S-shaped portion of the internal
carotid artery [ICA]), sympathetic fibers closely associated
with the carotid artery, cranial nerves III (oculomotor), IV (trochlear), V-1 (ophthalmic
division of trigeminal), and VI (abducens), and, as
already noted, a venous plexus. The venous plexus communicates with
the contralateral CS through an ostium. Parasympathetic fibers also
travel through the CS from the Edinger-Westphal nucleus as part of the
oculomotor nerve. Remember that a second set of parasympathetics enters
the orbit via the sphenopalatine ganglion (see below). In most cases, the maxillary division of the trigeminal nerve (V-2) is
closely related to, but not quite within, the posterior and inferior portion
of the CS.3,5 SYMPATHETIC AND PARASYMPATHETIC INNERVATION, CILIARY GANGLION Ciliary Ganglion The ciliary ganglion is a flattened, quadrangular structure located deep
in the orbit, temporal to the optic nerve and 1 cm anterior to the orbital
apex (Fig. 14).28 True ganglion cells and sustentacular or satellite spindle cells, along
with the axons of the entering and emerging nerve fibers, form the substance
of the ganglion. Rare chemodectomas and primary orbital carcinoids
possibly may arise from ciliary ganglion.29–32 Degeneration, trauma, inflammation, or viral infection of the ganglion
can cause an efferent (Adie's tonic) pupil; the dissection of the
short posterior ciliary nerves necessary to perform optic nerve sheath
fenestration often results in a temporary sectoral tonic pupil.33  Fig. 14. Autonomic nerve supply of the orbit. A. Parasympathetic supply. Parasagittal view of the right orbit shows the
position of the ciliary ganglion on the lateral aspect of the optic nerve. Parasympathetic
fibers to the globe travel through the superior
orbital fissure with the nerve to the inferior oblique muscle and synapse
within this ganglion. Sympathetic and nasociliary fibers pass through
the ciliary ganglion without synapse. Parasympathetic fibers to the
lacrimal gland synapse outside the orbit in the pterygopalatine ganglion, which
lies in the pterygopalatine fossa. Fibers then join the maxillary
division of the trigeminal nerve to travel along the lateral orbit
with the zygomaticotemporal nerve before finally joining the lacrimal
nerve and entering the lacrimal gland. B. Sympathetic supply. First-order neurons travel from the hypothalamus inferiorly
within the spinal cord to synapse at the ciliospinal center
of Budge (C8 to T2). Second-order neurons travel over the apex of the
lung and around the subclavian artery to enter the paravertebral sympathetic
chain and then synapse at the superior ciliary ganglion. From there, a
plexus of third-order neurons forms surrounding the external and
internal carotid artery. The external carotid artery fibers are responsible
for vasoconstriction and sweating of most of the face. Within
the CS, internal carotid artery fibers destined for the globe travel
for a short course with the abducens nerve, then join the nasociliary
nerve and enter the orbit through the superior orbital fissure. Long posterior
ciliary nerves synapse in the dilator muscle of the iris. A second
group of fibers enters the orbit through the optic canal as a plexus
around the ophthalmic artery. These fibers then travel diffusely
through the orbital soft tissue to supply orbital vessels, the superior ( Müller's) and inferior tarsal muscles, the sweat glands
of the forehead, and possibly the main lacrimal gland. (A modified from Doxanas MT, Anderson RL: Clinical Orbital Anatomy, p 149. Baltimore, Williams & Wilkins, 1984. B modified from Weinstein JM, Zweifel TJ, Thompson HS: Congenital Horner's
syndrome, p 1076. Arch Ophthalmol 98:1074, 1980) Fig. 14. Autonomic nerve supply of the orbit. A. Parasympathetic supply. Parasagittal view of the right orbit shows the
position of the ciliary ganglion on the lateral aspect of the optic nerve. Parasympathetic
fibers to the globe travel through the superior
orbital fissure with the nerve to the inferior oblique muscle and synapse
within this ganglion. Sympathetic and nasociliary fibers pass through
the ciliary ganglion without synapse. Parasympathetic fibers to the
lacrimal gland synapse outside the orbit in the pterygopalatine ganglion, which
lies in the pterygopalatine fossa. Fibers then join the maxillary
division of the trigeminal nerve to travel along the lateral orbit
with the zygomaticotemporal nerve before finally joining the lacrimal
nerve and entering the lacrimal gland. B. Sympathetic supply. First-order neurons travel from the hypothalamus inferiorly
within the spinal cord to synapse at the ciliospinal center
of Budge (C8 to T2). Second-order neurons travel over the apex of the
lung and around the subclavian artery to enter the paravertebral sympathetic
chain and then synapse at the superior ciliary ganglion. From there, a
plexus of third-order neurons forms surrounding the external and
internal carotid artery. The external carotid artery fibers are responsible
for vasoconstriction and sweating of most of the face. Within
the CS, internal carotid artery fibers destined for the globe travel
for a short course with the abducens nerve, then join the nasociliary
nerve and enter the orbit through the superior orbital fissure. Long posterior
ciliary nerves synapse in the dilator muscle of the iris. A second
group of fibers enters the orbit through the optic canal as a plexus
around the ophthalmic artery. These fibers then travel diffusely
through the orbital soft tissue to supply orbital vessels, the superior ( Müller's) and inferior tarsal muscles, the sweat glands
of the forehead, and possibly the main lacrimal gland. (A modified from Doxanas MT, Anderson RL: Clinical Orbital Anatomy, p 149. Baltimore, Williams & Wilkins, 1984. B modified from Weinstein JM, Zweifel TJ, Thompson HS: Congenital Horner's
syndrome, p 1076. Arch Ophthalmol 98:1074, 1980)
|
The ciliary ganglion contains three roots, only one of which actually synapses
within the ganglion: - Sensory: carries sensory fibers from the cornea, iris, and ciliary body
to the nasociliary nerve (CN V-1)
- Parasympathetic: arrives via the nerve to the inferior oblique muscle (inferior
division of CN III) to synapse in the ciliary ganglion, then
exit as the short ciliary nerves to supply the iris sphincter and ciliary
body (see Fig. 14A)
- Sympathetic: enters via the nasociliary nerve through ciliary ganglion
to innervate blood vessels of the globe and iris dilator muscle as the
long ciliary nerves (see Fig. 14B).
SYMPATHETIC INNERVATION OF THE ORBIT The sympathetic chain travels into the calvarium as a plexus surrounding
the ICA.6 Within the CS, nerve fibers leave the carotid siphon to enter the orbit
through two routes (see Fig. 14B): - Nerve fibers join the abducens nerve within the CS for a short course, then
travel with the nasociliary nerve to enter the orbit through the
SOF and pass through the ciliary ganglion as described6
- Nerve fibers join the ophthalmic artery to enter the orbit through the
optic canal to supply Müller's muscle, the orbital vessels, and
possibly the lacrimal gland.
Damage to any portion of the sympathetic chain results in Horner's
syndrome (i.e., miosis and ptosis, possibly with anhydrosis depending on the location
of the lesion). In general, a lesion of the sympathetics in the CS will
at most result in anhydrosis of the forehead (the cheek and remainder
of the face are supplied by a different sympathetic route). PARASYMPATHETIC INNERVATION OF THE ORBIT Parasympathetic fibers supply different structures in the orbit by two
separate routes (see Fig. 14A): - Ocular fibers travel with motor neurons from the Edinger-Westphal subnucleus
of the oculomotor nerve (specifically, the inferior division of
CN III) through the CS and then with the nerve of the inferior oblique
before synapsing in the ciliary ganglion to supply the globe.
- Efferent secretory fibers to the lacrimal gland exit from the superior
salivatory nucleus via the nervus intermedius to travel with the facial
nerve (VII) to the level of the external genu. At the genu, the secretory
fibers divide to supply the lacrimal gland and the salivary glands. The
fibers to the lacrimal gland travel as the greater superficial
petrosal nerve through the vidian canal to synapse in the sphenopalatine
ganglion. From the sphenopalatine ganglion, postganglionic fibers
then join with the infraorbital nerve (CN V-2) to join the zygomaticotemporal
nerve, and finally join the lacrimal nerve (see Fig. 14A).
Thus, parasympathetic supply to the lacrimal gland is distinct from that
of the globe and is more closely related to the innervation of the parotid
and accessory salivary glands. Short-circuiting or misdirected
innervation results in the gustolacrimal reflex (“crocodile tears”).34 The complex parasympathetic innervation of the orbit is summarized in Table 7. TABLE SEVEN. Parasympathetic Pearls
Parasympathetic fibers to the globe travel with CN III
Fibers to the lacrimal gland never travel through the CS, superior orbital
fissure, or ciliary ganglion
Fibers to the lacrimal gland synapse outside the orbit
Parasympathetic fibers to the lacrimal gland travel with CN VII, CN V-2 (zygomaticotemporal
n.), and finally with CN V-1 (lacrimal n.)
CLINICAL PRESENTATIONS AT THE ORBITAL APEX Before neuroimaging by CT and MRI, several clinical syndromes were used
to describe lesions at specific sites of the cranio-orbital junction. Because
of the widespread availability of neuroimaging today, these various
clinical subtleties are less important. However, a clear understanding
of cranio-orbital anatomy requires a knowledge of possible clinical
manifestations. In general, cranio-orbital syndromes may be described as superior orbital
fissure syndrome, orbital apex syndrome, and cavernous sinus syndrome (Fig. 15).7,8 The distinction between these presentations depends on the involvement
of CN V-2, the sympathetic nerves, and the optic nerve (Table 8). It is much easier simply to call the constellation of signs of cranio-orbital
disease the parasellar or sphenocavernous syndrome, rather than
attempting to be too specific in an area where the clinical presentations
are seldom as distinct as described in Table 8.  Fig. 15. Parasellar syndrome. A. Composite photograph of an elderly patient with periorbital pain in the
distribution of the right supraorbital nerve along with complete ptosis
and absent levator function (upper left and upper right). When the
eyelid was lifted, she complained of diplopia (center). Note the complete
external ophthalmoplegia (remaining panels). MRI revealed a mass
eroding the anterior clinoid process and extending into the CS and surrounding
brain parenchyma. Given the bony erosion, a diagnosis of Tolosa-Hunt
would be inappropriate in this case. A transorbital craniotomy
for biopsy revealed metastatic adenocarcinoma. A systemic workup failed
to reveal a primary site of involvement. The patient' orbital signs
responded to radiation therapy. B and C. Axial and coronal MRI of a different patient who presented in an identical
fashion. Note the enlargement of the right CS (arrows). Systemic workup was negative, and the patient responded rapidly to intravenous
corticosteroids. The lesion disappeared on subsequent scans
with no evidence of recurrence after 2 years. A diagnosis of “presumed
Tolosa-Hunt syndrome”is acceptable in this case. Fig. 15. Parasellar syndrome. A. Composite photograph of an elderly patient with periorbital pain in the
distribution of the right supraorbital nerve along with complete ptosis
and absent levator function (upper left and upper right). When the
eyelid was lifted, she complained of diplopia (center). Note the complete
external ophthalmoplegia (remaining panels). MRI revealed a mass
eroding the anterior clinoid process and extending into the CS and surrounding
brain parenchyma. Given the bony erosion, a diagnosis of Tolosa-Hunt
would be inappropriate in this case. A transorbital craniotomy
for biopsy revealed metastatic adenocarcinoma. A systemic workup failed
to reveal a primary site of involvement. The patient' orbital signs
responded to radiation therapy. B and C. Axial and coronal MRI of a different patient who presented in an identical
fashion. Note the enlargement of the right CS (arrows). Systemic workup was negative, and the patient responded rapidly to intravenous
corticosteroids. The lesion disappeared on subsequent scans
with no evidence of recurrence after 2 years. A diagnosis of “presumed
Tolosa-Hunt syndrome”is acceptable in this case.
|
TABLE EIGHT. Parasellar Syndromes
Superior Orbital Fissure Syndrome:
Varying degrees of CNs III, IV, V-1, and VI palsies
Varying degrees of proptosis and orbital congestion
Note that CN II (visual function is normal) and CN V-2 are spared.
Orbital Apex Syndrome:
Superior orbital fissure syndrome + CN II involvement (vision is compromised)
Cavernous Sinus Syndrome:
Varying degrees of the aforementioned signs, with the following: Involvement of CN V-2 indicates posterior cavernous sinus location because
of the proximity of V-2 to the posterior portion of the CS.
Combination of sympathetic involvement (Horner's) and CN III or VI
palsies is said to be a specific indicator of cavernous sinus lesions.
The differential diagnosis for parasellar syndrome is listed in Table 9, but two points are worth stressing. First, the most common neoplasias
by far of this region are meningiomas and pituitary adenomas. Second, the
diagnosis of Tolosa-Hunt syndrome is often misused. This specific
entity is rigorously defined as granulomatous inflammation around the
carotid siphon (resulting in the clinical findings of a painful parasellar
syndrome).9,10 In general, Tolosa-Hunt syndrome is responsive to corticosteroid therapy11,12 but is not equivalent to orbital apical pseudotumor (nongranulomatous
inflammation). Further, Tolosa-Hunt syndrome is a diagnosis of exclusion
and should never be applied liberally.13 Remember that other entities, including meningiomas, aneurysms, and even
infection, have been reported to respond to corticosteroids (i.e., misdiagnosed as Tolosa-Hunt syndrome). TABLE NINE. Differential Diagnosis of Cranio-orbital Lesions
Inflammatory, Noninfectious
Thyroid-related orbitopathy
Idiopathic inflammatory pseudotumor
Adenoma/carcinoma
Sarcoidosis
Inflammatory, Infectious
Bacterial Adjacent sinusitis
Septic embolus
Subperiosteal orbital abscess
Orbital cellulitis
Paranasal sinus pyocele
Viral Herpes zoster (rarely, simplex)
Fungal Mucor
Aspergillus
Vascular
Carotid/ophthalmic artery aneurysms
Arteriovenous malformations
Carotid—cavernous fistulas
Dural—sinus fistulas
Aseptic cavernous sinus thrombosis
Vasculitic
Temporal arteritis
Wegener's granulomatosis
Neoplasia
Meningioma
Pituitary adenoma
Lymphoma
Craniopharyngioma
Nasopharyngeal carcinoma
Neurolemmoma
Glioma
Chordoma
Chondroma/chondrosarcoma
Multiple myeloma/leukemia
Metastatic disease
Carcinomatous meningitis
Trauma
Fractures
Hematoma
Tolosa-Hunt syndrome
Miscellaneous/Systemic
Fibrous dysplasia
Diabetes/hypertension
Rheumatoid arthritis/SLE
Dermoid
Mucocele
MOTOR AND SENSORY NERVES Tables 10 and 11 summarize the important features of each of the cranial nerves supplying
the orbit (Fig. 16, see Fig. 12). Several points are worth mentioning. First, the optic nerve assumes
an S-shaped course within the orbit. Because the intraorbital nerve is
about 25 mm long and the distance from the back of the globe to the optic
foramen is 18 mm, 7 mm of slack remains. This degree of potential
mobility allows the nerve to remain unaffected during ocular rotations
and provides a cushion for axial proptosis (Fig. 17). TABLE TEN. Cranial Nerve Features
| Nerve | Features |
| Optic (II) | Divided into three sections: |
| | · Intraorbital: 24–25 mm long |
| | · Canalicular: 8–10 mm long |
| | · Intracranial: 10–16 mm long |
| | Contains about 1.5 million fibers |
| | Diameter: |
| | · 1.5 mm at optic nerve head |
| | · 3.0 mm posterior to globe (acquires dural sheath) |
| Oculomotor (III) | Divides into a superior and inferior division, usually within the cavernous
sinus: |
| | · Superior division: levator, superior-rectus |
| | · Inferior division: medial, inferior, inferior oblique |
| | · Injury to the oculomotor nerve may result in miswiring between the
divisions, manifesting as an “aberrant III regeneration syndrome” |
| | Enters orbit through the SOF and the annulus of Zinn |
| | Carries the parasympathetic fibers to the globe (Edinger-Westphal nucleus) |
| Trochlear (IV) | Has the longest intracranial course (~75 mm) |
| | Contains the fewest fibers (1500) |
| | Commonly injured in closed head trauma |
| | Enters through SOF, but superolateral to annulus of Zinn |
| | · Usually not affected by retrobulbar anesthetic blocks |
| Abducens (VI) | Arises in pons |
| | Tethered by petrosphenoidal (Gruber's) ligament within Dorello's
canal |
| | · Commonly injured in closed head trauma |
| | The only CN not tethered to the lateral dural wall within the cavernous
sinus |
| | · Partially tethered to the carotid siphon, but otherwise has an unpredictable
central course through the CS |
| | Most inferior nerve to pass through the SOF |
| Facial (VII) | The upper face has bilateral innervation. |
| | The lower face has contralateral innervation. |
| | Provides motor supply to the eyelid protractors (closes the eyelids): |
| | · Orbicularis oculi m. |
| | · Procerus m. |
| | · Corrugator supraciliaris m. and one eyelid retractor: frontalis
m. |
| | Carries the parasympathetic fibers to the lacrimal gland (nervus intermedius) |
| | Most commonly affected CN in sarcoidosis (optic nerve is #2) |
| | Course over the face defines the locations of the Nadbath, O'Brien, and
van Lindt blocks. | TABLE ELEVEN. The Trigeminal Nerve
Ophthalmic Division (V-1)
Enters the orbit through the SOF
Provides the major sensory input from the upper face and orbit
Divides into three nerves within the cavernous sinus or orbital apex
Frontal nerve
Supplies medial upper lid and conjunctiva, forehead, scalp, frontal sinuses, and
side of nose
Emerges from the orbit as the supraorbital and supratrochlear nerves
Lacrimal nerve
Supplies conjunctiva and skin in region of lacrimal gland + postganglionic
parasympathetics for reflex tearing
Nasociliary nerve
Only portion of the ophthalmic division to enter the orbit through the
annulus of Zinn
Passes through ciliary ganglion (without synapsing)
Sensory innervation from globe and cornea
Joined by sympathetic vasomotor fibers and synapsing parasympathetic motor
fibers in the ciliary ganglion.
Branches: Long ciliary nerves (2)
Anterior and posterior ethmoidal nerves: nasal mucosa, turbinates, lateral
nasal wall, sphenoid and posterior ethmoid sinuses
Infratrochlear nerve: tip of nose (Hutchinson's sign), canaliculi, medial
canthus
Maxillary Division (V-2)
Travels just inferior to the cavernous sinus to enter the face through
the foramen rotundum
Enters the pterygopalatine fossa just inferior to the orbit
Postganglionic secretory fibers to the lacrimal gland join from the sphenopalatine
ganglion to travel with the zygomatic branches
Zygomaticofacial and zygomaticotemporal nerves
Enter the orbit from the pterygopalatine fossa through the inferior orbital
fissure
Carry sensory input from the skin overlying the lateral orbit
Carry parasympathetic fibers to the lacrimal gland
Infraorbital nerve
Travels along the inferior orbital fissure to enter the infraorbital canal
Exits from the infraorbital foramen 4–10 mm inferior to the orbital
rim
Mandibular Division (V-3)
Enters the face through the foramen ovale
Contains both sensory and motor fibers
Supplies motor input to the muscles of mastication
Sensory supply: jaw, anterior two thirds of tongue, external ear, tympanic
membrane.
Trigeminal Reflexes
Corneal blink
V to VII
Oculocardiac
V to XI (vagal) most notable with tension on medial rectus muscle
always warn the anesthesiologist before you pull on extraocular muscles
intraoperatively
reflex extinguishes after repeated stimulations
Corneolacrimal
V to VII responsible for reflex tearing
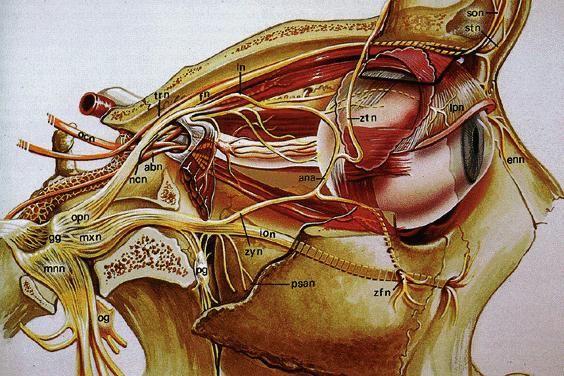 Fig. 16. Sensory supply to the orbit. Parasagittal view shows the trigeminal nerve
branches of the ophthalmic division (opn): ncn, nasociliary nerve; ln, lacrimal
nerve; fn, frontal nerve; stn, supratrochlear nerve; son, supraorbital
nerve; shown but not labeled: long ciliary nerve, anterior
ethmoidal nerve. Branches of the maxillary division (mxn) are also
shown: ion, infraorbital nerve; zyn, zygomatic nerve; ana, anastamosis
with lacrimal nerve; zfn, zygomaticofacial nerve; ztn, zygomaticofrontal
nerve. Note the sympathetic chain webbed around the carotid siphon. Gg, geniculate
ganglion; mnn, mandibular division; pg, pterygoid (sphenopalatine) ganglion; abn, abducens nerve; trn, trochlear nerve; ocn, oculomotor
nerve. (Modified from Rootman J, Stewart B, Goldberg RA: Orbital Surgery: A conceptual
Approach, p. 117. Philadelphia, Lippincott-Raven, 1995) Fig. 16. Sensory supply to the orbit. Parasagittal view shows the trigeminal nerve
branches of the ophthalmic division (opn): ncn, nasociliary nerve; ln, lacrimal
nerve; fn, frontal nerve; stn, supratrochlear nerve; son, supraorbital
nerve; shown but not labeled: long ciliary nerve, anterior
ethmoidal nerve. Branches of the maxillary division (mxn) are also
shown: ion, infraorbital nerve; zyn, zygomatic nerve; ana, anastamosis
with lacrimal nerve; zfn, zygomaticofacial nerve; ztn, zygomaticofrontal
nerve. Note the sympathetic chain webbed around the carotid siphon. Gg, geniculate
ganglion; mnn, mandibular division; pg, pterygoid (sphenopalatine) ganglion; abn, abducens nerve; trn, trochlear nerve; ocn, oculomotor
nerve. (Modified from Rootman J, Stewart B, Goldberg RA: Orbital Surgery: A conceptual
Approach, p. 117. Philadelphia, Lippincott-Raven, 1995)
|
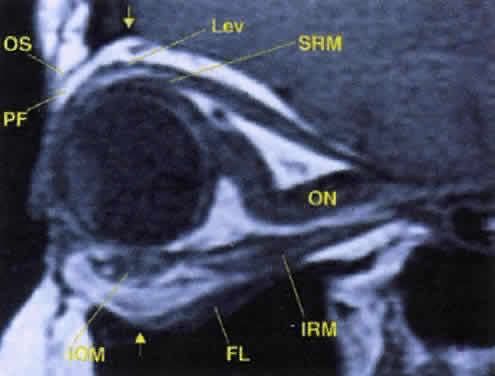 Fig. 17. The optic nerve (ON). A parasagittal MR image demonstrates the 7-mm excess of intraorbital
optic nerve, seen as an S shape. Also note the perpendicular relation
of the inferior oblique muscle (IOM) to the inferior rectus muscle (IRM). Other findings of anatomic interest in this image include the levator (Lev), the superior rectus muscle (SRM), the orbital septum (OS) arising from the arcus marginalis, and the preaponeurotic fat pad (PF) between the septum and the levator. Note that the orbital floor (FL) is angled upward by 15° to 20° from anterior to posterior. Fig. 17. The optic nerve (ON). A parasagittal MR image demonstrates the 7-mm excess of intraorbital
optic nerve, seen as an S shape. Also note the perpendicular relation
of the inferior oblique muscle (IOM) to the inferior rectus muscle (IRM). Other findings of anatomic interest in this image include the levator (Lev), the superior rectus muscle (SRM), the orbital septum (OS) arising from the arcus marginalis, and the preaponeurotic fat pad (PF) between the septum and the levator. Note that the orbital floor (FL) is angled upward by 15° to 20° from anterior to posterior.
|
Second, the motor fibers to the EOMs usually enter the inner aspect of
each muscle at the junction of the posterior one third and the anterior
two thirds of the muscle's length. The exception is the nerve to
the inferior oblique, which runs along the lateral aspect of the inferior
rectus muscle to enter the inferior oblique muscle near the globe's
equator (see Fig. 14). This nerve may be damaged with manipulation of inferior orbital soft
tissue during repair of an orbital floor fracture. Because the parasympathetic
fibers of the pupil also travel with the nerve to the inferior
oblique at the orbital apex, any anterior traction may cause contusion
to these more posterior fibers, resulting in a postoperative Adie's
pupil.14 VASCULAR SUPPLY Arteries The tissues of the orbit and periorbital region derive their blood supply
from two sources—the internal and external carotid arteries.15 Although the majority of orbital blood supply comes from the ICA, anastomoses
with external carotid supply are numerous. The ICA enters the calvarium through the foramen lacerum, runs near the
posterior clinoid process, and then makes a sharp turn to enter the CS
with the abducens nerve (see Fig. 13). As already noted, within the CS the ICA has an S-shaped course called
the carotid siphon. As the ICA exits the CS, it gives off its first
major intracranial branch, the ophthalmic artery. Before giving off the ophthalmic artery, the ICA has several minor branches
that supply the meninges, including the dura of the lateral wall
of the CS. An abnormal communication between the arterial and venous supply
of the CS results in either a carotid-cavernous fistula or a dural-sinus
fistula (Fig. 18A). Because of the larger caliber of the ICA, a carotid-cavernous fistula
is usually symptomatic secondary to a high flow state, possibly manifesting
as orbital/ocular ischemia and increased intraocular pressure. This
type of fistula is most commonly encountered in younger patients
after blunt trauma and may require invasive neuroradiologic treatment (Fig. 18B). Conversely, a dural-sinus fistula is typically a low-flow state because
the abnormal communication forms between the small-caliber dural arterial
feeders of the lateral CS wall and the venous plexus of the CS. Such
fistulas are usually seen in older individuals as a spontaneous
event. Depending on the severity of symptoms, most dural sinus fistulas
are simply followed by observation because of the high rate of spontaneous
closure. 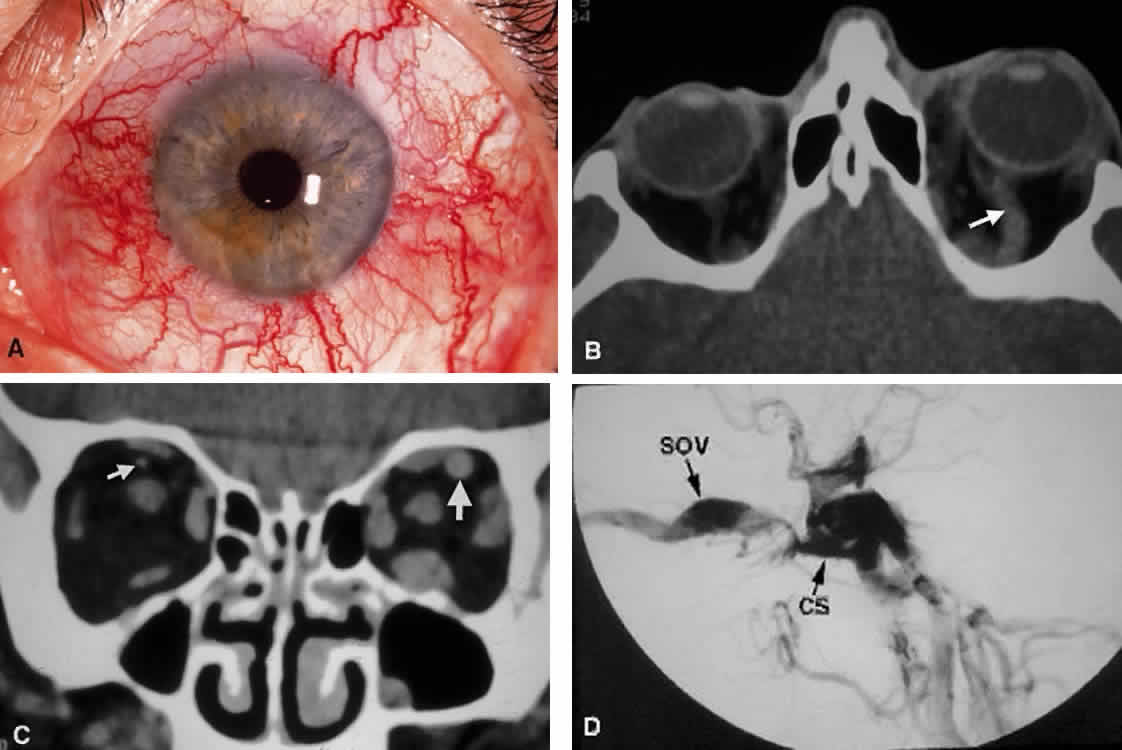 Fig. 18. Carotid-cavernous fistula. A. Clinical photograph demonstrating tortuosity of the arteriolized episcleral
veins, extending up to the limbus (the vascular congestion of conjunctivitis
usually ends 1 mm short of the limbus). B. Axial CT shows the difference in caliber between the uninvolved superior
ophthalmic vein and the involved vein (arrows). C. Coronal image likewise shows the difference in venous size (arrows). Also note the enlargement of the extraocular muscle on the involved
side, indicative of orbital congestion. The superior ophthalmic vein is
always found beneath the superior rectus muscle, to which it is tethered
by a hammock-like fascial slip. On the involved side, orbital congestion
and enlargement of the vein cause distortion of this anatomic
relation. D. Parasagittal arteriography image of a different patient shows abnormal
arterial filling of the CS, extending anteriorly into the orbit through
an engorged superior ophthalmic vein (SOV). Fig. 18. Carotid-cavernous fistula. A. Clinical photograph demonstrating tortuosity of the arteriolized episcleral
veins, extending up to the limbus (the vascular congestion of conjunctivitis
usually ends 1 mm short of the limbus). B. Axial CT shows the difference in caliber between the uninvolved superior
ophthalmic vein and the involved vein (arrows). C. Coronal image likewise shows the difference in venous size (arrows). Also note the enlargement of the extraocular muscle on the involved
side, indicative of orbital congestion. The superior ophthalmic vein is
always found beneath the superior rectus muscle, to which it is tethered
by a hammock-like fascial slip. On the involved side, orbital congestion
and enlargement of the vein cause distortion of this anatomic
relation. D. Parasagittal arteriography image of a different patient shows abnormal
arterial filling of the CS, extending anteriorly into the orbit through
an engorged superior ophthalmic vein (SOV).
|
The ophthalmic artery enters the optic canal inferolateral to the optic
nerve, carrying with it a sympathetic plexus from the ICA. The intraorbital
course of the ophthalmic artery is highly variable. In about 83% of
cases, the artery crosses over the optic canal from lateral to medial
and continues to travel superomedially in the orbit to its terminal
branches. While still within the orbit, the ophthalmic artery gives off several branches.16 These are most easily subdivided into three groups: ocular, muscular, and
extraorbital (Table 12, Fig. 19). TABLE TWELVE. Three Arterial Groupings (Circles) of the Orbit
Ocular (Posterior):
Central retinal
Short posterior ciliaries (15–20)
Long posterior ciliaries (2)
Orbital (Middle):
Muscular or anterior ciliaries (7)
Lacrimal
Extraorbital (Anterior)
Anterior & posterior ethmoidals
Supraorbital
Terminal branches of the ophthalmic artery (supratrochlear, infratrochlear, and
dorsal nasal)
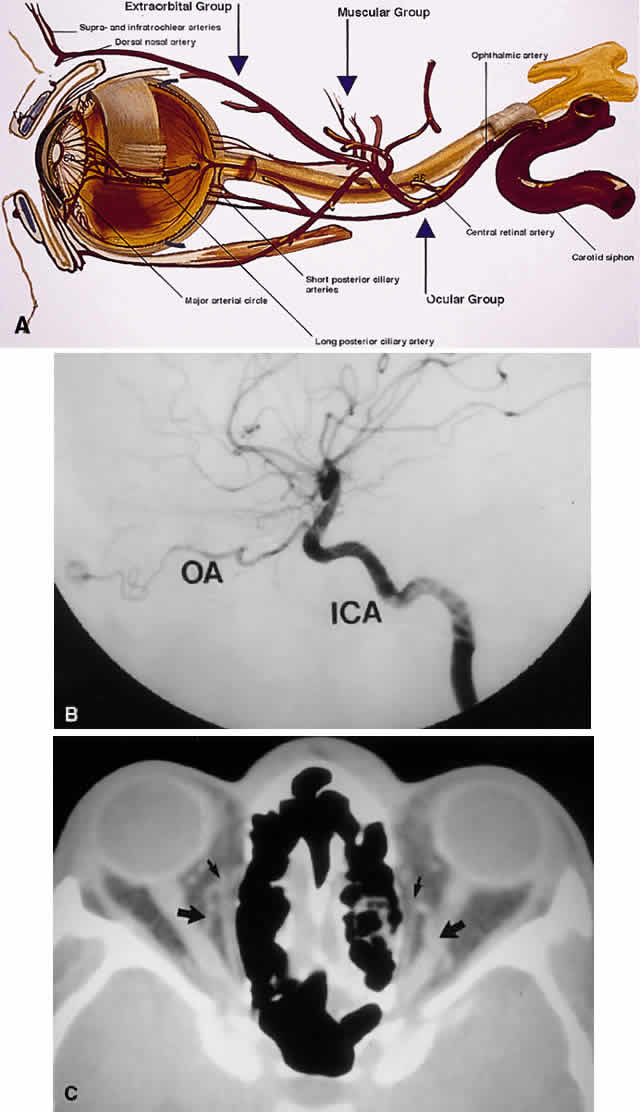 Fig. 19. Arterial supply of the orbit. A. The ophthalmic artery enters the orbit to give off its ocular supply, followed
by branches to the extraocular muscles and lacrimal gland, finally
ending superomedially as an extraorbital supply to the eyelids and
glabella. B. Parasagittal arteriography view shows the ophthalmic artery (OA) branching
from the internal carotid artery (ICA) just above the carotid siphon. Note
that although the carotid siphon lies within the CS, the ophthalmic
artery branches off just above the CS. The kink of the ophthalmic
artery within the orbit indicates its course over the optic nerve
from medial to lateral. C. Axial CT image. A fortuitous cut shows the ophthalmic artery looping over
the optic nerve (large arrows). Small arrows indicate the ethmoidal arteries branching off the ophthalmic
arteries to travel through the lamina papyracea. (A modified from Zide BM, Jelks GW: Surgical Anatomy of the Orbit, p 11. New
York, Raven Press, 1985) Fig. 19. Arterial supply of the orbit. A. The ophthalmic artery enters the orbit to give off its ocular supply, followed
by branches to the extraocular muscles and lacrimal gland, finally
ending superomedially as an extraorbital supply to the eyelids and
glabella. B. Parasagittal arteriography view shows the ophthalmic artery (OA) branching
from the internal carotid artery (ICA) just above the carotid siphon. Note
that although the carotid siphon lies within the CS, the ophthalmic
artery branches off just above the CS. The kink of the ophthalmic
artery within the orbit indicates its course over the optic nerve
from medial to lateral. C. Axial CT image. A fortuitous cut shows the ophthalmic artery looping over
the optic nerve (large arrows). Small arrows indicate the ethmoidal arteries branching off the ophthalmic
arteries to travel through the lamina papyracea. (A modified from Zide BM, Jelks GW: Surgical Anatomy of the Orbit, p 11. New
York, Raven Press, 1985)
|
The optic nerve derives its blood supply from three sources: the central
retinal artery, the pial perforators, and the short posterior ciliary
arteries.17 Note that the two long posterior ciliary vessels do not supply the optic
nerve. Instead, they travel anteriorly within their scleral canal to
supply the major arterial circle of the globe located at the iris root. The
major arterial circle is also supplied by the terminal branches
of the seven muscular arteries, which enter each rectus muscle with
the motor nerve at the posterior third of the muscle. Note that the lateral
rectus muscle has only one muscular artery but derives an additional
blood supply from the lacrimal artery. The extraorbital or anterior arterial circle includes the ethmoidal arteries, which
act to tether the ophthalmic artery to the medial wall of
the orbit, and terminal branches which exit the orbit anteriorly to anastomose
with the external carotid arterial supply of the face. The anterior
and posterior ethmoidal vessels are important landmarks during
medial orbitotomy. Because their ostia run through the frontoethmoidal
suture, they are found at the level of the cribriform plate (skull base) (see Fig. 7). The anterior to posterior location of these arteries is also an important
guide to the optic foramen: the anterior ethmoidal artery is located 24 mm
posterior to the anterior lacrimal crest, the posterior ethmoidal
artery is 12 mm posterior to the anterior ethmoidal artery, and
the optic foramen is 6 mm posterior to the posterior ethmoidal artery. Thus, remembering
the numbers 24-12-6 is helpful as a guide to the optic
foramen. The terminal branches of the ophthalmic artery are the supratrochlear, infratrochlear, and
dorsal nasal arteries, which supply the glabella and
medial canthus and form the medial half of the palpebral arcades (Fig. 20A). The dorsal nasal artery (ICA) anastomoses with the facial artery (external
carotid artery) to form the angular artery, often encountered during
dacryocystorhinostomy. 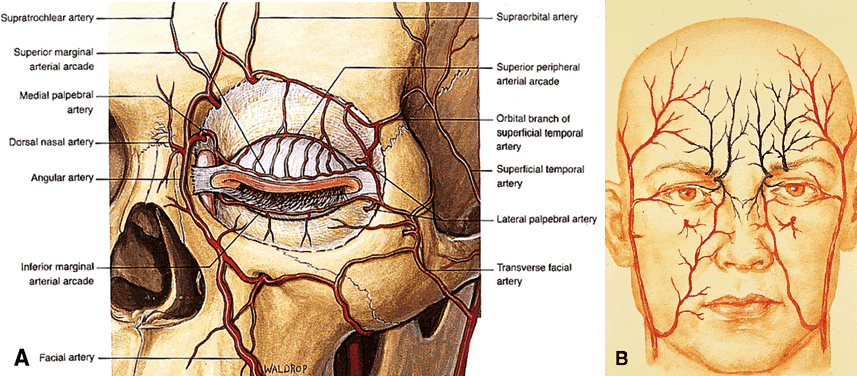 Fig. 20. The arterial supply of the eyelids and midface. A. The upper eyelid contains two arcades, whereas the lower typically has
one main arcade with or without a more rudimentary secondary arcade. Note
that the palpebral arteries pierce the orbital septum to communicate
with the anterior orbit. B. Major anastomoses of the internal (green) and external (red) carotid arterial
supplies include the transverse facial and dorsal nasal arteries; the
zygomatic/superficial temporal and lacrimal/lateral palpebral
arteries; and the frontal branch of the superficial temporal and supraorbital
arteries. Note that the infraorbital artery is a branch of the
external carotid artery, arising from the internal maxillary artery within
the pterygopalatine fossa. (A from Dutton JJ: Atlas of Clinical and Surgical Orbital Anatomy, p 79. Philadelphia, WB
Saunders, 1994) Fig. 20. The arterial supply of the eyelids and midface. A. The upper eyelid contains two arcades, whereas the lower typically has
one main arcade with or without a more rudimentary secondary arcade. Note
that the palpebral arteries pierce the orbital septum to communicate
with the anterior orbit. B. Major anastomoses of the internal (green) and external (red) carotid arterial
supplies include the transverse facial and dorsal nasal arteries; the
zygomatic/superficial temporal and lacrimal/lateral palpebral
arteries; and the frontal branch of the superficial temporal and supraorbital
arteries. Note that the infraorbital artery is a branch of the
external carotid artery, arising from the internal maxillary artery within
the pterygopalatine fossa. (A from Dutton JJ: Atlas of Clinical and Surgical Orbital Anatomy, p 79. Philadelphia, WB
Saunders, 1994)
|
The terminal arteries of the orbit exit around the orbital rim to form
three main anastomoses with the external carotid arterial supply of the
face, namely with the facial artery, the superficial temporal artery, and
the maxillary artery (see Fig. 20B). The eyelids are supplied chiefly by palpebral arcades that course across
the lids from medial and lateral arteries. In most cases, the upper
eyelid contains two arcades, the marginal and peripheral, whereas the
lower eyelid contains only one arcade, the peripheral. The peripheral
arcades are found at the peripheral border of each tarsal plate, lying
between the eyelid retractors. In the upper eyelid, the peripheral arterial
arcade is commonly encountered during levator surgery between the
levator aponeurosis and Müller's muscle. The medial palpebral
artery pierces the orbital septum and follows a tortuous course through
the medial fat pad of the upper eyelid as it anastomoses with deeper
orbital vessels.35 Clamping of the medial fat pad before excision is important in preventing
excessive bleeding during upper eyelid surgery. A summary of important vascular characteristics of the orbit is found in Table 13. TABLE THIRTEEN. Vascular Pearls
- The first major branch of the internal carotid artery is the ophthalmic
artery, and the first branch of the ophthalmic artery is the central
retinal artery.
- The orbital arteries can be grouped into three circles: the posterior (supplying
the eyeball), the middle (supplying the extraocular muscles), and
the anterior (terminal branches supplying the face).
- The infraorbital artery is a branch of the internal maxillary artery, which
is in turn a branch of the external carotid artery. The infraorbital artery is not a branch of the ophthalmic
artery.
- The major drainage of the orbit is via the superior ophthalmic vein. The
inferior ophthalmic vein plays a major role only in pathologic conditions (e.g., carotid—cavernous fistula).
Veins Within the soft tissue of the orbit, venous drainage is distinctly separate
from the arterial supply; a similar arrangement is found intracranially. Although
the veins are found in a relatively reproducible pattern
within the orbital septal system, the arteries travel more haphazardly
within orbital fat.18 All orbital veins are valveless; this may facilitate more rapid posterior
spread of infectious processes within the anterior orbit. The major venous drainage of the orbit is the superior ophthalmic vein
and the CS (Fig. 21). The superior ophthalmic vein follows a medial-to-lateral route posteriorly
along the superior orbit, tethered beneath the superior rectus
muscle by a fascial sling.19 At the orbital apex, it is usually joined by the inferior ophthalmic vein, which
also communicates through more minor branches with the pterygopalatine
plexus through the IOF. The central retinal vein typically
drains directly into the CS without joining the superior ophthalmic vein. Anteriorly, the
orbit also drains into the angular vein of the facial
plexus. 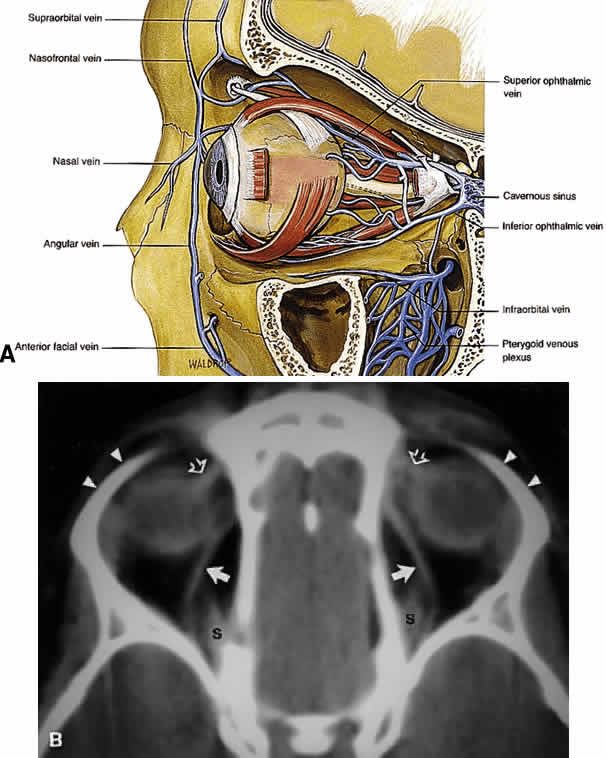 Fig. 21. A. Venous drainage of the orbit. Major drainage is supplied by the superior
ophthalmic vein, which drains into the CS. Note that the central retinal
vein usually drains directly into the CS. Drainage through the pterygoid
plexus is minor under normal conditions but becomes very important
during outflow problems through the CS (carotid-cavernous or dural-cavernous
fistulas). B. Axial CT reveals the course of the superior ophthalmic veins (closed arrows) beneath the superior rectus muscles (S). Also imaged are the superior
orbital rims (arrowheads) and the superior oblique muscles (open arrows) as each passes through the trochlea. (A from Dutton JJ: Atlas of Clinical and Surgical Orbital Anatomy, p 79. Philadelphia, WB
Saunders, 1994) Fig. 21. A. Venous drainage of the orbit. Major drainage is supplied by the superior
ophthalmic vein, which drains into the CS. Note that the central retinal
vein usually drains directly into the CS. Drainage through the pterygoid
plexus is minor under normal conditions but becomes very important
during outflow problems through the CS (carotid-cavernous or dural-cavernous
fistulas). B. Axial CT reveals the course of the superior ophthalmic veins (closed arrows) beneath the superior rectus muscles (S). Also imaged are the superior
orbital rims (arrowheads) and the superior oblique muscles (open arrows) as each passes through the trochlea. (A from Dutton JJ: Atlas of Clinical and Surgical Orbital Anatomy, p 79. Philadelphia, WB
Saunders, 1994)
|
Lymphatics To date, no lymphatic system has been identified within the human orbit, although
recent studies that identified lymphatic drainage in primate
orbits make this an intriguing possibility.20 In the eyelids, lymphatic drainage occurs through a deep (posterior lamellar) and
superficial (anterior lamellar) system. The medial aspect
of the eyelids drains into the submandibular lymph nodes, whereas the
lateral aspect and most of the upper eyelid drain into the preauricular
and parotid nodes. EXTRAOCULAR MUSCLES AND ORBITAL FASCIAL SYSTEM The EOMs insert 5.5 to 8 mm behind the limbus of the globe. Specific details
regarding the dimensions and functions of each EOM are found elsewhere. Useful
points are summarized in Table 6. The EOMs are striated and composed of aerobic, twitch-type Fibrillenstruktur
fibers and anaerobic, tonic-type Felderstruktur fibers.36,37 The most common affliction of the EOMs is inflammatory myositis, which
is seen most typically in thyroid-related orbitopathy and idiopathic
inflammatory pseudotumor. Rhabdomyosarcoma, a tumor with focal striated
muscle differentiation that generally occurs in children, appears to
arise from indifferent orbital mesenchyme rather than from preformed
and completely differentiated striated muscle fibers. Landmark anatomic investigations by Koornneef38–40 have shown that there are highly elaborate and reproducible connections
of the epimysium of the EOMs with the fibrous connective tissue system
of the orbital fat, the epibulbar fascia of Tenon's capsule, and
the periosteum (periorbita) (Fig. 22). This extensive yet reproducible network of connective tissue provides
support for the delicate neurovascular structures traversing the orbit, both
facilitates and restricts the movement of EOMs in a coordinated
fashion, and compartmentalizes the orbit into various spaces. Because
of the inherent complexity of the orbital connective tissue system, any
disruption in one area of the orbit may cause significant dysfunction
of more distant areas. For example, these connections become clinically
important in the understanding of restricted motility after orbital
wall fracture and orbital decompressions for Graves' orbitopathy, as
well as the superior sulcus and lower eyelid deformities that follow
enucleation and orbital implant placement.  Fig. 22. Fascial network of the orbit, oblique coronal views. A. Section through the equator of the globe. Note the diffuse attachments
from the lateral rectus muscle sheath to the lateral retinaculum, collectively
called the lateral rectus check ligament. Also shown are the
complex attachments between each oblique muscle and its corresponding
rectus muscle neighbor. B. Section through the posterior globe. The superior rectus-levator complex
is clearly shown, with attachments to the orbital roof. C. Section at the lamina cribrosa. The superior ophthalmic vein begins to
course more laterally, toward the superior rectus-levator complex. Attachments
of the medial and lateral rectus muscle sheaths to their corresponding
orbital walls are indicated. The lateral rectus attachments
continue along the entire length of the lateral orbital wall. Note that
the intraconal fat lies in discrete lobules separated by fascial sleeves. The
orbital veins are contained within these sleeves, whereas the
orbital arteries follow an independent course through the fat lobules. D. Section through the midorbit (anterior portion of the optic nerve). The
superior ophthalmic vein is now shown in its fascial sling below the
superior rectus muscle. More posteriorly, the orbital fascial system
deteriorates, with a less defined intraconal space. (Dutton JJ: Atlas of Clinical and Surgical Orbital Anatomy, pp 103–105. Philadelphia, WB
Saunders, 1994) Fig. 22. Fascial network of the orbit, oblique coronal views. A. Section through the equator of the globe. Note the diffuse attachments
from the lateral rectus muscle sheath to the lateral retinaculum, collectively
called the lateral rectus check ligament. Also shown are the
complex attachments between each oblique muscle and its corresponding
rectus muscle neighbor. B. Section through the posterior globe. The superior rectus-levator complex
is clearly shown, with attachments to the orbital roof. C. Section at the lamina cribrosa. The superior ophthalmic vein begins to
course more laterally, toward the superior rectus-levator complex. Attachments
of the medial and lateral rectus muscle sheaths to their corresponding
orbital walls are indicated. The lateral rectus attachments
continue along the entire length of the lateral orbital wall. Note that
the intraconal fat lies in discrete lobules separated by fascial sleeves. The
orbital veins are contained within these sleeves, whereas the
orbital arteries follow an independent course through the fat lobules. D. Section through the midorbit (anterior portion of the optic nerve). The
superior ophthalmic vein is now shown in its fascial sling below the
superior rectus muscle. More posteriorly, the orbital fascial system
deteriorates, with a less defined intraconal space. (Dutton JJ: Atlas of Clinical and Surgical Orbital Anatomy, pp 103–105. Philadelphia, WB
Saunders, 1994)
|
To promote a clearer understanding, Dutton has succinctly summarized the
orbital connective tissue system by dividing it into three components: Tenon's
capsule, the anterior orbital suspensory system, and the
posterior orbital suspensory system.41 The three components should not be considered as distinct anatomic entities, because
complex attachments unite this triad into one comprehensive
unit. Tenon's capsule begins anteriorly just posterior to the limbus with
firm attachments to the underlying episclera, investing the globe in
an elastic and vascular connective tissue cover. The capsule also encompasses
the anterior portions of the EOMs. Posteriorly, Tenon's
capsule surrounds the optic nerve, interdigitating with the dural sheath. Other
than its anterior and posterior attachments to the globe, Tenon's
capsule is only loosely adherent to the underlying sclera, thereby
cushioning the movement of the globe. An intermuscular fibrous membrane connects the four rectus muscles around
the globe to create a distinct intraconal space, but in the deeper
orbital tissues this membranous system is not complete, so that the distinction
between a central and a peripheral orbital space is lost toward
the orbital apex. Anteriorly, the intermuscular fibers also blend
with Tenon's capsule, creating a sort of rotating sleeve around the
globe during ocular rotations. The anterior orbital suspensory system is primarily concerned with providing
support for the globe and fixation for eyelid structures. In addition, fascial
support exists for the lacrimal gland (Sommering's
ligaments) and the superior oblique muscle in the area of the trochlea. The
four main structures of the anterior system are Whitnall's
suspensory ligament, Lockwood's ligament, and the medial and lateral
canthal tendons. These structures are discussed in detail elsewhere. The
anterior suspensory system also provides anchoring points for
the check ligaments of the EOMs, limiting their overaction during ocular
rotations. As already noted, the orbital septal system becomes less defined more posteriorly. The
well-defined intraconal space encountered anteriorly blends
with the surrounding extraconal space as one approaches the orbital
apex. The annulus of Zinn, discussed earlier, forms dense adhesions
to the periosteum of the orbital apex around the optic canal and SOF (see Fig. 12). In addition, fibers pass inferiorly along the IOF to interdigitate with
Müller's orbital muscle, which spans the fissure. As the levator palpebrae superioris (levator) muscle and superior rectus
muscle course anteriorly, diffuse connections form to the orbital roof, creating
an effective suspensory system. Further, diffuse fascial
connections form between the levator and superior rectus muscles, allowing
precise coordination of upper eyelid retraction with upgaze. Note
that on imaging studies, the close association of these two muscles often
makes them look like a single anatomic unit; the term “levator-superior
rectus complex”is often found in radiologic reports (see Fig. 17). A fascial sling forms beneath the superior rectus muscle to provide
suspensory support for the superior ophthalmic vein. The medial rectus muscle has few important attachments to the posterior
medial orbital wall, in contrast to the lateral rectus muscle, which
has diffuse attachments to the lateral orbital wall from the lateral canthal
tendon anteriorly to the annulus of Zinn posteriorly. Presumably, the
extensive fascial attachments of the lateral rectus muscle sheath
provide a firm attachment sleeve within which the lateral rectus muscle
can function while still subtending a significant arc around the
globe.41 The orbital muscle of Müller spans the IOF, separating the orbit from
the pterygopalatine fossa below. The function of this smooth muscle
in humans is unclear. It may simply represent an anatomic relic of evolution: Dutton
notes that the orbital floor in lower mammals is poorly
defined posteriorly and in need of muscular support.41 Some anatomists also ascribe a vasculosympathetic function to the muscle, given
its proximity to the inferior ophthalmic vein. LACRIMAL SYSTEM Drainage System Drainage of tears from the eyelids into the nose requires a patent drainage
system (Fig. 23) comprising the puncta, canaliculi, lacrimal sac, and nasolacrimal duct, proper
positioning of the eyelids against the globe, and an intact
lacrimal pump function.42,43 If any of these components fails, epiphora will result. 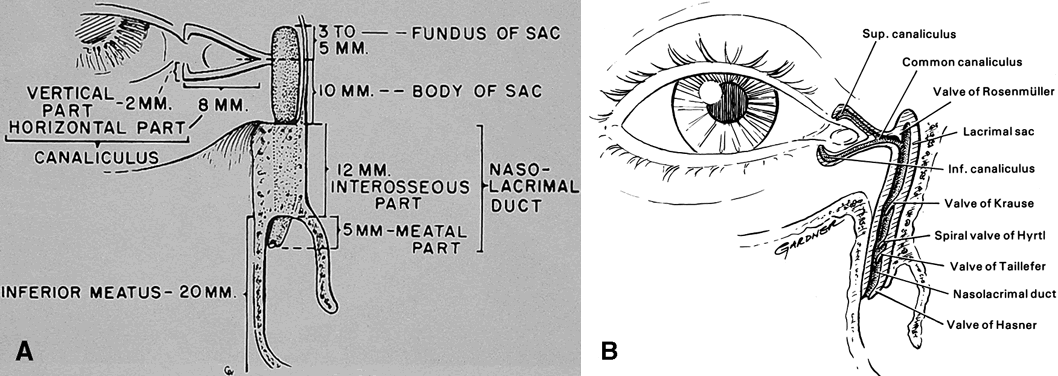 Fig. 23. Lacrimal drainage system. A. Measurements. B. Valves of the lacrimal sac and nasolacrimal duct. (A from Jones LT, Wobig JL: Surgery of the Eyelids and Lacrimal System. Birmingham, Aesculapius, 1976. B from Bergin DJ: Anatomy of the eyelids, lacrimal system, and orbit. In
McCord CD, Tanenbaum M, Nunery WR [eds]: Oculoplastic Surgery [3rd
ed]. New York, Raven Press, 1995). Fig. 23. Lacrimal drainage system. A. Measurements. B. Valves of the lacrimal sac and nasolacrimal duct. (A from Jones LT, Wobig JL: Surgery of the Eyelids and Lacrimal System. Birmingham, Aesculapius, 1976. B from Bergin DJ: Anatomy of the eyelids, lacrimal system, and orbit. In
McCord CD, Tanenbaum M, Nunery WR [eds]: Oculoplastic Surgery [3rd
ed]. New York, Raven Press, 1995).
|
The puncta are defined as the openings of the canaliculi onto the eyelid
margin (see Fig. 6). Each punctum measures about 0.3 mm in diameter and is found 0.5 to 1.0 mm
medial to the end of the tarsal plate. The upper punctum is usually
found slightly nasal to the lower punctum, each measuring 6 mm and 6.5 mm
from the medial commissure, respectively. Thus, the puncta do
not overlap each other during eyelid blink. Peripunctal tissue is avascular
and rich in elastic tissue. This last point is documented clinically
when observing the rapid collapse of the puncta in younger patients
immediately after probing. The canaliculi begin anatomically at the puncta. The vertical portion measures
about 2 mm, with an ampulla (2.5 mm in diameter) forming just
beneath the punctal opening. The horizontal portion measures 8 mm in length, surrounded
by the medial portions of the muscle of Riolan (also
known as Horner's muscle, an extension of the pretarsal orbicularis) and
traveling through the fiber network of the medial canthal tendon. The
diameter of each canaliculus is only 1 mm. In more than 90% of
individuals, the canaliculi join to form a common canaliculus known
as the ampulla of Maier, which then enters the lacrimal sac at the common
internal punctum. The common internal punctum forms the first major
valve of the lacrimal system, the valve of Rosenmuller, and acts to
prevent reflux of tears back into the canaliculi during lacrimal pump
function. The lacrimal sac is found within its bony fossa, formed by the anterior
and posterior lacrimal crests (see Fig. 7A and B). It measures 12 to 15 mm in length and has strong attachments over its
lateral surface from the medial canthal tendon and fibers of the orbicularis (Horner's
and Jones' muscles). The portion of the sac found
above the medial canthal tendon is defined as the fundus, measuring 3 to 5 mm
in height. Inferiorly, the sac opens into the nasolacrimal
duct at the lacrimal ostium. (Note the proximity of the inferior oblique
muscle origin, an important consideration during surgery of the lacrimal
sac.) The pseudostratified nonkeratinized columnar epithelium of
the lacrimal sac is thrown into numerous folds that form a screwlike
passageway for tear outflow. Although each fold has been given various
eponyms (see Fig. 23B), these are not important to remember; rather, understand that the folds
as a unit perform the important function of efficiently driving tear
flow in a unilateral direction down the nasolacrimal duct. The walls
of the sac are rich in elastic tissue, thereby allowing for the constant
collapse and reformation of the sac during the blink reflex. The nasolacrimal duct travels within its canal for 12 mm through the nasofrontal
process of the maxillary bone, opening intranasally into the
inferior meatus, found just beneath the inferior turbinate. The opening
of the nasolacrimal duct is about 16 to 25 mm posterior to the tip
of the nose in adults, and about half that distance in children. The duct
travels in a posterior and lateral direction,24 angulating 15° and 5°, respectively. As already noted, the inferior
opening of the duct is defined as the valve of Hasner. The lacrimal pump mechanism is described elsewhere. In addition to the
dynamic process of the pump, gravity also assists in tear outflow. SECRETORY SYSTEM The aqueous portion of the tear film is produced by several glandular units
found within the eyelids and anterior orbit. Considerable controversy
exists regarding the innervation and contribution of each set of
glands.28 One theory put forth by Jones defines the main lacrimal gland as a “reflex”secretor (i.e., dependent on neural stimulation for tear production), whereas the accessory
lacrimal glands found along the inner aspect of the eyelids are
called “basic”secretors, producing tears at a constant, unchanging
rate.29 This theory forms the basis for the Schirmer tests of tear production. In the fully developed orbit, the lacrimal gland is normally impalpable
and is situated in a small fossa behind the superotemporal orbital rim. It
is not an encapsulated structure but rather is an aggregated collection
of lobules of secretory tissue set in the superotemporal orbital
fat, with interlobular ducts converging into the main excretory ducts
in the superotemporal fornix. Within the secretory acini are located
inner columnar to cuboidal zymogen-bearing cells, on the outer aspect
of which are applied contractile myoepithelial cells. Mucus-producing
elements are not present. Myoepithelial cells are not present in the
terminal intralobular ductules or in the interlobular ducts. The acinar
elements are not capable of regenerating after inflammatory insults, and
the regenerative capacity of the gland resides in the terminal ductules. Lymphoid tissue is present in the substantia propria of the conjunctiva, and
lymphocytes and plasma cells are lightly dispersed among the secretory
acini of the lacrimal gland, but the deep orbital tissue is devoid
of a standing population of lymphoid cells. Based on a study of the
immunoarchitecture of the lacrimal gland using monoclonal antibodies
as markers, plasma cells are the predominant mononuclear cell type, making
up 54%, and are located in the interstitium of the gland. Immunoglobulin
A (IgA) vastly predominates over other immunoglobulin types; lymphocytes
of the substantia propria of the conjunctiva and of the lacrimal
gland secrete IgA, which has a secretory piece added to it as it
moves across the ducts of the gland or the conjunctival epithelium. T
cells make up 40% of all mononuclear cells, with T8 suppressor/cytotoxic
cells outnumbering T4 helper cells. Occasional small lymphoid aggregates
without germinal centers may also be seen at the juncture of intralobular
and interlobular ducts, in which T4 helper cells predominate
and a smattering of B cells are found. Lymphoid tumors, representing
reactive hyperplasias and true lymphomas, consequently are derived from
blood-borne lymphocytes that emigrate from the orbital vascular system
into the orbital connective tissues, generally into the orbital fat. To
date, there are no identifiable endothelial-lined lymphatics within
the orbit, although the lacrimal gland itself contains lymphatic
channels that drain to the preauricular and cervical nodes. The fact that
the lacrimal gland has a standing population of lymphocytes and plasma
cells—which are not, however, organized into lymph nodes (as
may be seen in the parotid gland)—may explain the relative frequency
of the development of idiopathic dacryoadenitis and lymphoid tumors
of the gland. Inflammatory pseudotumors, lymphoid tumors, inflammations
such as sarcoidosis and Sjögren syndrome, and epithelial tumors
are the main types of pathology that arise from or involve the lacrimal
gland. The main lacrimal gland is split into two lobes, the palpebral and the
orbital, by the lateral horn of the levator aponeurosis2 (Fig. 24). The palpebral lobe measures about one-third the volume of the orbital
lobe, and its subconjunctival location is easily identified during slit-lamp
examination. The orbital lobe lies within its fossa, a concavity
found within the lateral portion of the orbital roof. Fibrous septa
known as Sommering's ligaments run from the gland to attach it
more firmly to the periosteum of the lacrimal gland fossa. Whitnall's
ligament also adds support for lacrimal gland suspension.30 (The lacrimal sac fossa is distinctly separate from the lacrimal gland
fossa; the term “lacrimal fossa”is discouraged because it
is confusing in its nonspecificity.) The shape of the orbital lobe is
rather like that of a pancake (see Fig. 21B). The orbital lobe is usually limited to its fossa, but it is not uncommon
to encounter posterior tails of lacrimal gland tissue traveling toward
the orbital apex on routine CT/MRI studies.1 Likewise, the palpebral lobe may extend as inferiorly as the lateral canthal
tendon in some individuals.31  Fig. 24. The lateral horn of the levator aponeurosis splits the lacrimal gland into
the orbital and palpebral lobes as it sweeps laterally to insert into
the lateral canthal tendon of the eyelid. (Zide BM, Jelks GW: Surgical Anatomy of the Orbit, p 35. New York, Raven
Press, 1985) Fig. 24. The lateral horn of the levator aponeurosis splits the lacrimal gland into
the orbital and palpebral lobes as it sweeps laterally to insert into
the lateral canthal tendon of the eyelid. (Zide BM, Jelks GW: Surgical Anatomy of the Orbit, p 35. New York, Raven
Press, 1985)
|
Tear outflow passes from the orbital to the palpebral lobe through the
levator aponeurosis; aggressive dissection in this area during levator
surgery can thus damage the outflow mechanism. The palpebral lobe opens
into the superolateral conjunctival fornix through 10 to 12 ductules. Innervation
to the lacrimal gland remains controversial32 but can be summarized succinctly as sensory innervation from the lacrimal
nerve (V-1) and parasympathetic innervation from the pterygopalatine
ganglion.23 The role of sympathetic innervation is not completely understood. The accessory lacrimal glands or basic secretors are usually grouped as
the glands of Krause, found along the conjunctival fornices, and the
glands of Wolfring, found along the superior tarsal border of the upper
eyelid. The glands of Wolfring may be injured during ptosis repair by
a posterior approach. The glands of Popov are located within the substance
of the caruncle. Two additional components of the tear film are mentioned briefly. The outer
lipid layer is produced by the meibomian glands, which form excavations
within the tarsus and open at the eyelid margin (and by minor contributions
from the glands of Zeis and Moll of the eyelash follicle). The
inner mucin layer is made by the conjunctival goblet cells. |




 6.5 mm, length
6.5 mm, length 












