The first examination of CNV using ICG angiography was performed in 1976 by Patz and associates.10 Using this early film-based system, they were able to identify only 2 of 25 choroidal neovascular lesions. In a follow-up report by Bischoff and Flower of 100 ICG angiograms in patients with age-related macular degeneration, “delayed or irregular choroidal filling”11 was detected in some patients. Using ICG angiography, Hayashi and colleagues12,13 were able to detect CNV and to confirm the findings of fluorescein angiography in patients with well-demarcated or classic CNV. They demonstrated that leakage of ICG from CNV was slow compared with the rapid leakage of fluorescein. Follow-up reports using digital imaging systems as well as scanning laser ophthalmoscopy suggested that ICG angiography might be more useful in the area of occult CNV by providing enhanced imaging of the abnormal vessels.14–17
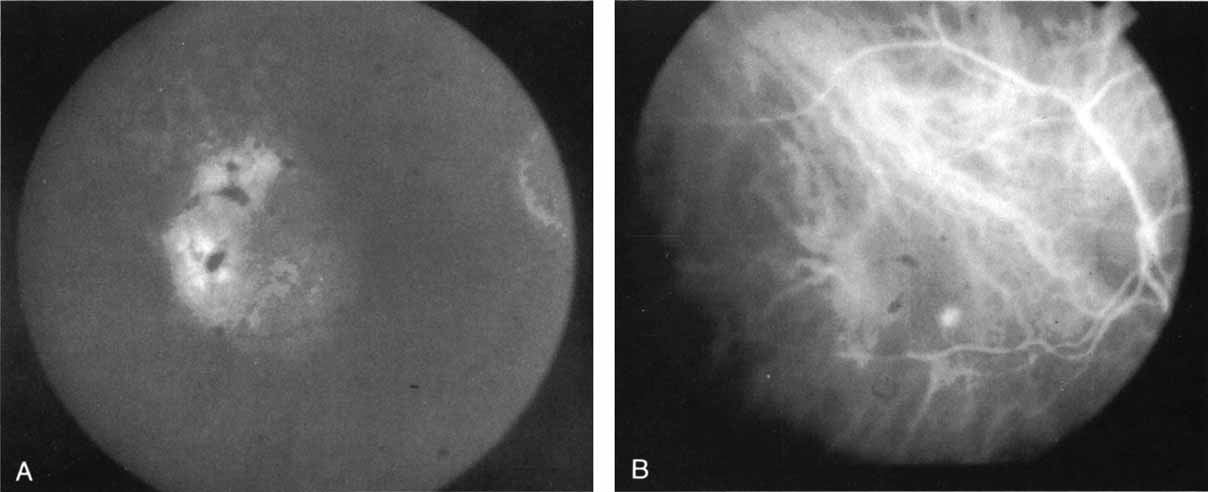
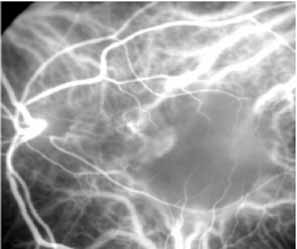
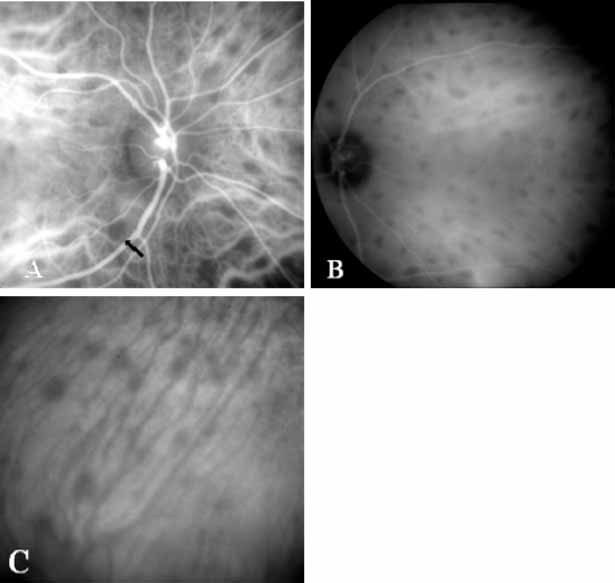
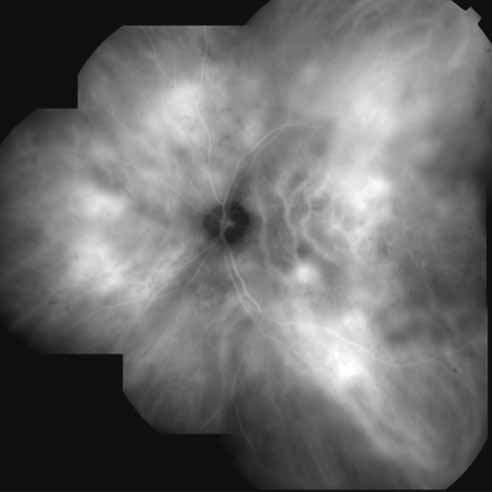
In a landmark article, Yannuzzi and associates18 demonstrated that ICG videoangiography was extremely useful in identifying well-demarcated localized areas of neovascularization in what had been classified as occult CNV by standard fluorescein angiography.19–24 In this study, 39% of 129 patients with “occult CNV” originally diagnosed as determined by fluorescein angiography were given a revised diagnosis of “well-defined neovascular lesions” based on the information obtained from the ICG study (Fig. 1). One study revealed that approximately 40% of patients with occult CNV diagnosed actually presented with early, well-defined focal areas of fluorescence on ICG videoangiography.25 They further defined two potential subgroups of occult CNV: those with and those without serous pigment epithelial detachments (PEDs) accompanying the occult neovascular process. They also pointed out that ICG angiography offered a potential advantage in identifying neovascular lesions when there was clinical evidence of recurrent CNV after previous laser photocoagulation treatment.
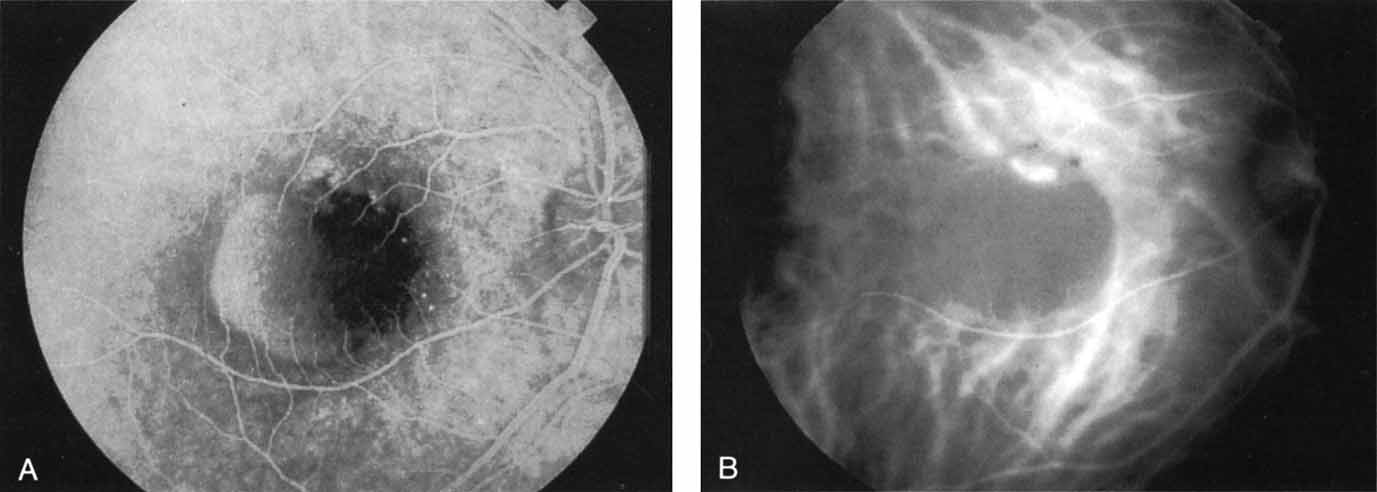
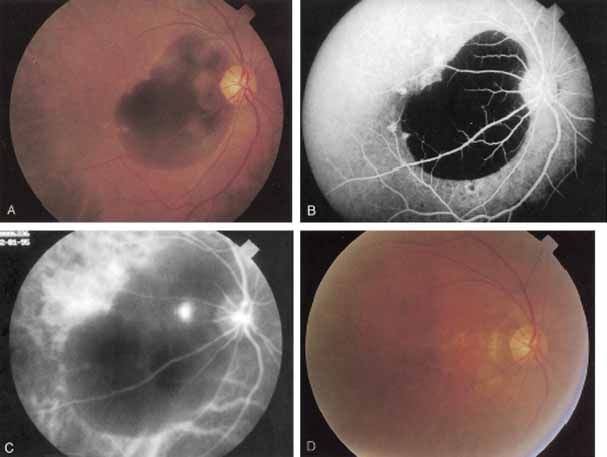
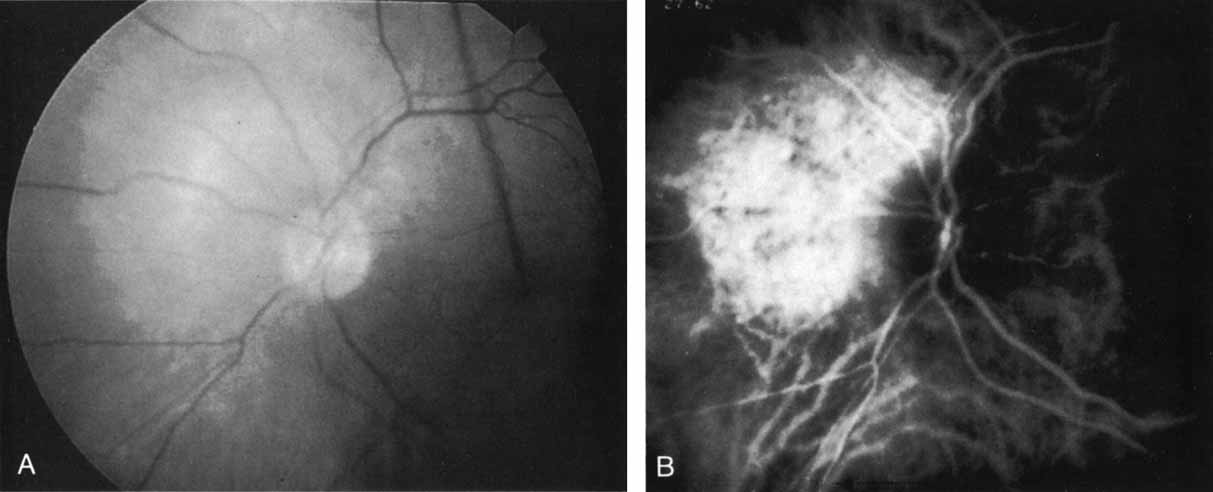
Subsequently, Yannuzzi and colleagues25 and Guyer and associates26 evaluated the usefulness of ICG angiography in identifying localized areas of CNV in patients with occult CNV with or without a serous PED. In a careful evaluation of more than 680 patients, they identified 22% of patients with localized lesions that might be amenable to laser therapy—lesions that would otherwise be classified as untreatable based on guidelines for laser photocoagulation (Figs. 2 and 3). As a result of this improved imaging technique, as many as two to three times the number of patients would have been potentially eligible for laser treatment than would have been treatable based on fluorescein angiography alone.
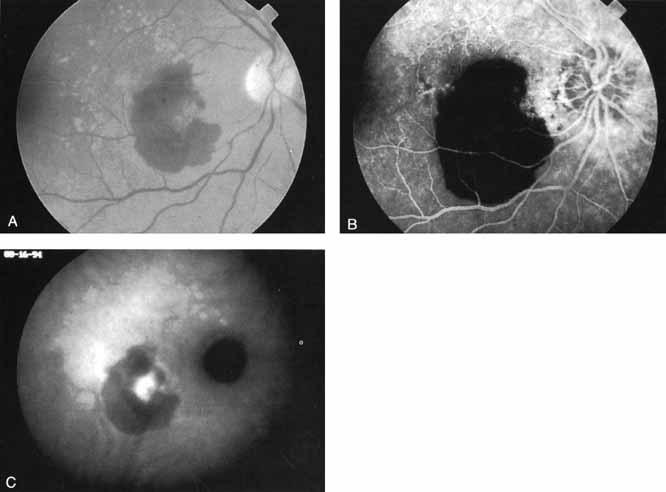
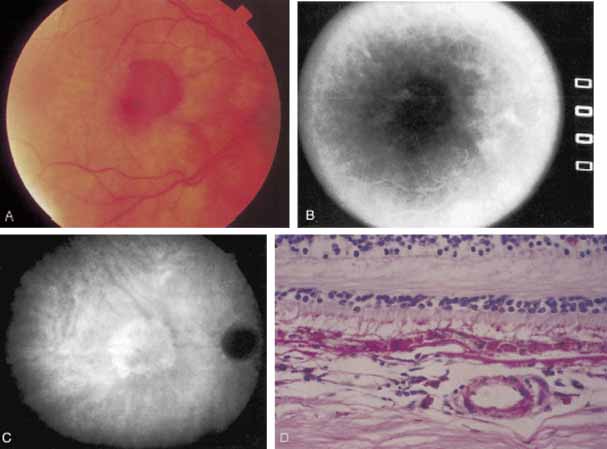
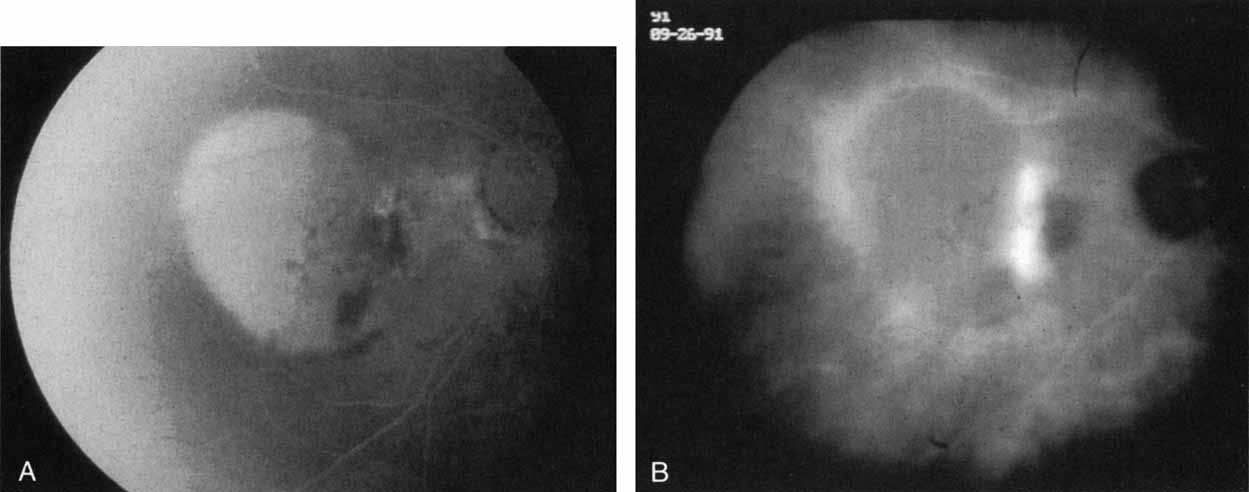
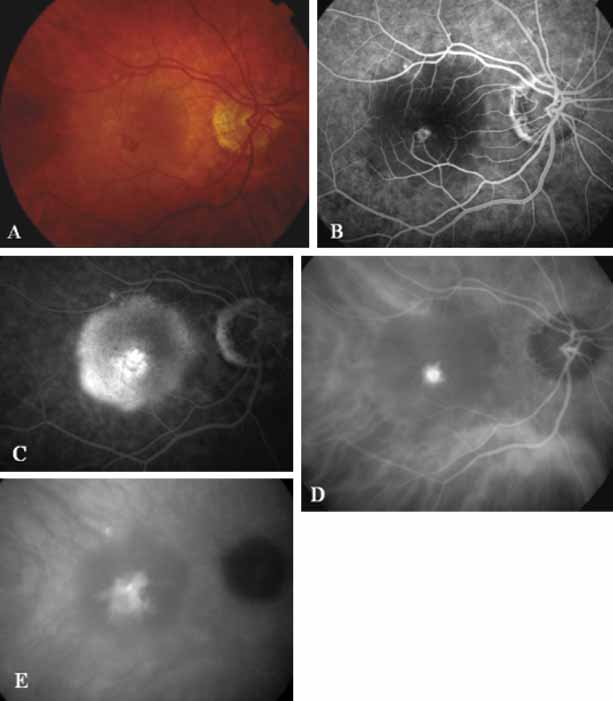
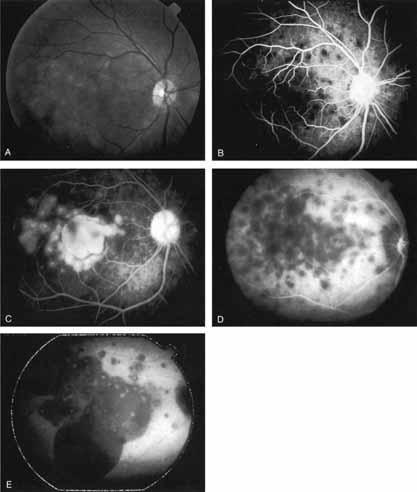
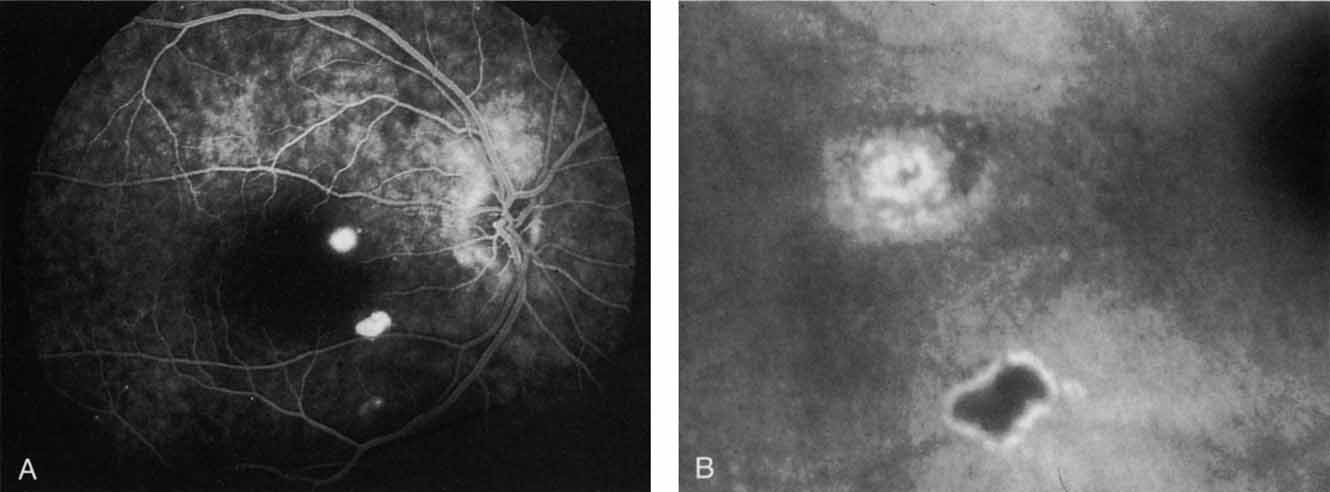
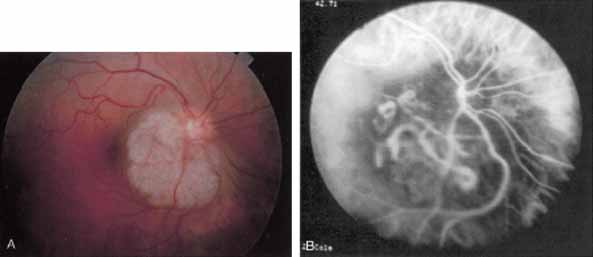
An important report by Chang and co-workers27 lends support to the rationale for interpreting the hyperfluorescence seen on ICG angiography as CNV. In this clinicopathologic study, a patient was identified who had experienced subretinal hemorrhage with early signs of occult CNV on fluorescein angiography (Fig. 4A). Fluorescein angiography demonstrated blocked fluorescence (Fig. 4B). The ICG study, however, demonstrated late staining in a well-circumscribed fashion, which the authors interpreted as a “plaque” of occult CNV (Fig. 4C). When the patient died, this area was evaluated and studied histopathologically and compared with the picture seen on ICG angiography. The area of hyperfluorescence on the ICG study corresponded precisely to a thin layer of fibrovascular tissue beneath the pigment epithelium and neurosensory retina, confirming that the late-staining tissue imaged with ICG angiography was truly a neovascular membrane (Fig. 4D).
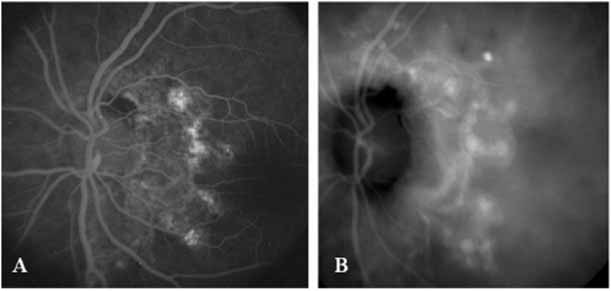
With this clinical diagnostic and histopathologic information available, pilot studies were performed to determine the practicality of using ICG angiographic guidance in the treatment of occult CNV. Slakter and associates28 performed laser photocoagulation treatment on 79 eyes with occult CNV. The occult CNV was successfully eliminated in 57% of patients who underwent ICG-guided treatment (Figs. 5 and 6). The authors found the success rate to be higher (66%) for patients with CNV not associated with PEDs than for those with PEDs (43%). Visual acuity improvement or stabilization was achieved in 57% of all patients. Recurrences were more frequent and more difficult to control in those patients who had associated PEDs on initial clinical presentation. Additional independent studies have reported similar diagnostic and treatment outcomes with the use of ICG angiography in patients with occult CNV.29,30
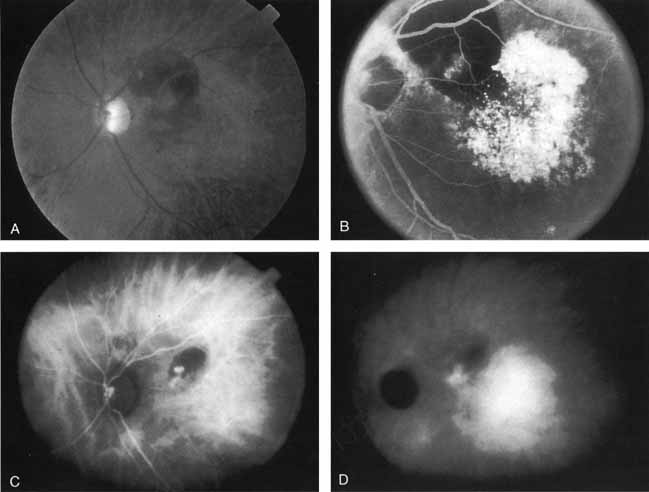
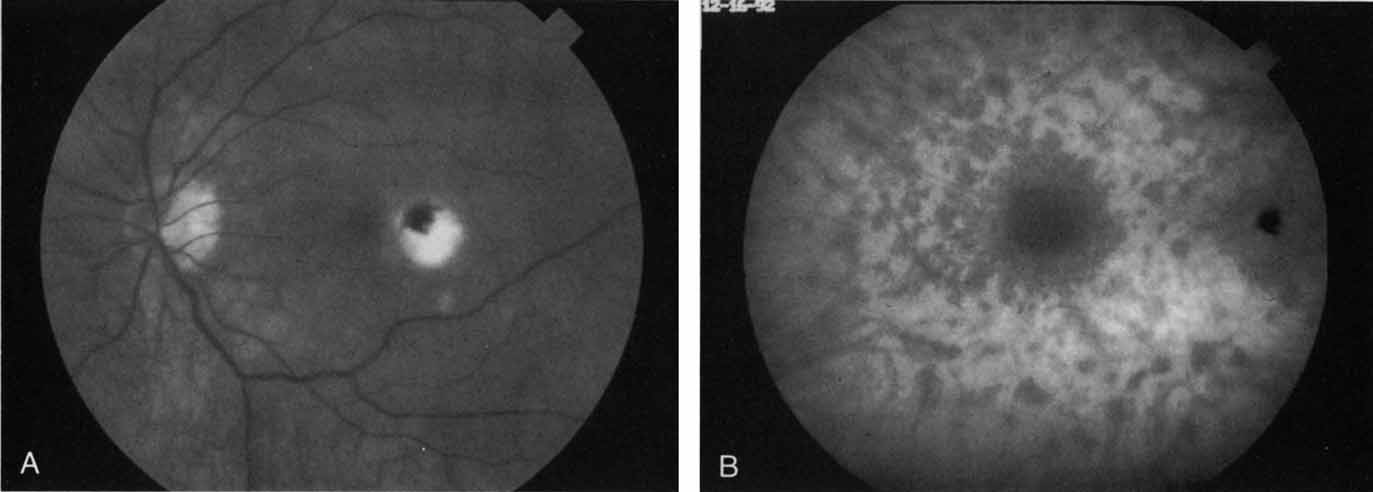
Sorenson and colleagues31 reported on the diagnostic and therapeutic ability of ICG angiography in patients who had clinical signs of recurrent CNV but who were found to have occult membranes on fluorescein angiography. In a group of 66 patients, 97% were identified as having localized areas of hyperfluorescence on ICG angiography consistent with recurrent CNV (Fig. 7). In laser photocoagulation treatment performed in a subgroup of 29 patients, 62% achieved anatomic resolution and stabilization of the exudative process over time. Visual acuity improved in 66%, 45% of whom achieved a visual acuity of 20/100 or better.
In essence, the technique of ICG-guided laser treatment is a repetition of traditional principles employed with fluorescein angiographic imaging. The technique involves identification of a staining subretinal lesion imaged with ICG angiography, photocoagulation under ICG guidance, obliteration of the lesion, and resolution of the secondary serosanguineous complications. Obviously, stabilization or improvement of vision is a desired outcome.
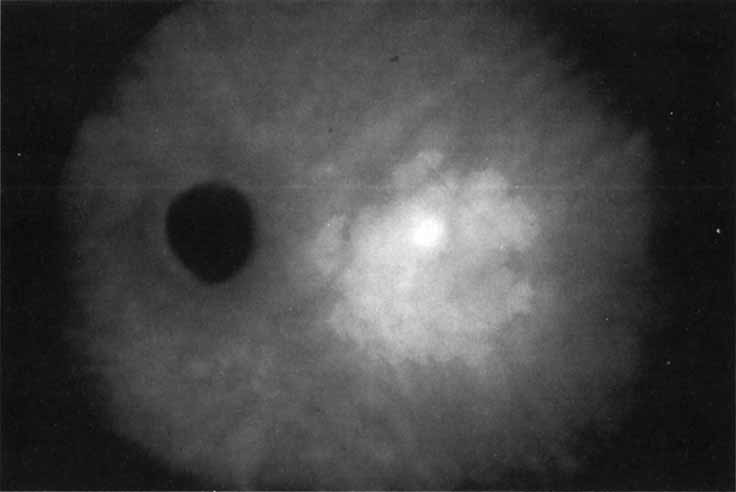
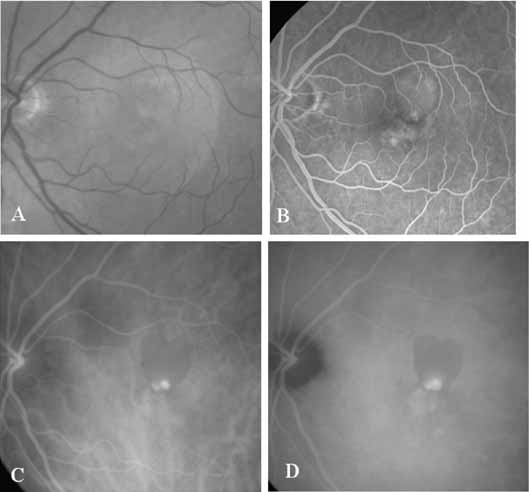
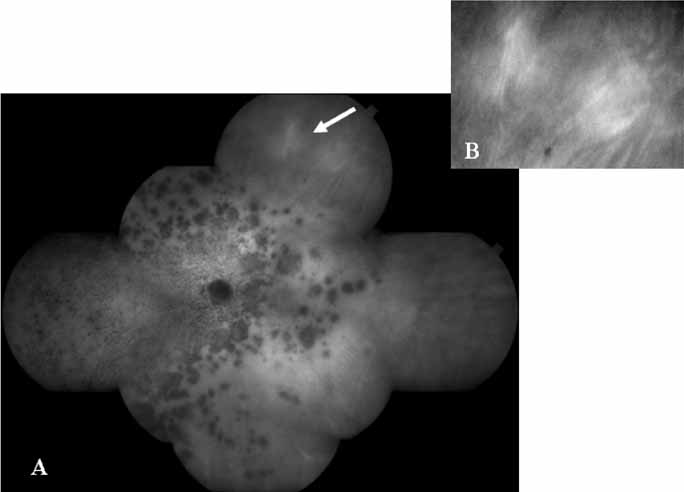
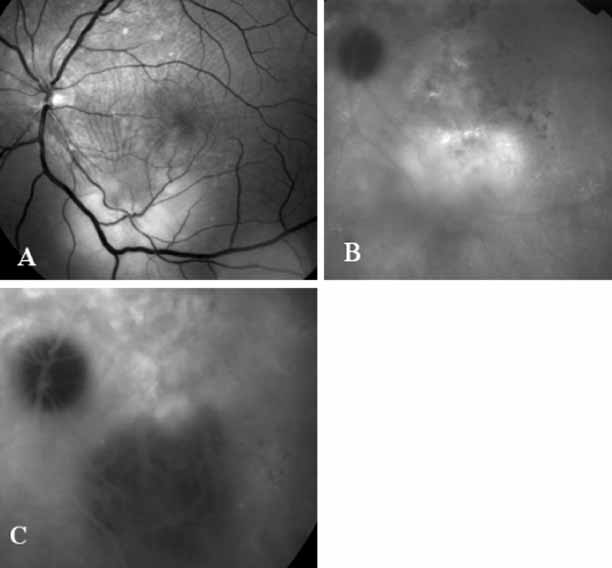
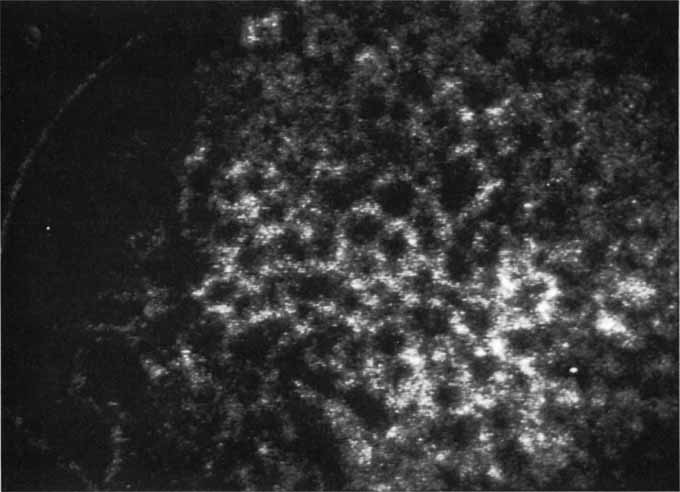
Unlike fluorescein angiography, however, ICG angiographic studies have pointed out that certain principles of laser photocoagulation treatment may not hold with this new imaging capability. This is particularly true with the concept that all areas of CNV need to be obliterated to achieve a successful anatomic result. A careful evaluation of ICG angiograms in some patients with occult CNV has revealed that two forms of neovascular lesions may exist: (1) localized, intensely hyperfluorescent leaking areas of “active” CNV; and (2) more subtle and larger areas of hyperfluorescence with minimal leakage, representing “quiescent” portions of the neovascular complex (Fig. 8). A pilot study by Guyer and colleagues22 demonstrated that localized photocoagulation treatment applied to the active area of CNV alone might result in successful and long-term anatomic stabilization and improvement in the visual acuity in some patients (Fig. 9). A subsequent review of these lesions has demonstrated that they may represent a subpopulation of occult CNV known as polypoidal choroidal neovascularization, described later.
Flower revealed that feeder vessels (FVs) identified by dynamic ICG angiography, but not by FA, may be amenable to treatment with argon green laser.32 ICG angiography is then performed at regular intervals after treatment to determine the treatment response. A second laser treatment is administered if ICG angiography reveals a patent FV. A 40% success rate was found, initially, with a more recent report of 75% success.33 It appears that dynamic ICG angiography, especially high-speed ICG, detects smaller FVs and can therefore be used in treatment of these vessels. (Fig. 10)
|
More recently, ICG angiography has begun to play an important role in the management of patients with occult CNV. In particular, the identification and management of two subsets of occult CNV, namely retinal angiomatous proliferation (RAP)34 and polypoidal choroidal neovascularization (PCV),35 are greatly enhanced with ICG angiography. RAP is a distinct subgroup of neovascular AMD in which angiomatous proliferation within the retina is the first manifestation of the process of neovascularization. As the neovascularization proliferates into the outer retina and subretinal space, the angiomatous proliferation is then surrounded by dilated retinal vessels, hemorrhages (preretinal, intraretinal, and subretinal), and exudates. One or more of the related, compensatory retinal vessels perfuse and drain the neovascularization, occasionally forming a retinal–retinal anastomosis (RRA). In these patients, the same indistinct staining seen in occult CNV is present on a fluorescein angiogram. Therefore, most cases of RAP require the use of ICG angiography to make the diagnosis (Fig. 11).34
ICG angiograms of RAP lesions reveal a focal area of intense hyperfluorescence corresponding to a so-called hot-spot of neovascularization with some late extension of the leakage within the retina caused by intraretinal neovascularization (IRN) within the deep layers of the retina. As the IRN progresses down into the subretinal space, the neovascularization present in the choroid joins the IRN to form a large, neovascular complex. At this stage, clinical and angiographic evidence of a vascularized PED (V-PED) is often present. ICG is the preferred method of imaging a V-PED because the serous component of the PED remains hypofluorescent while the vascular component displays hyperfluorescence. ICG angiography may be able to capture direct communication between the retinal and choroidal component of the neovascular complex as they meet to form a retinal choroidal anastomosis (RCA).34 Treatment options of RAP lesions include: traditional laser photocoagulation of the stage 1 and early stage 2 lesions, surgical excision of stage 2 lesions in conjunction with laser diathermy, as well as PDT, alone and in combination with other treatments, particularly triamcinolone acetonide.36,37 Other therapies, both singly and in combination, are currently undergoing investigation for this unique form of neovascular AMD.
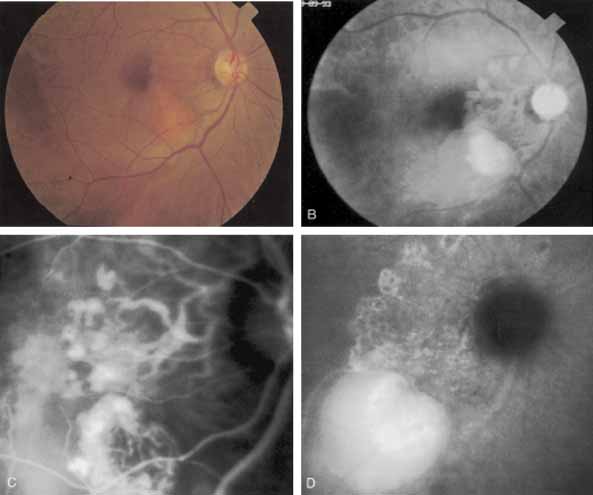
Although initially reported exclusively in middle-aged, black females, PCV has since been recognized as a variant of CNV that can be found in all patients with AMD. This entity is characterized by the presence of an inner choroidal vascular network ending in an aneurysmal bulge clinically seen as a red–orange, spheroid, polyp-like structure. Leakage and bleeding from the choroidal vascular abnormalities result in multiple, recurrent, serosanguinous RPE detachments.25,39,40,41 ICG can be used to identify and characterize the vascular abnormality with high sensitivity and specificity.19–21,40–59 Early-phase images of the lesions show a distinct network of vessels within the choroid. Patients with juxtapapillary involvement show a radial, arching pattern with an inner network of vascular channels extending and connecting with smaller, spanning branches that are more numerous and increasingly prominent at the edge of the PCV lesion.
Larger choroidal vessels in the PCV lesion begin to fill before retinal vessels but appear to fill at a slower rate than the retinal vessels. The lesion initially appears noticeably hypofluorescent relative to the surrounding, uninvolved choroid, but soon after the network is first visible with ICG angiography, small, hyperfluorescent “polyps” can be seen within the choroid. These polypoidal structures correspond to the red–orange choroidal excrescence seen clinically. They leak slowly into the surrounding hypofluorescent area, creating increasing hyperfluorescence. In the late phases of the ICG angiogram, a uniform washout pattern is seen as the dye disappears from the polypoidal vascular structure; the late-staining characteristic of occult CNV is not seen in PCV. PCV may be localized to the macular area without any peripapillary involvement. It may present as a network of small branching vessels ending in polypoidal dilation best-imaged with ICG angiography. (Fig. 12)
ICG angiography has clearly provided new and important diagnostic information, identifying well-defined, potentially laser-treatable lesions in approximately 30% of occult cases.21,30 In addition, the successful elimination of the exudation in patients with occult CNV by obliteration of the hyperfluorescent lesion with laser photocoagulation treatment suggests that ICG-guided laser treatment of occult CNV may be a useful technique in the management of some of these difficult cases (Fig. 13).
Perhaps most importantly, ICG angiography has provided a better overall understanding of the complexity of patients with occult CNV. Certainly this diagnosis may be an oversimplification of the nature of the condition. The presence or absence of a serous PED accompanying this disorder may have a bearing not only on the treatment course but also on the natural history of the disease. In addition, not all areas of CNV may be equivalent: areas that are more actively proliferating may represent a larger threat to visual acuity than the more quiescent plaques of CNV. These larger areas of quiescent vessels may, in fact, be compatible with long-term good visual acuity and may not require therapy. Undoubtedly, much more remains to be learned regarding the nature of these complex lesions, but ICG angiography has clearly been demonstrated to play an important role in the evaluation and management of these patients.