THE CILIARY BODY
The ciliary body performs three principal functions: (1) It produces and secretes aqueous humor into the posterior chamber of the eye; (2) it contains the smooth muscle that acts on the crystalline lens, via the zonular fibers, to shift the focus of the eye from far to near (accommodation); and (3) by extension of certain smooth muscle fibers and their tendons into the adjacent trabecular meshwork, it can assist in the drainage of aqueous humor from the eye. There is also some evidence supporting a role for the ciliary body in the production and slow turnover of certain molecular components of the vitreous humor.3
The ciliary body extends posteriorly from the root of the iris to the ora serrata. In the adult eye, the distance from the surgical limbus to the ora serrata is approximately 7 mm on the temporal side of the globe and 6 mm on the nasal side. The ciliary body is grossly subdivided into two portions, the pars plicata and the pars plana.
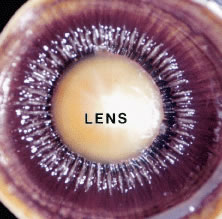
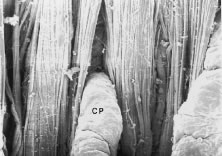
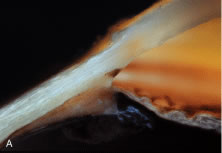
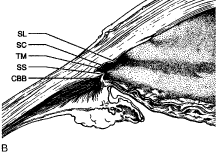
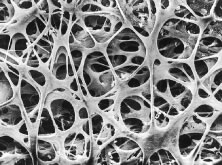
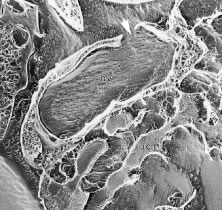
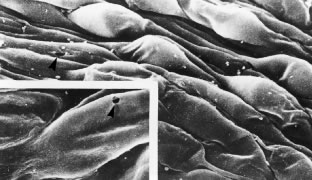
The pars plicata exhibits 70 to 80 corrugated, fin-like ridges on its inner surface that are arranged around the circumference of the crystalline lens (Fig. 1). Each individual ridge is referred to as a ciliary process, and these are divided into categories of major and minor processes based on their relative height. Major processes predominate, and they extend into the posterior chamber of the eye approximately 1 mm. Minor processes are about one third as high. In the scanning electron micrograph shown in Figure 2 these structures and their relationships are clearly discernible. It is important to appreciate, however, that the zonular fibers supporting the crystalline lens have been removed from the surface of this specimen. These fibers form a continuous carpet over the inner surface of the pars plana and are then channeled into the valleys between adjacent processes in the pars plicata on their way to the lens capsule. As such, in vivo, the valleys between adjacent ciliary processes are largely filled with zonules leaving only the crest of each process exposed to the posterior chamber (Fig. 3).
The pars plana, as its name implies, exhibits a flat inner surface. This portion of the ciliary body is much thinner and less vascularized than the pars plicata, making it the safest point of entry into the eye for vitreoretinal surgical procedures.
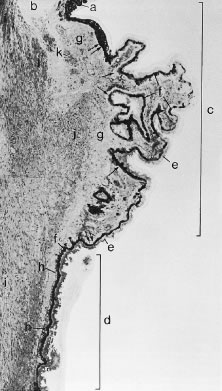
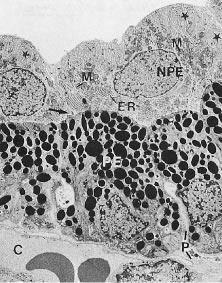
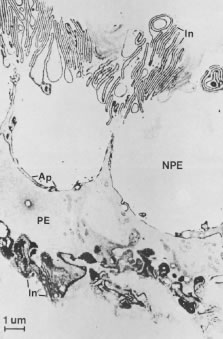
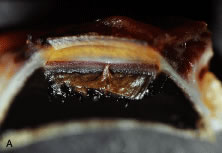
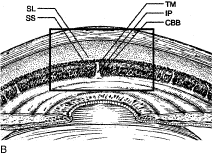
In meridional sections, the ciliary body appears as shown in Figure 4. The entire surface of the ciliary body, facing the inside of the eye, is lined by a bilayered epithelium. These two layers are named for their relative content of melanin pigment. The layer closest to the posterior chamber is devoid of pigment and is called the nonpigmented ciliary epithelium. The layer closest to the ciliary body stroma is called the pigmented ciliary epithelium for its melanin content. The nonpigmented ciliary epithelium is continuous with the posterior pigmented epithelium of the iris anteriorly and with the neurosensory retina posteriorly at the ora serrata. The pigmented ciliary epithelium continues anteriorly as the anterior myoepithelium of the iris (which includes the iris sphincter muscle) and posteriorly as the pigmented epithelium of the retina. The double layer of epithelium covering the ciliary body actually represents two simple epithelia that are joined at their apical surfaces following the invagination of the optic cup during embryogenesis. As a result, the basement membrane of the nonpigmented ciliary epithelium faces the posterior chamber and that of the pigmented ciliary epithelium joins both epithelial layers to the ciliary body stroma.
The ciliary body stroma is composed of a loose connective matrix that supports the nerves and blood vessels that course within it. This connective tissue matrix extends into the core of each ciliary process. The ciliary body stroma is directly continuous with the stroma of the iris anteriorly and with the choroidal stroma posteriorly. Because the anterior surface of the iris has no epithelial covering, substances released into the ciliary body stroma have access to the anterior chamber by diffusing from the ciliary body stroma to the iris surface.4,5 Similarly, fluid entering the ciliary body stroma from the anterior chamber can reach the choroid by traveling along the uveoscleral outflow pathway described later. Between the loose connective tissue stroma and the inner surface of the sclera is a complex smooth muscle called the ciliary muscle, also described later.
PRODUCTION OF AQUEOUS HUMOR
To provide anatomic correlations for the physiology of aqueous humor formation, it is most convenient to describe the process in two steps: (1) elaboration of a plasma filtrate from which aqueous humor is derived, and (2) formation of aqueous humor from this filtrate. Although these steps are not independent, the first is related primarily to the ciliary body microvasculature and the second to the ciliary epithelium.
Ciliary Body Microvasculature
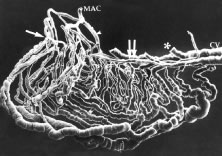
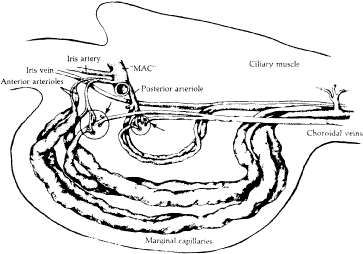
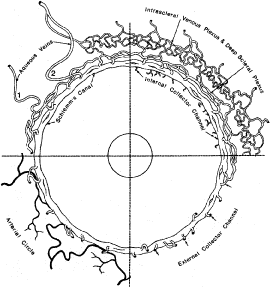
When viewed in cross section, each ciliary process contains a fibrovascular core that is continuous with the ciliary body stroma and covered by the bilayered ciliary epithelium (Fig. 5). The arterioles that serve the ciliary body stroma, like those serving the iris, arise from the discontinuous major circle of the iris.6 Each major process is served by a set of anterior and posterior arterioles7 (Figs. 6 and 7). The anterior arterioles supply the large diameter capillaries, near the crests of the processes, whereas the posterior arterioles supply the smaller caliber capillaries deep within each process. The direction of blood flow in these systems is from anterior to posterior, toward a network of choroidal veins. The blood from the entire ciliary body ultimately leaves the eye via the vortex veins.
|
Blood flow in the ciliary body is most likely regionalized and under autonomic control. Scanning electron microscopic studies have revealed the presence of localized constrictions in casts of afferent arterioles in the ciliary body microvasculature that may reflect the presence of a sphincter-like system for controlling blood flow.7–10 Through these mechanisms, alterations in blood flow can influence aqueous humor production by increasing or decreasing the amount of filtrate made available to the ciliary epithelium.
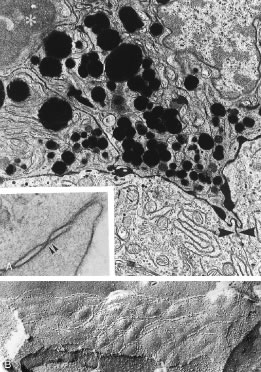
The capillaries derived from the posterior arterioles that serve the ciliary muscle are nonfenestrated and do not leak plasma proteins under normal conditions (Fig. 8). By contrast, the capillaries derived from the anterior arteriole, which pass within the stromal core of each ciliary process, lack tight junctions and are lined by fenestrated endothelial cells (Fig. 9). Using tracers for plasma protein leakage such as horseradish peroxidase (HRP), the capillaries of the ciliary body stroma are seen to be very permeable to macromolecules, as well as to ions and fluid (Figs. 8 and 10 ). As such, these vessels are limited in their capacity to serve as a selective permeability barrier. That function is one of several reserved for the ciliary epithelium.
The Ciliary Epithelium
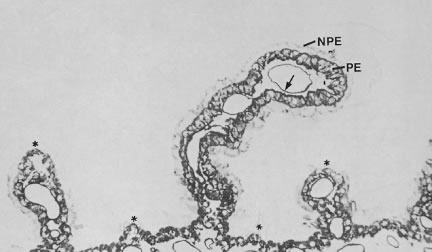
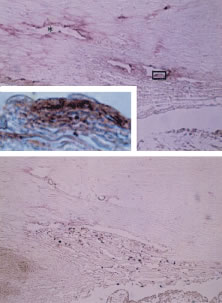
As noted earlier, the two cell layers that constitute the ciliary epithelium are named for their relative content of melanin pigment. The layer closest to the ciliary body stroma is the pigmented ciliary epithelium and that closest to the posterior chamber of the eye is the nonpigmented ciliary epithelium (Fig. 11). The morphology of the ciliary epithelium varies along the surface of the ciliary body, in accord with the different demands placed on it in various locations.1 From this analysis, it appears that it is primarily the epithelia at the tips of the ciliary processes that are involved in the production of aqueous humor. In these areas, immunoelectron microscopic studies have clearly documented both Na-K-ATPase activity (Fig. 12) and carbonic anhydrase activity11,12 (Fig. 13). Both are known to be central to the production of aqueous humor and one of them, carbonic anhydrase, is regularly targeted for pharmacologic inhibition to reduce intraocular pressure in glaucoma.13
THE BLOOD-AQUEOUS BARRIER IN THE CILIARY BODY
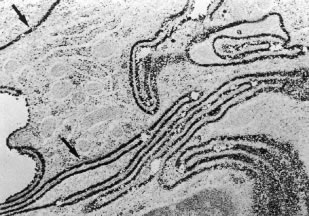
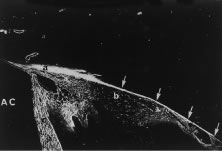
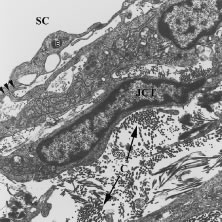
Aqueous humor is a clear nutrient fluid, derived from plasma, that provides for the metabolic needs of the avascular tissues of the eye. Although free amino acid levels in aqueous humor are nearly equivalent to those found in plasma,14 the protein content of aqueous humor in the anterior chamber is less than 1% of that found in plasma.15 Proteins create turbidity and turbidity scatters light, which degrades the optical efficiency of the eye. To prevent this and to ensure that potential antigens in the bloodstream are prevented from reaching the anterior and posterior chambers of the eye and the lens, vitreous, and retina, a selective barrier must be positioned between the bloodstream and the aqueous humor. As noted earlier, the microvasculature of the ciliary body stroma is composed of fenestrated capillaries that readily leak fluids, ions, and plasma proteins to provide the reservoir from which the ciliary epithelium secretes aqueous humor. To ensure that the protein-laden fluid in the ciliary body stroma and the composition of the aqueous humor remain different, a barrier to macromolecular diffusion is interposed between the ciliary body stroma and the posterior chamber by the ciliary epithelium.16 As noted earlier (see Figs. 8 to 10), following injection of HRP in monkeys, this protein readily leaves the lumen of the fenestrated capillaries of the ciliary body stroma.17 The protein diffuses to the ciliary epithelium and easily moves between adjacent pigmented ciliary epithelial cells, filling the intercellular cleft18 (Fig. 14A). On reaching the interface between the juxtaposed apical surfaces of the pigmented and nonpigmented layers, the HRP is again able to freely permeate the intercellular cleft between these cell layers. But when the HRP attempts to diffuse along the intercellular cleft between adjacent nonpigmented ciliary epithelial cells, its progress toward the aqueous humor in the posterior chamber is blocked by an apicolateral junctional complex composed of a zonula occludens (tight junction), a zonula adherens, and desmosome19,20 (see Fig. 14A). In sections, the tight junction is seen as a series of apparent fusion points between the membranes of adjacent nonpigmented ciliary epithelial cells (see Fig. 14,Inset). Using the technique of freeze-fracture, however, allows an en face view of the tight junctions, revealing that these fusion points are actually cross sections of a complex system of branching and anastomosing protein strands that surround the entire circumference of each cell.19 This web of strands spans the intercellular cleft and is mirrored on the adjacent cell, which blocks the intercellular cleft around the entire circumference of each cell, to limit diffusion of macromolecules (see Fig. 14B). Disruption of this junctional complex has been shown to occur in experimental anterior uveitis, resulting in increased amounts of protein in the aqueous humor.21 This extra protein scatters the light of the slit lamp and is described clinically as flare.
Although not involved directly in the barrier properties of the ciliary epithelium, another intercellular junction, the gap junction, has been shown to play an important role in aqueous production. Gap junctions are known to provide a means for metabolic and electrotonic coupling of cells. In the ciliary epithelium, cells of both layers are interconnected by these junctions and they provide a low resistance pathway for the passage of current and ions between cells, allowing them to function as a syncytium in the coordinated secretion of aqueous humor.22,23 When the ciliary body is inflamed, it has been shown that gap junctions largely disappear, contributing to a reduction in aqueous secretion and to the often lower intraocular pressure that accompanies inflammation of the anterior uvea.21 Attempts to exploit gap junction uncoupling as a means of reducing aqueous production in glaucoma have yet to be done.
THE CILIARY MUSCLE
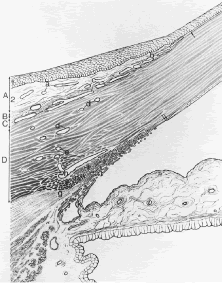
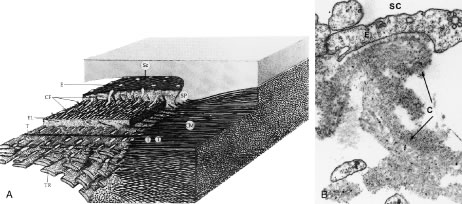
Most of the added thickness of the pars plicata region of the ciliary body results from its layers of smooth muscle. The ciliary muscle is innervated by parasympathetic fibers that originate in the Edinger-Westphal nucleus, leaving the midbrain with cranial nerve (CN) III to be conveyed from its nerve branch to the inferior oblique and then to the ciliary ganglion. Here these fibers synapse, and postganglionic fibers are conveyed to the eye and, therefore, to the ciliary body. Classically three layers of smooth muscle are described as shown in Figure 15. The smooth muscle cells that constitute the outermost of these three layers run in an anterior-posterior direction, and, thus, in meridional sections of the eye, these fibers are cut in longitudinal section. As such, this layer is called the longitudinal bundle of the ciliary muscle (Brucke's muscle). These fibers originate in stellate prongs, called epichordal stars that anchor into the inner surface of the sclera in the posterior portion of the pars plana (see Fig. 15). From this origin, the longitudinal bundle extends anteriorly to insert on the posterior surface of the scleral spur, a projection of scleral tissue that extends inward from the scleral surface like a shelf at the posterior limit of the trabecular meshwork. As described later, contraction of this portion of the ciliary muscle appears to pull on the scleral spur. Doing so results in improved outflow of aqueous humor through the trabecular meshwork. This is the presumed beneficial effect underlying the use of miotics in the treatment of glaucoma.
The middle layer of smooth muscle is called the radial bundle, and the innermost layer represents a confined band of smooth muscle cells that encircle the ciliary body. In meridional sections of the eye, these fibers are seen in cross section and are called the circular bundle (Muller's muscle, no relationship to the smooth muscle of the same name found within the eyelid). The circular bundle appears to play less of a role in facilitating aqueous outflow than in the process of accommodation.
These classical separations of anatomic and physiologic function are exaggerations of the way in which this complex muscle actually works. Intricate structural analyses of the various portions of this muscle have revealed that they are not separate and distinct.1
THE INTERSTITIUM OF THE CILIARY MUSCLE AND UVEOSCLERAL OUTFLOW
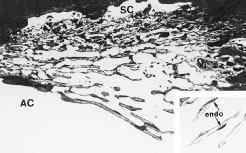
Only a few years ago a discussion of the ciliary body would not have made specific mention of the connective tissue interstices of the ciliary muscle. However, recent evidence suggests that the interstices of this muscle represent a part of an increasing important alternate pathway for the drainage of aqueous humor called the uveoscleral pathway. Unlike the pathway for aqueous outflow through the trabecular meshwork, discussed later, flow through the uveoscleral pathway is thought generally to be pressure independent.24,25 This pathway conveys aqueous humor from the anterior chamber into the portion of the ciliary muscle that is directly accessible from the anterior chamber angle. The fluid appears to then flow within the connective tissue fascicles that interweave with the smooth muscle fibers of the ciliary muscle. Ultimately, the fluid reaches the inner surface of the sclera. Although not an obvious feature, the ciliary body is separated from the sclera (over most of its length) by a potential space, the supraciliary space, which is continuous posteriorly with the suprachoroid. Having only minimal physical connections to the sclera, except at its anterior and posterior margins, the ciliary body is held against the outer wall of the eye primarily by the intraocular pressure. Aqueous moves posteriorly within the potential space of the suprachoroid as shown in Figure 16, leaving the eye by either diffusing through the sclera (uveoscleral) or by diffusing through the scleral emissaria for the vortex veins (uveovortex) flow.
Recently, certain prostanoid derivatives have been shown to be able to augment uveoscleral outflow, and they are proving to be a valuable addition to the glaucoma armamentarium.26,27 Several morphologic studies have suggested that the mechanism of action of these drugs may be through a partial dissolution of the ground substance in the interstices of the ciliary muscle that facilitates movement of fluid along this pathway.28