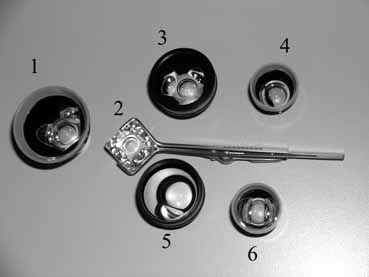
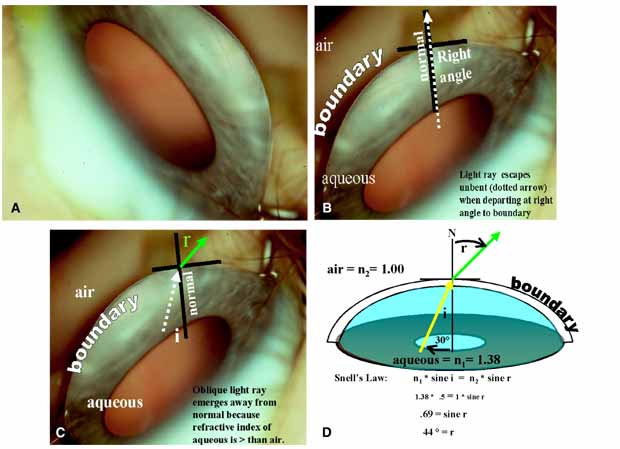
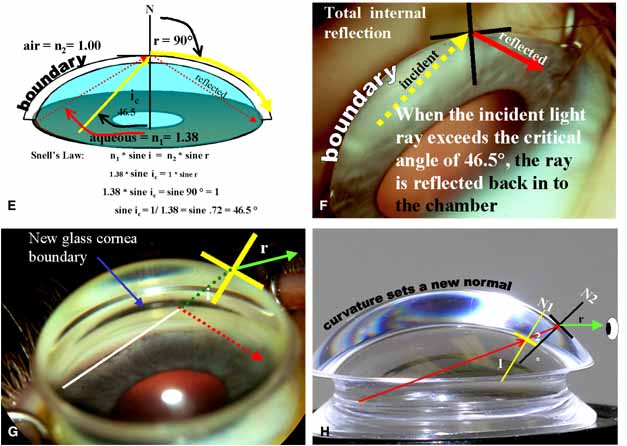
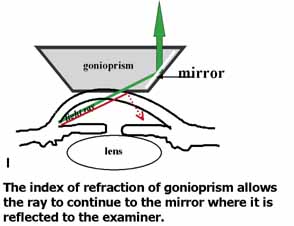
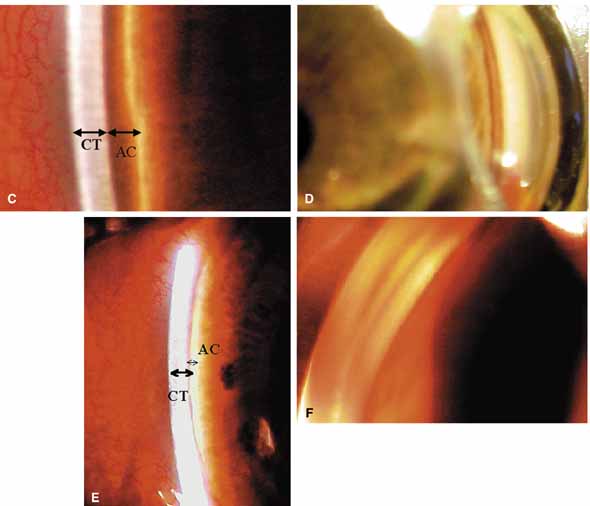
TABLE 1. Clinical Methods of Viewing the Anterior Chamber Angle
| Method of View | Sizes | Advantage | Disadvantage |
| I. Refractive | |||
| CONTACT LENS | |||
| Direct view | |||
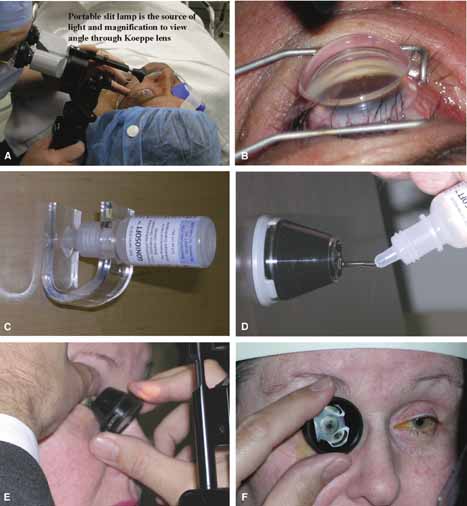
Koeppe |
Small medium and large | Convenient for EUA, no angle distortion, angle magnification, able to view fundus, angle photography excellent, requires hand held biomicroscope. | Patient must be in supine position, laborious examination, time consuming, patient dislikes, lid speculum required, examiner must change position. |
Barkan/Hoskins-Barkan |
Pediatric and adult | Surgical goniolens with blunted side allows access for goniotomy. | Same as Koeppe, angle photography more difficult than Koeppe. |
| II. Reflective | |||
| CONTACT LENS | |||
| Indirect view | |||
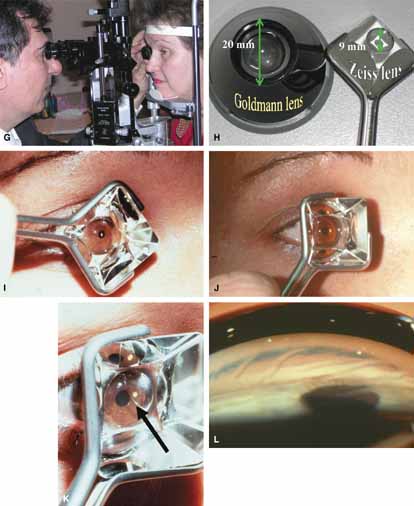
Goldmann 3-mirror |
Small/medium/large No handle to hold prism |
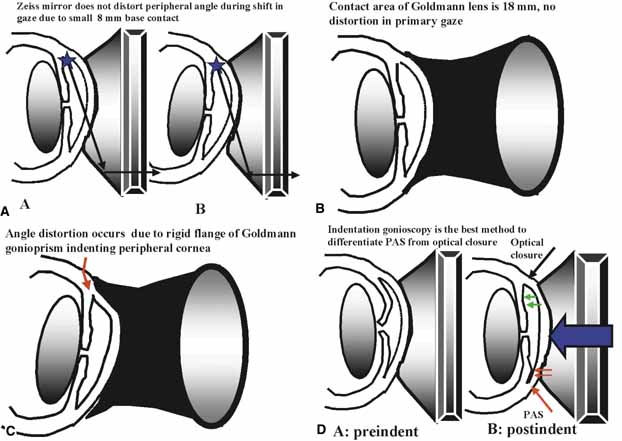
Excellent gonioprism for neophyte to learn angle anatomy, coupling gel necessary, viscous bridge creates suction effect stabilizing eye for examination and laser therapy. | Goniogel required which obscures patients vision and may compromise further same-day diagnostic tests, corneal abrasion in compromised cornea, part of angle hidden in narrow angled eyes, easy to distort peripheral angle. |
4-mirror lenses: Zeiss, Gaasterland, Posner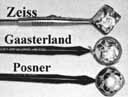 |
One size but variable handles to grasp the gonioprism
(round, flat) |
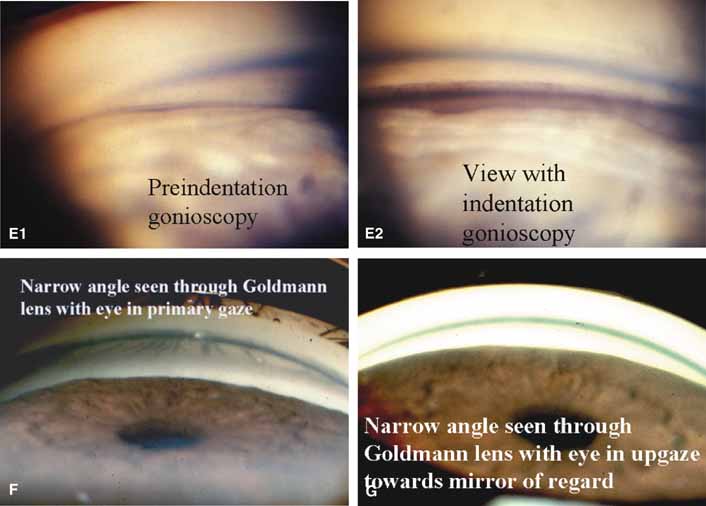
Rapid evaluation without goniogel, same day diagnostic tests not compromised, indentation or compression gonioscopy allows expert evaluation of narrow angled eyes with hidden anatomy, patient friendly, slit-lamp friendly with minimal movement to see 360 degrees. | Must first master Goldmann gonioprism, more hand–eye coordination necessary than for Goldmann gonioprism, easy to apply excessive force causing corneal folds with poor view of angle. |
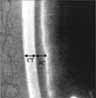
| III. Ultrasound Biomicroscopy (UBM) |  |
Imaging of anterior segment with UBM correlating ACD, iris position, lens, ciliary body and posterior chamber anatomy. | Expensive equipment, not clinically available for most physicians |
| IV. Scheimpflug photography | 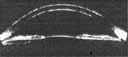 |
High resolution photography demonstrates position of lens and iris | Expensive and clinically difficult |
| V. Ophthalmic Endoscopy | Endoscope allows direct intraoperative visualization of chamber angle, useful with cloudy cornea and to find cyclodialysis clefts. | Must be performed in operating room under sterile conditions. |
EUA, examination under anesthesia; UBM, ultrasound biomicroscopy; ACD, anterior chamber depth.
These new clinical modalities inspire a modern-day definition of gonioscopy, namely: the evaluation and management of the eye based on the visualization of the anterior chamber angle constitute the field of gonioscopy. Gonioscopy currently consists of direct and indirect gonioscopic techniques performed with variable-sized gonioprisms, goniolenses, and viewing devices. Ophthalmic endoscopy is a relatively new technique that allows the surgeon to view the chamber angle directly during surgery. Imaging of the chamber angle with UBM and photography adds valuable angle information that is correlated with gonioscopy. Ophthalmologists who take advantage of these techniques and devices provide superior care through a rapid assessment of the angle situation. The focus of this chapter is classic gonioscopy, the benefits of which are currently the greatest for practitioners on a day-to-day clinical basis.
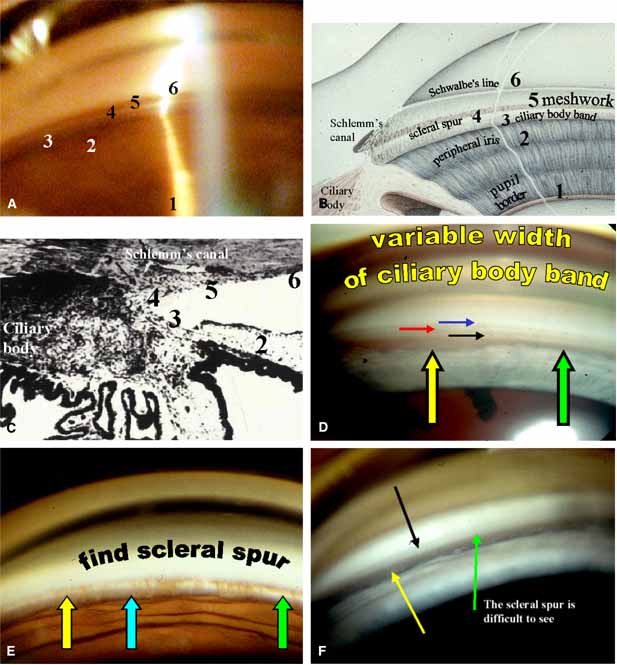
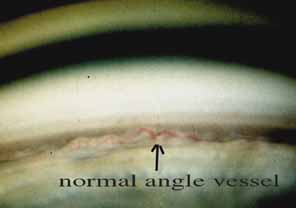
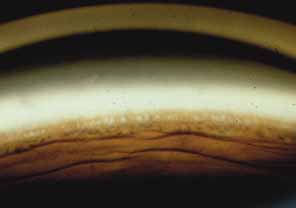
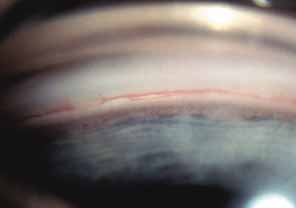
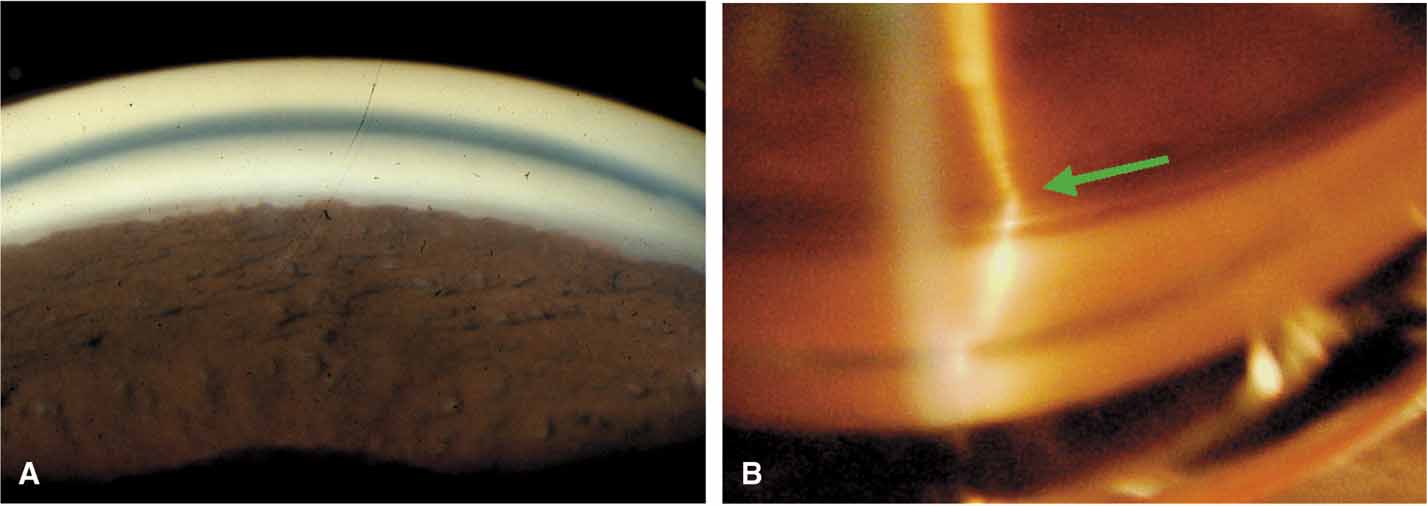
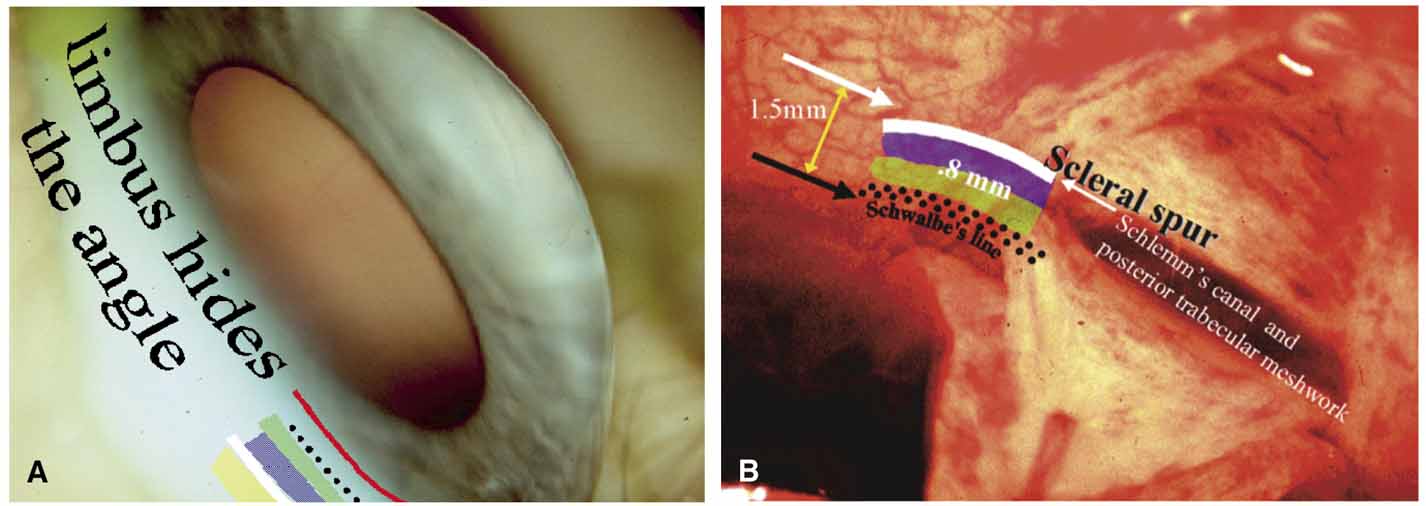
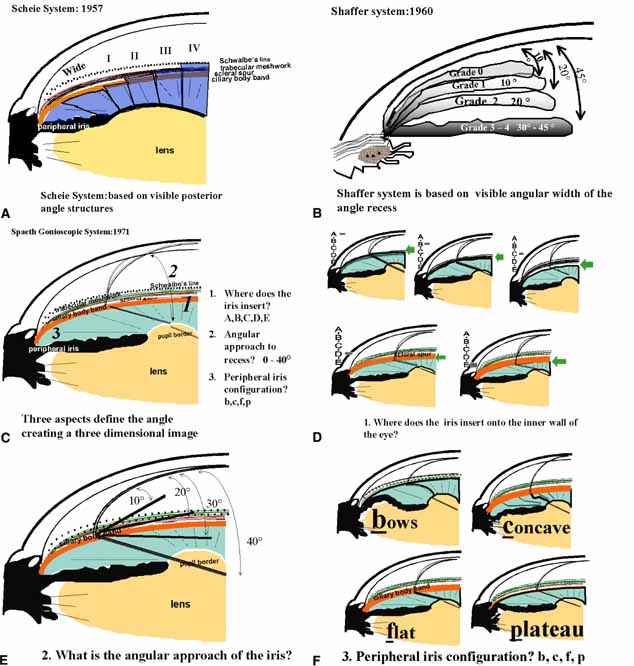
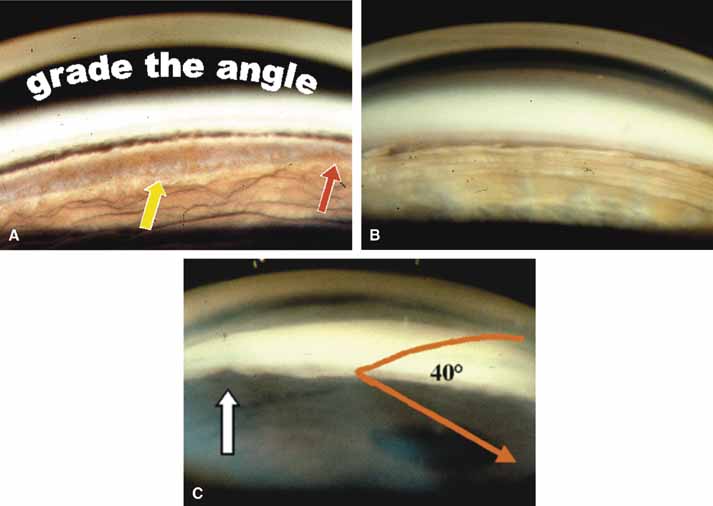
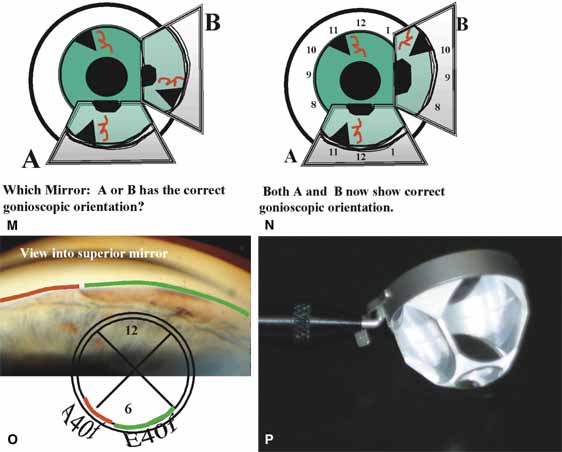
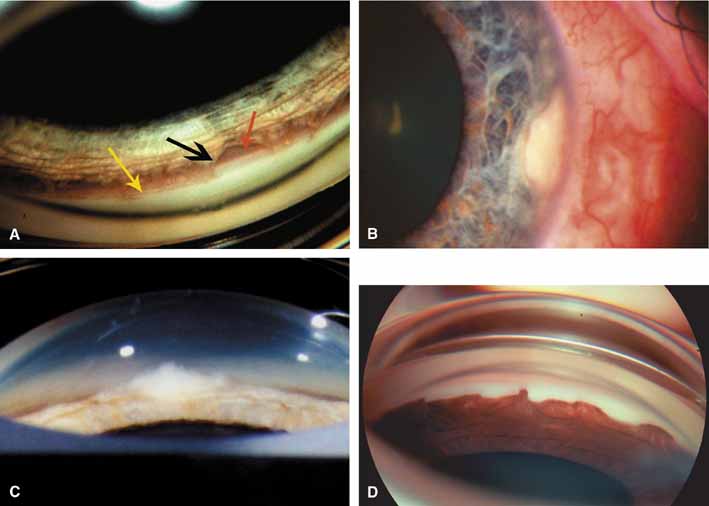
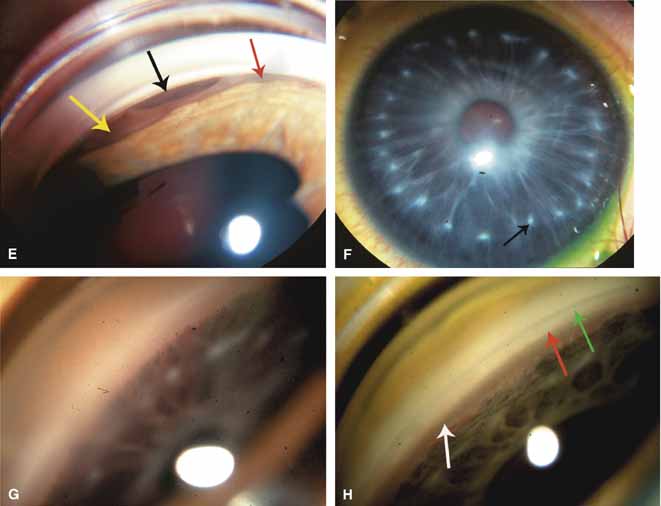
The ultimate goal of gonioscopy is to preserve or improve vision through the systematic evaluation and management of the anterior chamber angle. This requires the skill necessary to use a variety of instruments in order to accomplish a specific gonioscopic task (Fig. 1). In addition, the accurate recording and classification of visualized structures is imperative to document angle structures and note their changes over the lifetime of the patient. Physicians who integrate gonioscopy into their practice are able to examine, evaluate, document, and appreciate the appearance of the normal angle and its immense variability. A thorough understanding of normal is imperative in order to recognize and treat angle pathology.
|