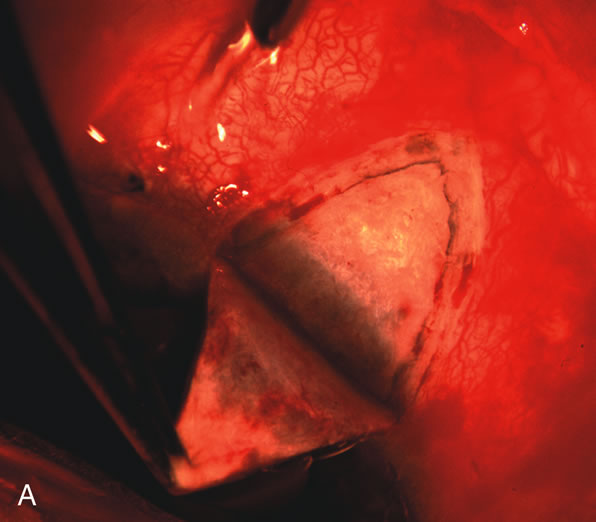
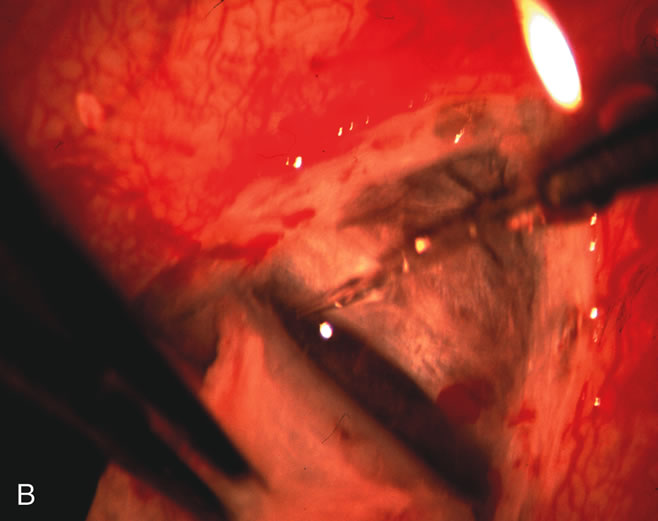
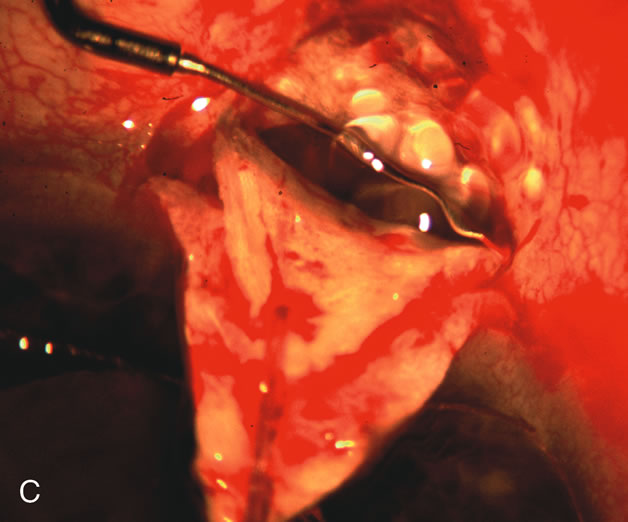
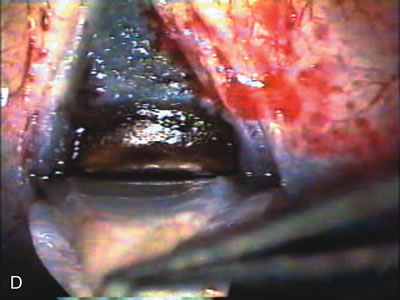
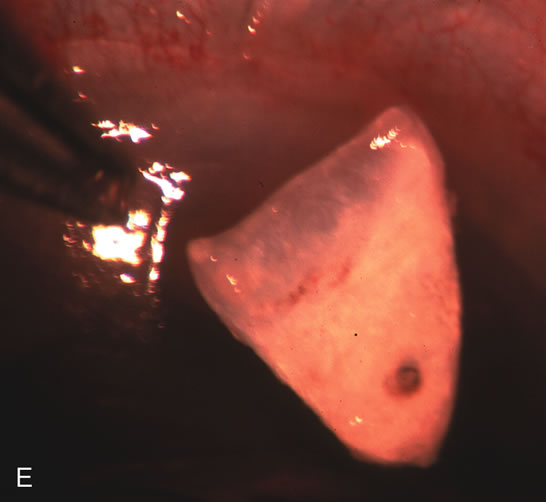
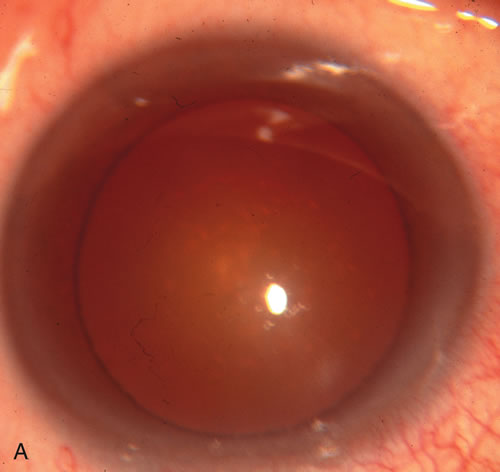
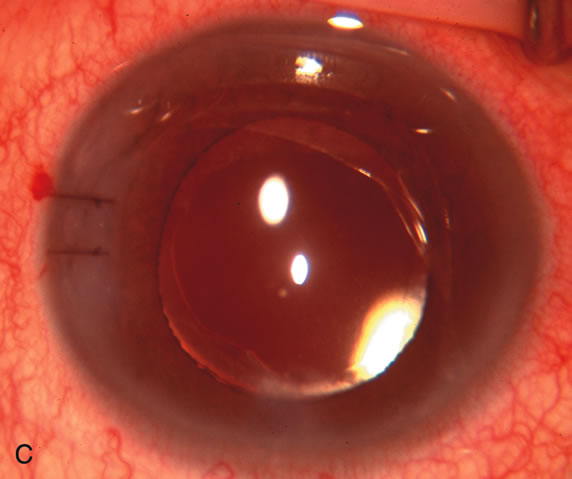
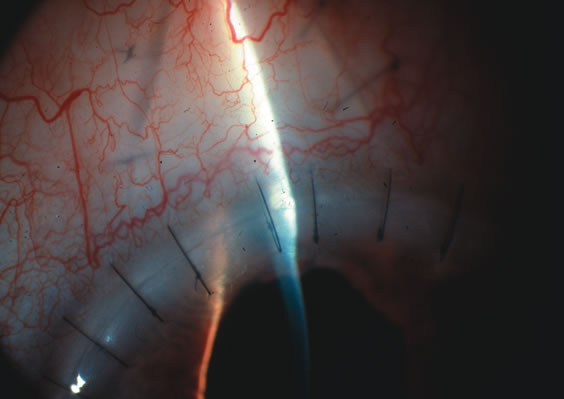
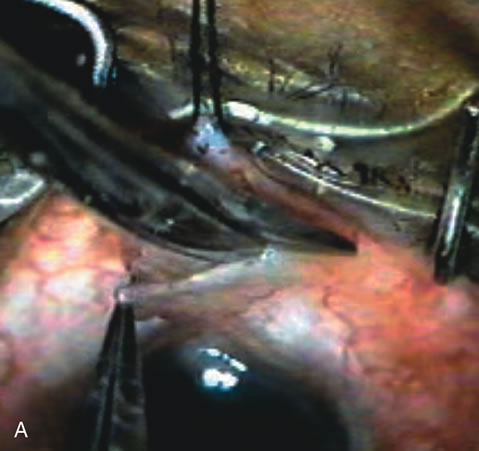
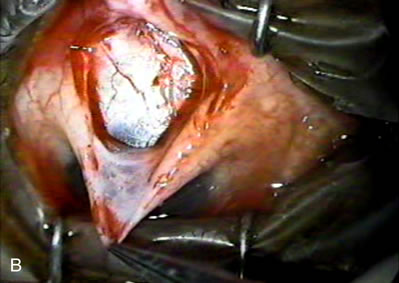
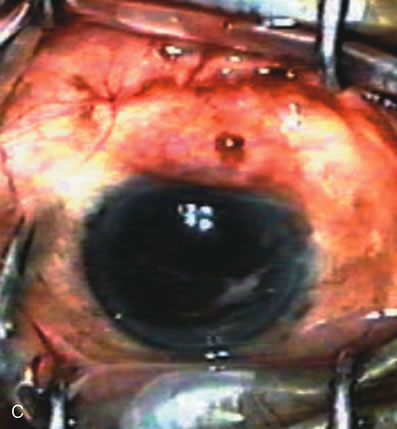
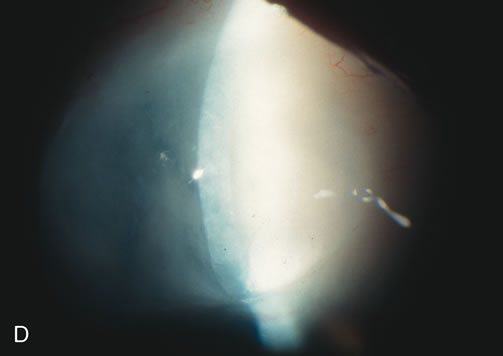
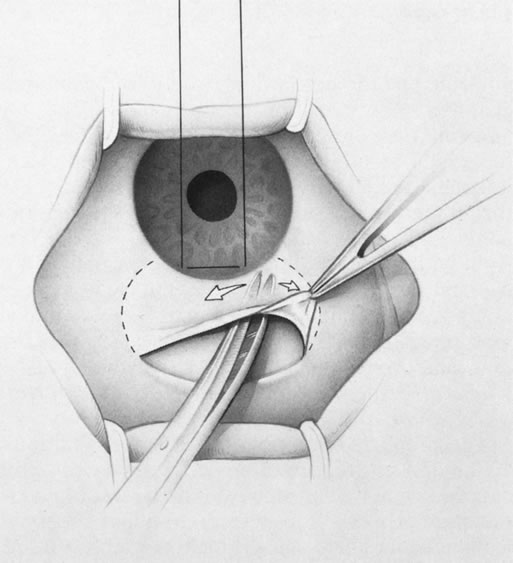
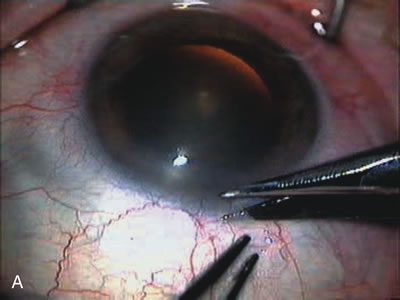
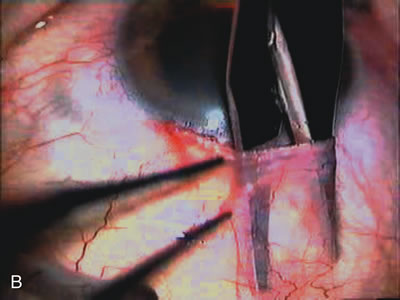
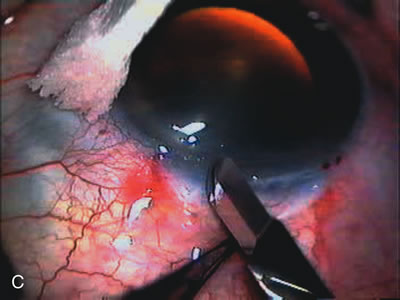
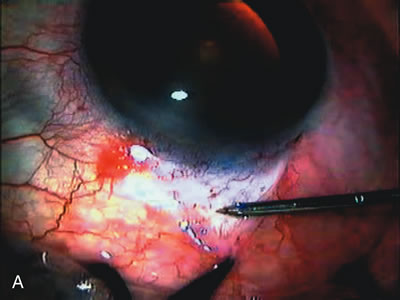
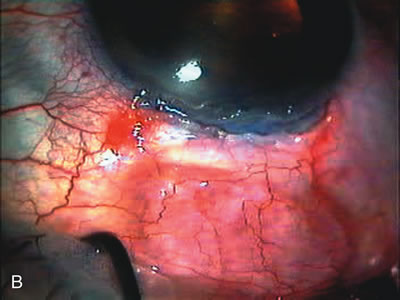
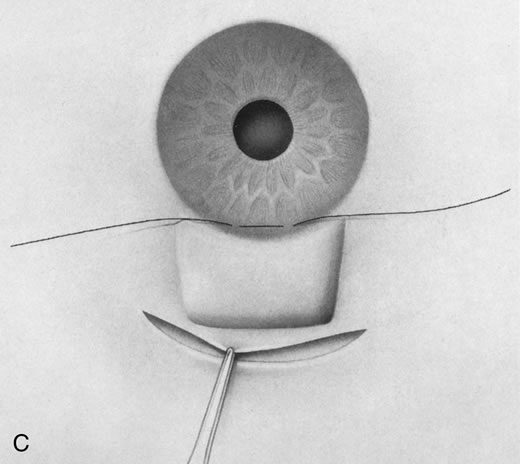
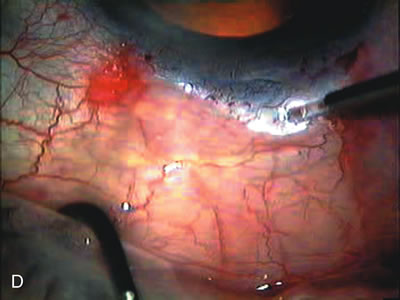
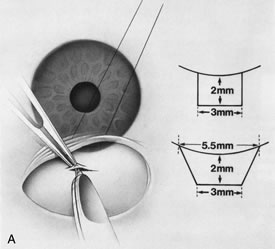
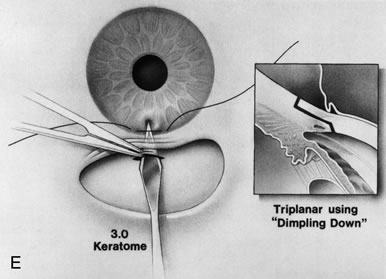
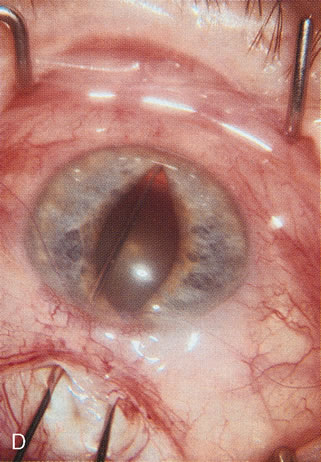
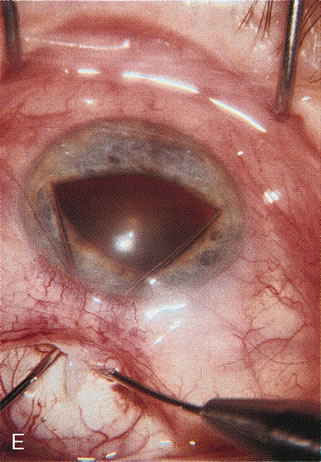
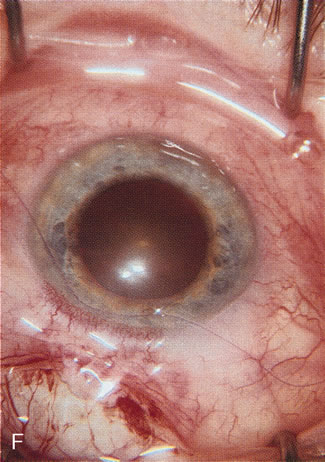
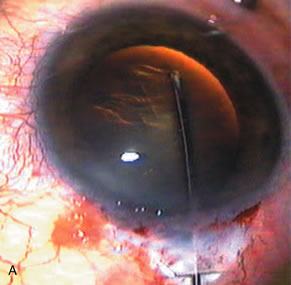
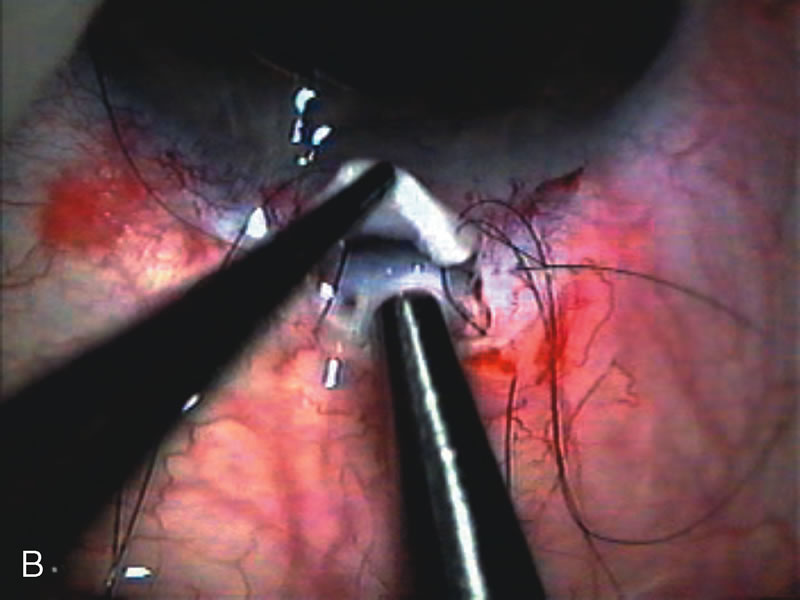
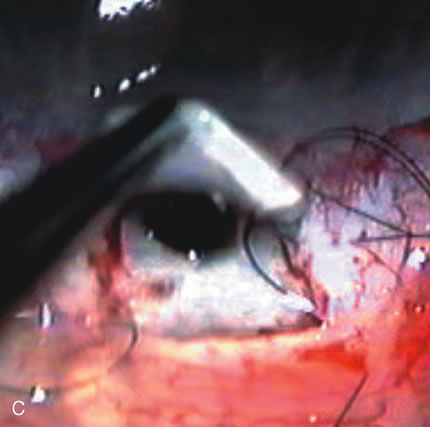
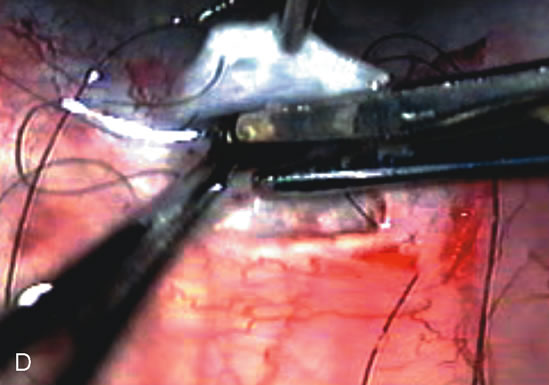
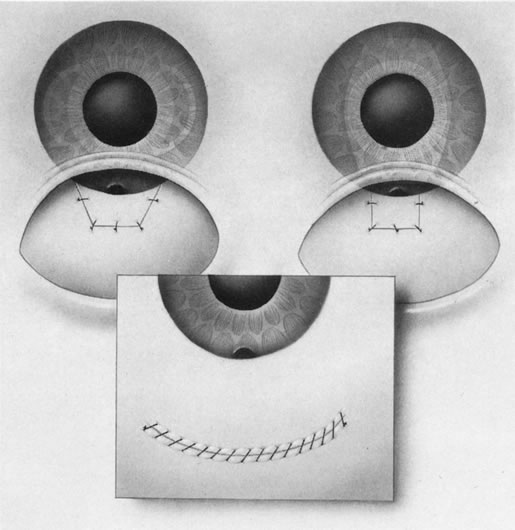
1. Vanbuskirk EM. The technology dilemma. J Glaucoma 5:77, 1996 2. Cunliffe IA, Dapling RB, Longstaff S: A prospective study examining the changes in factors that affect visual
acuity following trabeculectomy. Eye 6:618, 1992 3. Dietze PJ, Oram O, Kohnen T et al: Visual function following trabeculectomy: effect on corneal topography
and contrast sensitivity. J Glaucoma 6:90, 1997 4. Menapace R, Findl O, Georgopoulos M: The capsular tension ring:designs, applications, and techniques. J Cataract Refract Surg, 26:898, 2000 5. Friedman DS, Jampel HD, Lubomski LH et al: Surgical strategies for coexisting glaucoma and cataract. Ophthalmology 109:1902, 2002 6. Jampel HD, Friedman DS, Lubomski LH et al: Effect of technique on intraocular pressure after combined cataract and
glaucoma surgery. Ophthalmology, 109:2215, 2002 7. Chandler PA, Grant WM: General considerations. In Lectures on Glaucoma, pp 13–16. Philadelphia, Lea & Febiger, 1965 8. Drance SM: Doyne Memorial Lecture: Correlation of optic nerve and visual field defects
in simple glaucoma. Trans Ophthalmol Soc UK 95:288, 1975 9. Quigley HA, Anderson DR: The dynamics and location of axonal transport blockage by acute intraocular
pressure elevation in primate optic nerves. Invest Ophthalmol Vis Sci 15:606, 1976 10. Quigley HA, Addick EM, Green WR et al: Optic nerve damage in human glaucoma: II. The site of injury and susceptibility to damage. Arch Ophthalmol 99:635, 1981 11. Quigley HA: The future. In Spaeth GL, Varma R, Parker K (eds): The Optic Nerve in Glaucoma, p 347. Philadelphia, JB Lippincott, 1993 12. Quigley HA, Dunkelberger GR, Sanchez RM: Chronic experimental glaucoma causes selectively greater loss of larger
optic nerve fibers. Invest Ophthalmol Vis Sci 27:42, 1986 13. Spaeth GL: Appearance of the optic disc in glaucoma: A pathogenic classification. In New Orleans Academy of Ophthalmology: A Symposium on Glaucoma, p 114. St. Louis, CV Mosby, 1981 14. Caprioli J: Correlation between optic disc appearance and type of glaucoma. In Spaeth GL, Varma R, Parke K (eds): The Optic Nerve in Glaucoma, p 91. Philadelphia, JB Lippincott, 1993 15. Spaeth GL: Development of glaucomatous changes of the optic nerve. In Spaeth GL, Varma R, Parker K (eds): The Optic Nerve in Glaucoma, pp 63-81. Philadelphia, JB Lippincott, 1993 16. Crouch ER Jr, Frenkel M: Aminocaproic acid in the treatment of traumatic hyphema. Am J Ophthalmol 81:355,1976 17. Goldberg MF: Sickle erythrocytes, hyphema and secondary glaucoma. I. Diagnosis and treatment of sickled erythrocytes in human hyphemas. Trans Am Ophthalmol Soc 76:781, 1978 18. Goldberg MF, Tso MOM: Sickled erythrocytes, hyphema, and secondary glaucoma: VII. The passage of sickled erythrocytes out of the anterior chamber of the
human and monkey eye: Light and electron microscopic studies. Ophthalmic Surg 10:89, 1979 19. Trible JR, Anderson DR: Factors associated with intraocular pressure-induced acute visual
field depression. Arch Ophthalmolo, 115:1523-27, 1997 20. Javitt JC, Spaeth GL, Katz LJ et al: Acquired pits of the optic nerve. Increased prevalence in patients with low-tension glaucoma. Ophthalmology 97(8): 1038, 1990 21. Samuelson TW, Spaeth GL: Focal and diffuse glaucomatous visual field defects: Their relationship
to intraocular pressure. Ophthalmic Surg 24(8):519, 1993 22. Niesel P, Flammer J: Correlations between intraocular pressure, visual field and visual acuity, based
on 11 years of observations of treated chronic glaucomas. Int Ophthalmol 3(1):331, 1980 23. King D, Drance MD, Douglas G et al: Comparison of visual field defects in normal-tension glaucoma and
high-tension glaucoma. Am J Ophthalmol 101:204, 1986 24. Kurimoto Y, Kobayashi H, Sakaue H et al: Correlation between the optic disc shape and the visual field in glaucoma
patients: Objective and quantitative analysis using Heidelberg retinal
tomograph and Humphrey field analyzer. Invest Ophthalmol Vis Sci 35(4): 1345, 1994 25. Caprioli J, Spaeth GL: Comparison of visual field defects in the low-tension glaucomas
with those in the high-tension glaucomas. Am J Ophthalmol 97:730, 1984 26. Caprioli J, Sears M, Miller JM: Patterns of early visual field loss in open-angle glaucoma. Am J Ophthalmol 103:512, 1987 27. Costa VP, Smith M, Spaeth GL et al: Loss of visual acuity after trabeculectomy. Ophthalmology 100:599, 1993 28. Kolker A: Visual prognosis in advanced glaucoma: A comparison of medical and surgical
therapy for retention of vision in 101 eyes with advanced glaucoma. Trans Am Ophthalmol Soc 75:539, 1977 29. Hayashi K, Hayashi H, Nakao F, et al: Influence of cataract surgery on automated perimetry in patients with glaucoma. Am J Ophthalmol, 132:41, 2001 30. Fellman RL: Visual Fields. ed. Gross, RL, Clinical Glaucoma Management: Critical signs in diagnosis and Therapy, pp. 414-473, W.B. Saunders Co, Philadelphia, 2001 31. Kim YY, Kim JS, Shin DH et al: Effect of cataract extraction on blue-on-yellow visual field. Am J Ophthalmol 132:217, 2001 32. The AGIS Investigators: The advanced glaucoma intervention study,6: effect
of cataract on visual field and visual acuity. Arch Ophthalmol 118:1639, 2000 33. Chen PP, Budenz DL. The effects of cataract extraction on the visual field of eyes with chronic
open-angle glaucoma. Am J Ophthalmol, 125:325-33, 1998 34. Smith SD, Katz J, Quigley HA: Effect of cataract extraction on the results of automated perimetry in
glaucoma. Arch Ophthalmol 115:1515, 1997 35. Shin DH, Islander NG, Ahee JA et al: Long-term filtration and visual field outcomes after primary glaucoma
triple procedure with and without Mitomycin-C. Ophthalmology 109:1607, 2002 36. Lynn JR, Fellman RL, Starita RJ: Principles of perimetry. In Ritch R, Shields MB, Krupin R (eds): The Glaucomas, p 511 Mosby, St Louis, 1996 37. Bigar F, Witmer R: Corneal endothelial changes in primary acute angle-closure glaucoma. Ophthalmology 89:596,1982 38. Ritch R, Schlotzer-Schrehardt U: Exfoliation syndrome. Surv Ophthalmol 45:265-315, 2001 39. Naumann GO, Schlotzer-Schrehardt U, Kuchle M: Pseudoexfoliation syndrome for the comprehensive ophthalmologist. Intraocualr and systemic manifestations. Ophthalmology 105:951, 1998 40. Chen HSL, Steinmann WC, Spaeth GL: The effect of chronic miotic therapy on the results of posterior chamber
intraocular lens implantation and trabeculectomy in patients with glaucoma. Ophthalmic Surg 20(11):784, 1989 41. Sherwood MB, Grierson I, Miller L et al: Long-term morphologic effects of antiglaucoma drugs on the conjunctiva
and Tenon's capsule in glaucomatous patients. Ophthalmology 96:327, 1989 42. Mastropasqua L, Carpineto P, Ciancaglini et al: Intraocular pressure changes after phacoemulsifiocation and foldable silicone
lens implantation using Healon GV. Ophthalmologica, 212:315-21, 1998 43. Rich WJ, Radtke ND, Cohan BE: Early ocular hypertension after cataract extraction. Br J Ophthalmol 58:725, 1974 44. Krupin T, Feitl ME Bishop KI,: Postoperative intraocular pressure rise in open-angle glaucoma patients
after cataract or combined cataract-filtration surgery. Ophthalmology 96:579, 1989 45. Spaeth GL: The management of patients with cataract and glaucoma. Ophthalmic Surg 11:780, 1980 46. West J, Burke J, Cunliffe I et al: Prevention of acute postoperative pressure rises in glaucoma patients undergoing
cataract extraction with posterior chamber lens implant. Br J Ophthalmol 76:534, 1992 47. Ruiz RS, Rhem MN, Prager TC: Effects of carbachol and acetylcholine on intraocular pressure after cataract
extraction. Am J Ophthalmol 197:7, 1989 48. Pohjalainen T, Vesti E, Uusitalo RJ, Laatikainen L: Intyraocular pressure after phacoemulsification and intraocular lens implantation
in nonglaucomatous eyes with and without exfoliation. J Cataract Refract Surg, Mar 27(3):426-31, 2001 49. Pohjalainen T, Vesti E, Uusitalo RJ, Laatikainen L: Phacoemulsification and intraocular lens implantation in eyes with open-angle
glaucoma. Acta Ophthalmol Scand Jun 79(3):313-6, 2001 50. Schwenn O, Dick HB, Krummenauer F, et al Intraocular pressure after small incision cataract surgery: temporal sclerocorneal
versus clear corneal incision. J Cataract Refract Surg Mar:27:421, 2001 51. Savage JA, Thomas JV, Belcher CD et al: Extracapsular cataract extraction and posterior chamber intraocular lens
implantation in glaucomatous eyes. Ophthalmology 92:1506, 1988 52. Schwenn O, Dick HB, Krummenauer F et al: Healon5 versus Viscoat during cataract surgery: intraocular pressure, laser
flare and corneal changes. Graefes Arch Clin Exp Ophthalmol 238:861, 2000 53. Arshinoff SA, Albiani DA, Taylor-Laporte J: Intraocular pressure after bilateral cataract surgery using Healon, Hedalon5 and
Healon GV. J Cataract Refract Surg, 28:617, 2002 54. Ruiz RS, Rhem MN, Prager TC: Effects of carbachol and acetylcholine on intraocular pressure after cataract
extraction. Am J Ophthalmol 197:7, 1989 55. Shingleton BJ, Wadhwani RA, O'Donoghue MW et al:Evaluation of intraocular pressure in the immediate period after phacoemulsification. J Cataract Refract Surg 27:524, 2001 56. Rainer G, Menapace R, Schmetterer K, Findl O, et al:Effect of dorzolamide and latanoprost on intraocular pressure after small
incision cataract surgery. J Cataract Refract Surg 25:1624, 1999 57. Scherer WJ, Mielke DL, Tidwell PE, Hauber FA: Effect of latanoprost on intraocular pressure following cataract extraction. J Cataract refract Surg, 24:964, 1998 58. Byrd S, Singh K: Medical control of intraocular pressure after cataract surgery. J Cataract Refract Surg 24:1493, 1998 59. Rainer G, Menapace R, Findl O et al: Intraindividual comparison of the effects of a fixed dorzolamide-timolol
combination and latanoprost on intraocular pressure after small
incision cataract surgery. J Cataract Refract Surg 27:706, 2001 60. Robin AL: Short-term effects of unilateral 1% apraclonidine therapy. Arch Ophthalmol 106:912, 1988 61. Sterk CC,Renzenbrink-Bubberman AC, van Best JA: The effect of 1% aoraclonidine on intraocular pressure after cataract
surgery. Ophthalmic Surg Lasers 29:472, 1998 62. Kasetti SR, Shrivatsa PD, Subramaniam W et al: Preventing intraocular pressure increase after phacoemulsification and
the role of perioperative apraclonidine. J Cataract Refract Surg 28:2177, 2002 63. Whitehouse G: Brimonidine and postoperative pressure spikes in cataract surgery. Clin Experiment Ophthalmol 289:364, 2000 64. Rainer G, Menapace R, Findl O, et al: Effect of topical brimonidine on intraocular pressure after small incision
cataract surgery. J Cataract Refract Surg 27:1227, 2001 65. Carnahan MC, Platt LW: Serial paracentesis in the management of acute elevations of intraocular
pressure. Ophthalmology 109:1604, 2002 66. Brooks AMV, Gillies WE: Do any factors predict a favourable response to laser trabeculoplasty? Aust J Ophthalmol 12:149, 1984 67. Galin MA, Obstbaum SA, Asano Y et al: Laser trabeculoplasty and cataract surgery. Trans Ophthalmol Soc UK 104(1):72, 1985 68. Savage JA, Thomas JV, Belcher CD 3rd , Simmons RJ: Extracapsular cataract extraction and posterior chamber lens implantation
in glaucomatous eyes. Ophthalmology 92:1506, 1985 69. Fellman RL, Starita RJ, Spaeth GL, et al: Argon laser trabeculoplasty following failed trabeculectomy. Ophthalmic Surg 15:195, 1984 70. Laatikainen L: Late results of surgery on eyes with primary glaucoma and cataract. Acta Ophthalmol 49:281, 1971 71. Steuhl KP, Marahrens P, Frohn C et al: Intraocular pressure and anterior chamber depth before and after extracap-sular
cataract extraction with posterior chamber lens implantation. Ophthalmic Surg 23:233, 1992 72. Dimitrov PN, Mukesh BN, Taylor HR, McCarty CA:Intraocular pressure before and after cataract surgery in participants
of the Melbourne Visual Impairment Project. Clin Experiemtn Ophthalmol 29:128, 2001 73. Hansen MH, Gyldenkerne GJ, Otland NW et al: Intraocular pressure seven years after extracapsular cataract extraction
and sulcus implantation of a posterior chamber intraocular lens. J Cataract Refract Surg 21:676, 1995 74. Shingleton, BJ, Gamell LS, O'Donoghue MW et al: Long-term changes in intraocular pressure after clear corneal phacoemulsification: Normal
patients versus glaucoma suspect and glaucoma
patients. J Cataract Refract Surg 25:887,1999 75. Merkur A, Damji KF, Mintsioulisw G, Hodge WG: Intraocular pressure decrease after phacoemulsification in patients with
pseudoexfoliation syndrome. J Cataract and Refractive Surg 27:528, 2001 76. Wirbelauer C, Anders N, Pham DT et al: Intraocular pressure in nonglaucomatous eyes with pseudoexfoliation syndrome
after cataract surgery. Ophthalmic Surg Lasers 29:466, 1998 77. Jahn CE: Reduced intraocular pressure after phacoemulsification and posterior chamber
intraocular lens implantation. J Cataract Refract Surg 23:1260, 1997 78. Meyer MA, Savitt ML, Kopitas E: The effect of phacoemulsification on aqueous outflow facility. Ophthalmology 104:1221, 1997 79. Schwenn O, Dick HB, Krummenauer F et al: Intraocular pressure after small incsion cataract surgery:temporal sclerocorneal
versus clear corneal incision. J Cataract Refract Surg 27:421, 2001 80. Krupin T, Feitl ME Bishop KI,: Postoperative intraocular pressure rise in open-angle glaucoma patients
after cataract or combined cataract-filtration surgery. Ophthalmology 96:579, 1989 81. Hopkins JJ, Apel A, Trope GE, Rootman DS: Early intraocular pressure after phacoemulsification combined with trabeculectomy. Ophthalmic Surg Laser 29:273, 1998 82. Porges Y, Ophir A: Surgical outcome after early intraocular pressure elevation following combined
cataract extraction and trabeculectomy. Ophthalmic Surg Lasers 30:727, 1999 83. Bigger JF, Becker B: Cataracts and primary open-angle glaucoma: The effect of uncomplicated
cataract extraction on glaucoma control. Am Acad Ophthalmol Otol 75:260, 1971 84. Laatikainen L: Late results of surgery on eyes with primary glaucoma and cataract. Acta Ophthalmol 49:281, 1971 85. Steuhl KP, Marahrens P, Frohn C et al: Intraocular pressure and anterior chamber depth before and after extracapsular
cataract extraction with posterior chamber lens implantation. Ophthalmic Surg 23:233, 1992 86. Bobrow JC: Prospective intrapatient comparison of extracapsular cataract extraction
and lens implantation with and without trabeculectomy. Am J Ophthalmol 129:291, 2000 87. Anders N, Pham T, Holschbach A, Wollensak J: Combined phacoemuslfication and filtering surgery with the “no-stitch” technique. Arch Ophthalmol 115:1245, 1997 88. Kosmin AS, Wishart PK, Ridges PJ: Long-term intraocular pressure control after cataract extraction
with trabeculectomy:phacoemulsification versus extracapsular technique. J Cataract Refract Surg 24:249, 1998 89. Cohen JS: Combined cataract implant and filtering surgery with 5-fluorouracil. Ophthalmic Surg 21:181, 1990 90. Johnstone MA, Ziel CJ: Cataract surgery in the presence of a filtering bleb with postoperative 5-fluorouracil (5-FU). Invest Ophthalmol Vis Sci 33:948, 1992 91. Wong PC, Ruderman JM, Krupin T et al: 5-Fluorouracil (5-FU) after primary combined filtration
surgery. Am J Ophthalmol 117:149, 1994 92. Budenz DL, Pyfer M, Singh K et al: Comparison of phacotrabeculectomy with 5-fluorouracil, Mitomycin-C, and
without antifibrotic agents. Ophthalmic Surg Lasers 30:367, 1999 93. O'Grady JM, Juzych MS, Shin DH et al: Trabeculectomy, phacoemulsification and posterior chamber lens implantation
with and without 5-fluorouracil. Am J Ophthalmol 116:594, 1993 94. Hurvitz, LM: 5-fluorouracil supplemented phacoemulsification, posterior chamber
lens implantation, and trabeculectomy. Ophthalmic Surg 24:674, 1993 95. Cohen JS, Greff LJ, Novack GD, Wind BE: A placebo-controlled, double masked evaluation of Mitomycin C in
combined glaucoma and cataract procedures. Ophthalmology 103:1934, 1996 96. Carlson DW, Alward WL, Barad JP et al: A randomized study of Mitomycin augmentation in combined phacoemulsification
and trabeculectomy. Ophthalmology 104:719, 1997 97. Casson RJ, Salmon JF: Combined surgery in the treatment of patients with cataract and primary
open-angle glaucoma. J Cataract Refract Surg 27:1854, 2001 98. Shin DH, Ren J, Juzych MS et al: Primary glaucoma triple procedure in patients with primary open angle glaucoma: The
effect of Mitomycin C in patients with and without prognostic
factors for filtration failure. Am J Ophthalmol 125:346, 1998 99. Shin DH, Kim YY, Sheth N et al: The role of adjuctive Mitomycin C in seconday glaucoma triple procedure
as compared to primary glaucoma triple procedure. Ophthalmology 105:740, 1998 100. Rockwood EJ, Larive B, Hahn J: Outcomes of combined cataract extraction, lens implantation and trabeculectomy
surgeries. Am J Ophthalmol 130:704, 2000 101. Belyea DA, Dan JA, Lieberman MF, Stamper RL: Midterm follow-up results of combined phacoemulsification, lens
implantation, and mitomycin-C trabeculectomy procedure. J Glaucoma 6:90, 1997 102. Yamagami S, Hamada N, Araie M, Shirato S: Risk factors for unsatisfactory intraocular pressure control in combined
trabeculectomy and cataract surgery. Ophthalmic Surg Lasers 28:476, 1997 103. Tezel G, Kolker AE, Kass MA, Wax MB: Comparative results of combined procedures for glaucoma and cataract: I. Extracapsular cataract extraction versus phacoemulsification and foldable
versus rigid intraocular lenses. Ophthalmic Surg Lasers 28:539, 1997 104. Chia WLA, Goldberg I: Comparison of extracapsular and phacoemulsification cataract extraction
techniques when combined with intraocular lens placement and trabeculectomy:short
term results. Aust N Z J Ophthalmol 26:19, 1998 105. Shingleton BJ, Jacobson LM, Kuperwaser MC: Comparison of combined cataract and glaucoma surgery using planned extracapsular
and phacoemulsification techniques. Ophthalmology Surg lasers 26:414, 1995 106. Singh RP, Goldberg I, Mohsin M: The efficacy and safety of intraoperative and/or postoperative 5-fluorouracil
in trabeculectomy and phacotrabeculectomy. Clin Exp Ophthalmol 29:296, 2001 107. Siriwardena D, Kotecha A, Minassian D et al: Anterior chamber flare after trabeculectomy and after phacoemulsification. Br J Ophthalmol 84:1056, 2000 108. Beckers HJ, DeKroon KE, Nuijts RM, Webers CA: Phacotrabeculectomy. Doc Ophthalmol 100:43, 2000 109. Menapace R, Wedrich A, Muhlbauer-Ries et al: Phacotrabeculectomy with a small-optic PMMA implant:two-year
functional and m porphological results. Ophthalmologica 212:322, 1998 110. Caprossi A, Casprini F, Topsi GM, Balestrazzi A: Long-term results of combined 1-way phacoemulsification, intraocular
lens implantation and trabeculectomy. J Cataract Refract Surg 25:1641, 1999 111. Mamalis N, Lohner S, Rand AN, Crandall AS: Combined phacoemulsification, intraocular lens implantation, and trabeculectomy. J Cataract Refract Surg 22:467, 1996 112. Vyas AV, Bacon PJ, Percival SP: Phacotrabeculectomy: comparison of results from 3.5 and 5.2 mm incisions. Ophthalmic Surg Lasers 29:227, 1998. 113. Kosmin AS, Wishart PK, Ridges PJ: Silicone versus polymethacrylate lenses in combined phacoemulsification
and trabeculectomy. J Cataract Refract Surg 23:97, 1997 114. Donoso R, Rodriguez A: Combined versus sequential phacotrabeculectomy with intraoperative 5-fluorouracil. J Cataract Refract Surg 26:71, 2000 115. El-Sayyad FF, Helal MH, Khalil MM et al: Phacotrabeculectomy versus two-stage operation: a matched study. Ophthalmic Surg Lasers 30:260, 1999 116. Braga-Mele R, Cohen S, Rootman DS: Foldable silicone versus polymethylmethcrylate intraocular lenses in combined
phacoemulsification and trabeculectomy. J Cataract Refract Surg 26:1517, 2000 117. Wyse T, Meyer M, Ruderman JM, Krupin T, et al: Combined trabeculectomy and phacoemulsification: a one-site vs a
two-site approach. Am J Ophthalmol 125:334, 1998 118. Borggrefe J, Lieb W, Grehn F: A prospective randomized comparison of two techniques of combined cataract-glaucoma
surgery. Graefes Arch Clin Exp Ophthalmol 237:887, 1999 119. Rossetti L, Bucci L, Miglior S et al: Temporal corneal phacoemulsification combined with separate incision superior
trabeculectomy vs standard phacotrabeculectomy. A comparative study. Acta Ophthalmol Scand Suppl 224:39, 1997 120. el Sayyad F, Helal M, el-Maghraby et al: One-site verus 2-site phacotrabeculectomy: a randomized study. J Cataract Refract Surg 24:77, 1999 121. Spaeth GL: Glaucoma surgery. In Principles & Practice of Ophthalmic Surgery, p 319. Philadelphia, WB Saunders, 1990 122. Kass MA: Cataract extraction in an eye with a filtering bleb. Ophthalmology 89:871, 1988 123. Mietz H, Andresen A, Welsandt G, Krieglstein GK: Effect of cataract surgery on intraocular pressure in eyes with previous
trabeculectomy. Graefes Arch Clin Exp Ophthalmol 239:763, 2001 124. Halikiopoulos D, Moster MR, Azuara-Blanco A et al: The outcome of the functioning filter after subsequent cataract extraction. Ophthalmic Surg Lasers 32:108, 2001 125. Manoj B, Chako D, Khan MY: Effect of extracapsular extraction and phacoemulsification performed after
trabeculectomy on intraocular pressure. J Cataract Refract Surg 26:75, 2000 126. Rebolleda G, Munoz-Negrete FJ: Phacoemulsificatgion in eyes with functioning filtering blebs: a prospective
study. Ophthalmology 109:2248, 2002 127. Chen PP, Weaver YF, Budenz DL et al: Trabeculectomy function after cataract extraction. Ophthalmology 105:1928, 1998 128. Wygnanski-Jaffe T, Barak A, Melamed S, Glovinsky Y: Intraocular pressure increments after cataract extraction in glaucomatous
eyes with functioning filtering blerbs. Ophthalmic Surg Lasers 28:657, 1997 129. Crichton ACS, Kirker AW: Intraocular pressure and medication control after clear corneal phacoemulsification
and Acrysof posterior chamber intraocular lens implantation
in patients with filtering blebs. J Glaucoma 10:38, 2001 130. Bhattacharyya CA, WuDunn D, Lakhani V et al: Cataract surgery after tube shunts. J Glaucoma 9:453, 2000 131. Smith MF, Doyle JW: Use of tissue plasminogen activator to revive blebs following intraocular
surgery. Arch Ophthalmol 119:809, 2001 132. Mardelli PG, Lederer CM, Murray PL et al: Slit lamp needle revision of failed filtering blebs using Mitomycin C. Ophthalmology 103:1946, 1996 133. Hawkins AS, Glanagan JK, Brown SVL: Predictors for success of needle revision of failing filtration blebs. Ophthalmology 109:783, 2002 134. Sibayan SA, Igarashi S, Kasahara N et al: Cataract extraction as a means of treating postfiltration hypotony maculopathy. Ophthalmic Surg Lasers 28:241, 1997 135. Doyle JW, Smith MF: Effect of phacoemulsification surgery on hypotony following trabeculectomy
surgery. Arch Ophthalmol 118:763, 2000 136. Fellman RL: Trabeculotomy. In George L Spaeth (ed): Ophthalmic Surgery: Principles and Practice, 3rd ed, p XX. Philadelphia, WB Saunders, 2003 137. Tanihara H, Honjo M, Inatani M et al: Trabeculotomy combined with phacoemulsification and implantation of an
intraocular lens for the treatment of primary open angle glaucoma and
coexisting cataract. Ophthalmic Surg Lasers 28:810, 1997 138. Tanito M, Ohira A, Chihara E: Surgical outcome of combined trabeculotomy and cataract surgery. J Glaucoma 10:302, 2001 139. Tanito M, Ohira A, Chihara E: Factors leading to reduced intraocular pressure after combined trabeculotomy
and cataract surgery. J Glaucoma 11:3, 2002 140. Luke C, Dietlein TS, Jacobi PC et al: A prospective randomized trial of viscocanalostomy versus trabeculectomy
in open angle glaucoma: a one year follow-up study. J Glaucoma 11:294, 2002 141. Stegmann R, Pienaar A, Miller D: Viscocanalostomy for open-angle glaucoma in black African patients. J Cataract Refgract Surg 25:316, 1999 142. El Sayyad F, Helal M, El-Kholify H et al: Nonpenetrating deep sclerectomy versus trabeculectomy in bilateral primary
open angle glaucoma. Ophthalmology 107:1671, 2000 143. Gianoli F, Schyder CC, Bovey E, Mermoud A: combined surgery for cataract and glaucoma:phacoemulsification and deep
sclerectomy compared with phacoemulsification and trabeculectomy. J Cataract Refract Surg 25:340, 1999 144. Wishart MS, Shergill T, Porooshani H: Viscocanalostomy and phacoviscocanalostomy: long-term results. J Cataract Refract Surg 28:745, 2002 145. Gimbel HV, Penno EE, Ferensowicz M: Combined cataract surgery, intraocular lens implantation, and viscocanalostomy. J Cataract Refract Surg 25:1370, 1999 146. Hayashi K, Hayashi H, Nakao F, Hayashi F: Effect of cataract surgery on intraocular pressure control in glaucoma
patients. J Cataract Refract Surg 27:1779, 2001 147. Yang CH, Hung PT: Intraocular lens position and anterior chamber angle changes after cataract
extraction in eyes with primary angle-closure glaucoma. J Cataract Refract Surg 23:1109, 1997. 148. Gunning FP, Greve EL: Lens extraction for uncontrolled angle-closure glaucoma: Long term
follow-up. J Cataract Refract Surg 24:1347, 1998 149. Acton J, Salmon JF, Scholtz R: Extracapsular cataract extraction with posterior chamber lens implantation
in primary angle-closure glaucoma. J Cataract Refract Surg 23:930, 1997 150. Hayashi K, Hayashi H, Nakao F et al: Changes in anterior chamber angle width and depth after IOL implantation
in eyes with glaucoma. Ophthalmology 107:698, 2000 151. Fellman RL: Comment of: Changes in anterior chamber angle width and depth after IOL
implantation in eyes with glaucoma. Evidence-based Eye Care 2:53, 2001 152. Lai JS, Tham CC, Lam DS: The efficacy and safety of combined phacoemulsification, intraocular lens
implantation, and limited goniosynechialysis, followed by diode laser
peripheral iridoplasty, in the treatment of cataract and chronic angle
closure glaucoma. J Glaucoma 10:309, 2001 153. Jacobi PC, Dietlein TS, Luke C et al: Primary phacoemulsification and intraocular lens implantation for acute
angle closure glaucoma. Ophthalmology 109:1597, 2002 154. Cashwell LF, Martin CA: Axial length decreases accompanying successful glaucoma filtration surgery. Ophthalmology 106:2307, 1999 155. Kook MS, Kim HB, Lee SU: Short-term effect of Mitomycin-C augmented trabeculectomy
on acial length and corenal astigmatism. J Cataract Refract Surg 27:518, 2001 156. The advanced glaucoma intervention study group, The advanced glaucoma intervention
study, 8: risk of cataract formation after trabeculectomy. Arch Ophthalmol 119:1771, 2001 157. Iaccarino G, Rosa N, Romano M et al: Expulsive hemorrhage before phacoemulsification. J Cataract Refract Surg 28:1074, 2002 158. Cantor LB, Katz LJ, Spaeth GL: Complications of surgery in glaucoma: Suprachoroidal expulsive hemorrhage
in glaucoma patients undergoing intraocular surgery. Ophthalmology 92:1266, 1985 159. Ruderman JM, Harbin TS Jr, Campbell DG: Postoperative suprachoroidal hemorrhage following filtration procedures. Arch Ophthalmol 104:201, 1986 160. Givens K, Shields MB: Suprachoroidal hemorrhage after glaucoma filtering surgery. Am J Ophthalmol 103:689, 1987 161. Jaynes JH, Payne JW, Green WR: Clinicopathologic study of eyes obtained postmortem from a patient 6 and 2 years
after operative choroidal hemorrhage. Ophthalmic Surg 18:667, 1987 162. Welch JC, Spaeth GL, Benson WE: Massive suprachoroidal hemorrhage: Follow-up and outcome of 30 cases. Ophthalmology 95:1202, 1988 163. Speaker MG, Guerriero PN, Met JA et al: A case-control study of risk factors for intraoperative suprachoroidal
expulsive hemorrhage. Ophthalmology 98:202, 1990 164. Canning CR, Lavin M, McCartney ACE et al: Delayed suprachoroidal hemorrhage after glaucoma operations. Eye 3:327, 1989 165. Spaeth GL, Baez KA: Long-term prognosis of eyes having had operative suprachoroidal
expulsive hemorrhage. Ger J Ophthalmol 3:159, 1994 166. Bellows AR, Chylack LT, Huthchinson BT: Choroidal detachment:clinical manifestations, therapy and mechanism of
formation. Ophthalmology 88:1107, 1981. 167. Herndon LW: Shallow or flat anterior chamber. In Spaeth GL (ed): Ophthalmic surgery: Principles and Practice, 3rd ed. Philadelphia, WB Saunders, 2003 168. Fellman RL, Budenz D: Malignant glaucoma. J Glaucoma 8:149, 1999 169. Fellman RL: Flat chamber following glaucoma filtering procedures. In Spaeth GL, Katz LJ (eds): Current Therapy in Ophthalmic Surgery, p 330. Hamilton, Ontario, BC Decker, 1989 170. Lynn JR, Swanson WH, Fellman RL, Starita RJ.: Does glaucomatous visual filed loss continue despite surgically subnormal
IOP? Perimetry Update 1992.93, p 129. Proceedings of the Xth International Perimetric Society Meeting, Kugler Publications, Amsterdam / New York 171. The advanced glaucoma intervention study (AGIS): 7. The relationship between control of intraocular pressure and visual field
deterioration. The AGIS Investigators, Am J Ophthalmol, 130:429, 2000 172. Hoffman KB, Feldman RM, Budenz DL et al: Combined cataract extraction and Baerveldt glaucoma drainage implant: indications
and outcomes. Ophthalmology 109:1916, 2002 173. The advanced glaucoma intervention study, 8: risk of cataract formation
after trabeculectomy. Arch Ophthalmol 119:1771, 2001 174. Kozobolis VP, Siganos CS, Christodoulakis EV et al: Two-site phacotrabeculectomy with intraoperative mitomycin-C:Fornix-versus
limbus-based conjunctival opening in fellow
eyes. J Cataract Refract Surg 28:1758, 2002 175. Shingleton BJ, Chaudhry IM, O'Donoghue MW et al: Phacotrabeculectomy: limbus-based versus fornix-based conjunctival
flaps in fellow eyes. Ophthalmology 106:1152, 1999 176. Berestka JS, Brown SV: Limbus-versus fornix-based conjunctival flaps in combined
phacoemulsification and Mitomycin-C trabeculectomy surgery. Ophthalmology 104:187, 1997 177. Lemon LC, Shin DH, Kim C, et al: Limbus-based vs fornix-based conjunctival flap in combined
glaucoma and cataract surgery with adjunctive Mitomycin-C. Am J Ophthalmol 125:340, 1998 178. Agbeja AM, Dutton GN: Conjunctival incisions for trabeculectomy and their relationship to the
type of bleb formation—a preliminary study. Eye 1:738, 1987 179. el Sayyad F, el-Rashood A, Helal M et al: Fornix-based versus limbal-based conjunctival flaps in initial
trabeculectomy with postoperative 5-fluorouracil: four-year
follow up findings. J Glaucoma 8:124, 1999 180. Fellman RL: Trabeculectomy. London, Mosby International Ltd., 1999 181. Dinsmore SC, Modified stretch technique for small pupil phacoemulsification with topical
anesthesia. J Cataract Refract Surg 22:27, 1996 182. Graether JM. Graether pupil expander for managing the small pupil during surgery. J Cataract Refract Surg 22:530, 1996. 183. Bacskulin A, Kundt G, Guthoff R: Efficiency of pupillary stretching in cataract surgery. Eur J Ophthalmol 8:230, 1998. 184. Birchall W, Spencer AF: Misalignment of flexible iris hook retractors for small pupil cataract
surgery:effects on pupil circumference. J Cataract Refract Surg 27:20, 2001 185. Fine IH: Pupilloplasty for small pupil phacoemulsification. J Cataract Refract Surg 20:192, 1994 186. Yuguchi T, Oshika T, Sawaguchi S et al: Pupillary functions after cataract surgery using flexible iris retractor
in patients with small pupil. Jpn J Ophthalmol 43:20, 1999 187. Horiguchi M, Miyake K, Ohta I, Ito Y: Staining of the lens capsule for circular continuous capsulorhexis in eyes
with white cataract. Arch Ophthalmol 116:535, 1998 188. Jacob S, Agarwal AA, Agarwal AA et al: Trypan blue as an adjunct for safe phacoemulsification in eyes with white
cataract. J Cataract Refract Surg 28:1819, 2002 189. Shingleton BJ, Chaudhry IM, O'Donoghue MW: Phacotrabeculectomy: peripheral iridectomy or no peripheral iridectomy? J Cataract Refract Surg 28:998, 2002 190. Crestani A, DeNatale R, Steindler P: Phacotrabeculectomy with or without punch: preliminary results comparing
two techniques. Ophthalmologica 211:72, 1997 191. Wise JB: Mitomycin-C compatible suture technique for fornix-based
conjunctival flaps in glaucoma filtration surgery. Arch Ophthalmol 111:992, 1993 192. Philip WC, Yeung BYM, Yick DWF et al: Fornix-based trabeculectomy with Wise's suture technique in
Chinese patients. Ophthalmology, 107:2310–2313,2000 193. Traverso CE, Greenridge KC, Spaeth GL et al: Focal pressure:a new method to encourage filtration after trabeculectomy. Ophthalmol Surg, 15:62, 1984 194. Spaeth GL: Laser Suture Lysis. In Spaeth GL (ed): Ophthalmic Surgery: Principles and Practice, 3RD ed. Philadelphia, WB Saunders, 2003 195. Starita, RJ, Fellman RL, Spaeth GL et al: Short and long term effects of postoperative corticosteroids on trabeculectomy. Ophthalmology 92:938, 1985 |