1. Mann I: Development of the Human Eye. Philadelphia: Grune & Stratton, 1964 2. Barber AN: Embryology of the Human Eye. St Louis: CV Mosby, 1955 3. Dejean C, Leplat G, Hervouât F: L'Embryologie de l'Oeil et sa Teratologie, p 288. Paris: Masson et Cie, 1958 4. Duke-Elder S, Cook C: In Duke-Elder S (ed): System of Ophthalmology. St
Louis: CV Mosby, 1963 5. Bartelmez GW: The formation of neural crest from the primary optic vesicle in man. Contrib Embryol Carnegie Inst 35:603, 1954 6. Berson D: The development of the choroid and sclera in the eye of the foetal rat
with particular reference to their developmental interrelationship. Exp Eye Res 4:102, 1965 7. Clavert A: Role de la coupule optique et du cristallin dans la morphogenäse oculairé. Arguments
fournis par la teratogenäse. Arch Ophthalmol Rev Gen Ophthalmol 33:289, 1973 8. Coulombre AJ: Regulation of ocular morphogenesis. Invest Ophthalmol 8:25, 1969 9. Coulombre AJ, Coulombre JL: Lens development I. Role of lens in eye growth. J Exp Zool 156:39, 1964 10. Coulombre AJ, Coulombre JL: Mechanisms of ocular development. Int Ophthalmol Clin 15:7, 1975 11. Genis-Galvez JM: Role of the lens in the morphogenesis of the iris and cornea. Nature 210:209, 1966 12. Hay ED: Origin and role of collagen in the embryo. Am Zool 13:1085, 1973 13. Meier S, Hay ED: Control of corneal differentiation by extracellular materials. Collagen
as a promoter and stabilizer of epithelial stroma production.Dev Biol 38:249, 1974 14. Lopashov GV: Developmental Mechanisms of Vertebrate Eye Rudiments. New
York: Pergamon Press, 1963 15. Grainger R, Henry J, Henderson R: Reinvestigation of the role of the optic vesicle in embryonic lens induction. Development 102:517, 1988 16. Karkinen-Jaaskelainen M: Permissive and directive interactions in lens induction. J Embryol Exp Morphol 44:167, 1978 17. Kuno N, Kadomatsu K, Muramatsu T: Determination of the optimal time and dosage of all-trans retinoic acid
for induction of murine exencephaly. Teratology 60:63, 1999 18. McKeehan M: Cytological aspects of embryonic lens induction in the chick. J
Exp Zool 31, 1951 19. Selleck MA, Bronner-Fraser M: The genesis of avian neural crest cells: A classic embryonic induction. Proc Natl Acad Sci USA 93:9352, 1996 20. Streit A, Stern CD: Neural induction: A bird's eye view. Trends Genet 15:20, 1999 21. McKeehan MS: The relative ribonucleic acid content of lens and retina during lens induction
in the chick. Am J Anat 99:131, 1956 22. Cook CS, Nowotny AZ, Sulik KK: Fetal alcohol syndrome. Eye malformations in a mouse model. Arch Ophthalmol 105:1576, 1987 23. Cook C, Sulik K: Keratolenticular dysgenesis (Peters' anomaly) as a result of acute embryonic
insult during gastrulation. J Pediatr Ophthalmol Strabismus 25:60, 1988 24. Cook C: Experimental models of anterior segment dysgenesis. Ophthalm Paediatr Genet 10:33, 1989 25. Stromland K, Miller M, Cook C: Ocular teratology. Surv Ophthalmol 35:429, 1991 26. Cook C: Embryogenesis of congenital eye malformations. Vet Comp Ophthalmol 5:109, 1995 27. O'Rahilly R: The early development of the eye in staged human embryos. Contrib Embryol Carnegie Inst 38:1, 1966 28. Le Lievre C, Le Douarin N: Mesenchymal derivatives in the neural crest: Analysis of chimaeric quail
and chick embryos. J Embryol Exp Morphol 34:125, 1975 29. Jakobiec FA, Tannenbaum M: Embryological perspectives on the fine structure of orbital tumors. Int Ophthalmol Clin 15:85, 1975 30. Johnston M, Noden D, Hazelton R et al: Origins of avian ocular and periocular tissues. Exp Eye Res 29:27, 1979 31. O'Rahilly R. In Rohen J (ed): Eye Structure II Symposium p 557. Stuttgart: Schattauer
Verlag, 1965 32. Torczynski E, Jakobiec FA, Johnston MC et al: Synophthalmia and cyclopia: A histopathologic, radiographic and organogenetic
analysis. Doc Ophthalmol 44:311, 1977 33. Meier S: The distribution of cranial neural crest cells during ocular morphogenesis. Prog Clin Biol Res 82:1, 1982 34. Blankenship TN, Peterson PE, Hendrickx AG: Emigration of neural crest cells from macaque optic vesicles is correlated
with discontinuities in its basement membrane. J Anat 188:473, 1996 35. Dencker L, d'Argy R, Danielsson B et al: Saturable accumulation of retinoic acid in neural and neural crest derived
cells in early embryonic development. Dev Pharmacol Ther 10:212, 1987 36. Greenberg J, Seppa S, Hewitt A: Role of collagen and fibronectin in neural crest cell adhesion and migration. Dev Biol 87:259, 1981 37. Nataf V, Lecoin L, Eichmann A et al: Endothelin-B receptor is expressed by neural crest cells in the avian embryo. Proc Natl Acad Sci USA 93:9645, 1996 38. Noden D. In Tasman W, Jaeger E (eds): Duane's Foundations of Clinical
Ophthalmology, p 1. Philadelphia: JB Lippincott, 1993 39. Nye JS, McLone DG, Charrow J et al: Neural crest anomaly syndromes in children with spina bifida. Teratology 60: 179, 1999 40. Trainor PA, Tam PP: Cranial paraxial mesoderm and neural crest cells of the mouse embryo: Co-distribution
in the craniofacial mesenchyme but distinct segregation
in branchial arches. Development 121:2569, 1995 41. Meier S, Packard DS Jr: Morphogenesis of the cranial segments and distribution of neural crest
in the embryos of the snapping turtle, Chelydra serpentina. Dev Biol 102:309, 1984 42. Jacobson AG: Somitomeres: Mesodermal segments of vertebrate embryos. Development 104:209, 1988 43. Tam PPL, Meier S: The establishment of a somitomeric pattern in the mesoderm of the gastrulating
mouse embryo. Am J Anat 164:209, 1982 44. Meier S, Tam PPL: Metameric pattern development in the embryonic axis of the mouse. I: Differentiation
of the cranial segments. Differentiation 21:95, 1982 45. Meier SP: The development of segmentation in the cranial region of vertebrate embryos. Scan Electron Microsc 3:1269, 1982 46. Packard DS Jr, Meier S: An experimental study of the somitomeric organization of the avian segmental
plate. Dev Biol 97:191, 1983 47. Packard DS Jr, Meier S: Morphological and experimental studies of the somitomeric organization
of the segmental plate in snapping turtle embryos. J Embryol Exp Morphol 84:35, 1984 48. Tam PP: A study of the pattern of prospective somites in the presomitic mesoderm
of mouse embryos. J Embryol Exp Morphol 92:269, 1986 49. Tam PP, Meier S, Jacobson AG: Differentiation of the metameric pattern in the embryonic axis of the mouse. II: Somitomeric
organization of the presomitic mesoderm. Differentiation 21:109, 1982 50. Tam PP, Trainor PA: Specification and segmentation of the paraxial mesoderm. Anat Embryol (Berl) 189:275, 1994 51. Noden DM: The embryologic origins of avian craniofacial muscles and associated connective
tissues. Am J Anat 168:257, 1983 52. Wrenn JT, Wessels NK: An ultrastructural study of lens invagination in the mouse. J Exp Zool 171:359, 1969 53. Schook P: A review of data on cell actions and cell interactions during the morphogenesis
of the embryonic eye. Acta Morphol Neerl Scand 16:267, 1978 54. Beebe D, Latker C, Jebens J et al: Transport and steady-state concentration of plasma proteins in the vitreous
humor of the chicken embryo: Implications for the mechanism of the
eye growth during early development. Dev Biol 114:361, 1986 55. Woerdeman MW: The differentiation of the crystallin lens. J Embryol Exp
Morphol 301, 1953 56. Coulombre JL, Coulombre AJ: Lens development IV. Size, shape and orientation. Invest Ophthalmol 8:251, 1969 57. Hendrix RW, Zwaan J: Changes in the glycoprotein concentration of the extracellular matrix between
lens and optic vesicle associated with early lens differentiation. Differentiation 2:357, 1974 58. Zwaan J, Hendrix RW: Changes in cell and organ shape during early development of the ocular
lens. Am J Zool 13:1039, 1973 59. Beebe DC: Ocular Growth and Differentiation Factors. New York: John Wiley & Sons, 1985 60. Silver PHS, Wakely J: The initial stage in the development of the lens capsule in chick and mouse
embryo. Exp Eye Res 19:73, 1974 61. Beebe D, Compart P, Johnson M et al: The mechanism of cell elongation during lens fiber cell differentiation. Dev Biol 92:54, 1982 62. Grainger RM, Henry JJ, Saha MS et al: Recent progress on the mechanisms of embryonic lens formation. Eye 6:117, 1992 63. Graw J: Genetic aspects of embryonic eye development in vertebrates. Dev Genet 18:181, 1996 64. Muggleton-Harris AL, Higbee N: Factors modulating mouse lens epithelial cell morphology with differentiation
and development of a lentoid structure in vitro. Development 99:25, 1987 65. Menko AS, Philip NJ: Beta 1 integrins in epithelial tissues: a unique distribution in the lens. Exp Cell Res 218:516, 1995 66. Kuwabara T: The maturation of the lens cell: A morphologic study. Exp Eye Res 20:427, 1975 67. Wulle KG, Lerche W: Zur Feinstruktur der embryonalen menschlichen Linsenblase. Graefes Arch Klin Exp Ophthalmol 173:141, 1967 68. Wulle KG, Lerche W: Elektronenmikroskopischer Beitrag zur Embryonalentwicklung der menschlichen
Linse. Graefes Arch Klin Exp Ophthalmol 176:126, 1968 69. Zwaan J, Hendrix RW, Johnson RA: Lens invagination: A theory and a mathematical model. Ophthalmic Res 3:22, 1972 70. Zwann J: Fine structure of the developing lens. Int Ophthalmol Clin 15:39, 1975 71. Yamada T. In Hagen E, Wechsler W, Zilliken P et al (eds): Experimental
Biology and Medicine I, p 77. Basel: Karger, 1967 72. Lerche W, Wulle KG: Electron microscopic studies on the development of the human lens. Ophthalmologica 158: 296, 1969 73. Garcia-Porrero JA, Colvee E, Ojeda JL: The mechanisms of cell death and phagocytosis in the early chick lens morphogenesis: A
scanning electron microscopy and cytochemical approach. Anat Rec 208:123, 1984 74. Garcia-Porrero JA, Collado JA, Ojeda JL: Cell death during detachment of the lens rudiment from ectoderm in the
chick embryo. Anat Rec 193:791, 1979 75. Smelser GK: Embryology and morphology of the lens. Invest Ophthalmol 4:398, 1965 76. Brini A, Porte A, Stoeckel ME: Developpement de la cornée chez l'embryon de poulet: Etude en microscope
electronique. Doc Ophthalmol 20:309, 1966 77. Coulombre AJ. In Abercrombie M, Brachet J (eds): Advances in Morphogenesis, p 81. San
Diego: Academic Press, 1965 78. Dublin I: Vergleichend-embryologische Untersuchungen über die Fruhentwicklung
der Hornhaut und der Pupillarmembran bei Reptilien, V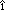 geln and Saugern. Acta Anat 76:381, 1970 geln and Saugern. Acta Anat 76:381, 1970 79. Ewer MS: Zur Fruhentwicklung des Stroma corneae und der Pupillarmembran beim Menschen. Acta Anat 75:37, 1970 80. Hay ED, Revel JP. In Wolsky A, Chen P (eds): Monographs in Developmental
Biology. Basal: A Karger, 1969 81. Kaye GI. In Rohen J (ed): Die Struktur des Auges, II Symposium, p 441. Stuttgart: Schattauer, 1965 82. Messeni ML: A study of the prenatal developmental of corneal mucopolysaccharides in
man. Boll Soc Ital Biol Sper 49:1166, 1973 83. Miyashita S: Electron microscopic studies on the cornea of fetuses. III. The human embryo
cornea. Nippon Ganka Gakkai Zasshi 68:526, 1964 84. Pei YF, Rhodin JAG: The prenatal development of the mouse eye. Anat Rec 168:105, 1970 85. Pei YF, Rhodin JAG: Electron microscopic study of the development of the mouse corneal epithelium. Invest Ophthalmol 10:811, 1971 86. Praus R, Brettschneider I: Glycosaminoglycans in embryonic and postnatal human cornea. Ophthalmic Res 7:452, 1975 87. Smelser GK: In Duke-Elder S (ed): The transparency of the cornea: A Symposium, p 23. Springfield, IL: Charles C Thomas, 1960 88. Schwarz W: Elektronenmikroskopische Untersuchungen fiber die Differenzierung der Cornea
and Sklera Fibrillen des Menschen. Z Zellforsch 38:78, 1953 89. Schwartz W. In Smelser G (ed): The Structure of the Eye, p 393. San Diego: Academic
Press, 1961 90. Zinn KM, Mockel-Pohl S: Fine structure of the developing cornea. Int Ophthalmol Clin 15:19, 1975 91. Wulle KG, Lerche W: Electron microscopic observations of the early development of the human
corneal endothelium and Descemet's membrane. Ophthalmologica 157:451, 1969 92. Wulle KG: Electron microscopy of the fetal development of the corneal endothelium
and Descemet's membrane of the human eye. Invest Ophthalmol 11:897, 1972 93. Wulle KG, Ruprecht KW, Windrath LC: Electron microscopy of the development of the cell junctions in the embryonic
and fetal human corneal endothelium. Invest Ophthalmol 13:923, 1974 94. Smelser GK, Ozanics V (eds): Symposium on the Cornea: Transactions of the
New Orleans Academy of Ophthalmology, p 20. St Louis: CV Mosby, 1972 95. Hay E: Development of the vertebrate cornea. Int Rev Cytol 63:263, 1980 96. Bahn CF, Glassman RM, MacCallum et al: Postnatal development of corneal endothelium. Invest Ophthalmol Vis Sci 27:44, 1986 97. Bee JA, Hay RA, Lamb EM et al: Positional specificity of corneal nerves during development. Invest Ophthalmol Vis Sci 27:38, 1986 98. Clarke ND, Bee JA: Innervation of the chick cornea analyzed in vitro. Invest Ophthalmol Vis Sci 37:1761, 1996 99. De Vries A, Gorgels TGMF, Berg RJW et al: Ultraviolet-B induced hyperplasia and squamous cell carcinomas in the cornea
of XPA-deficient mice. Exp Eye Res 67:53, 1998 100. Fitch JM, Gordon MK, Gibney EP: Analysis of transcriptional isoforms of collagen types IX, II, and I in
the developing avian cornea by competitive polymerase chain reaction. Dev Dyn 202:42, 1995 101. Toole B, Trelstad R: Hyaluronate production and removal during corneal development in the chick. Dev Biol 26:28, 1971 102. You LT, Kruse FE, Pohl J et al: Bone morphogenetic proteins and growth and differentiation factors in the
human cornea. Invest Ophthalmol Visual Sci 40:296, 1999 103. Hirsch M, Noske W, Prenant G et al: Fine structure of the developing avian corneal stroma as revealed by quick-freeze, deep-etch
electron microscopy. Exp Eye Res 69:267, 1999 104. Anseth A: Glycosaminoglycans in the developing corneal stroma. Exp Eye Res 1:116, 1961 105. Kitano S: An embryological study on the human corneal nerves. Jpn J Ophthalmol 1:48, 1957 106. Ozanics V, Rayborn M, Sagun D: Observations on the morphology of the developing primate cornea epithelium, its
innervation and anterior stroma. J Morphol 153:263, 1977 107. Whitear M: An electron microscopic study of nerves in the corneal epithelium. Experientia 13:287, 1957 108. Pouliquen Y, Faure JP, Bisson J et al: La zone fibrillaire accellulaire sous epitheliale de la cornée de
l'embryon de poulet: Ses rapports avec la formation de la membrane basale
de l'épithelium et de la membrane de Bowman. Arch Ophthalmol Paris 26:59, 1966 109. Ozanics V, Rayborn M, Sagun D: Some aspects of corneal and scleral differentiation in the primate. Exp Eye Res 22:305, 1976 110. Herrmann H: In Smelser G (ed): Structure of the Eye, p 421. San Diego: Academic
Press, 1961 111. Smelser GK, Ozanics V: The development of the trabecular meshwork in primate eyes. Am J Ophthalmol 71:366, 1971 112. Reme C, D'Epinay SL: Periods of development of the normal human chamber angle. Doc Ophthalmol 51:241, 1981 113. Hansson HA, Jerndal T: Scanning electron microscopic studies on the development of the iridocorneal
angle in human eyes. Invest Ophthalmol Vis Sci 10:252, 1971 114. Burian HM, Braley AE, Allen L: A new concept of the development of the angle of the anterior chamber of
the human eye. Arch Ophthalmol 55:439, 1956 115. Wulle KG: The development of the productive and draining system of the aqueous humor
in the human eye. Adv Ophthalmol 26:269, 1972 116. Anderson DR: The development of the trabecular meshwork and its abnormality in primary
infantile glaucoma. Trans Am Ophthalmol Soc 79:458, 1981 117. Kupfer C, Ross K: The development of outflow facility in human eyes. Invest Ophthalmol 10:513, 1971 118. Pandolfi M, Astedt B: Outflow resistance in the fetal eye. Acta Ophthalmol Copenh 49:344, 1971 119. Adamis AP, Molnar ML, Tripathi BJ et al: Neuronal-specific enolase in human corneal endothelium and posterior keratocytes. Exp Eye Res 41:665, 1985 120. Wulle KG: Electron microscopic observations of the development of Schlemm's
canal in the human eye. Trans Am Acad Ophthalmol Otolaryngol 72:765, 1968 121. Cook C, Sulik K, Wright K: In Wright KW (ed): Pediatric Ophthalmology and
Strabismus, p 3. St Louis: Mosby, 1995 122. Imaizumi M, Kuwabara T: Development of the rat iris. Invest Ophthalmol 10:733, 1971 123. Matsuo N, Smelser GK: Electron microscopic studies on the pupillary membrane: The fine structure
of the white strands of the disappearing stage of this membrane. Invest Ophthalmol 10:108, 1971 124. Ito M, Yoshioka M: Regression of the hyaloid vessels and pupillary membrane of the mouse. Anat Embryol 200: 403, 1999 125. Yamashita T, Sohal G: Development of smooth and skeletal muscle cells in the iris of the domestic
duck, chick and quail. Cell Tissue Res 244:121, 1986 126. Lai YL: The development of the sphincter muscle in the iris of the albino rat. Exp Eye Res 14:196, 1972 127. Tamura T, Smelser GK: Development of the sphincter and dilator muscles of the iris. Arch Ophthalmol 89:332, 1973 128. Lai YL: The development of the dilator muscle in the iris of the albino rat. Exp Eye Res 14:203, 1972 129. Hu F, Endo H, Alexander NJ: Morphological variations of pigment granules in eyes of the rhesus monkey. Am J Anat 136:167, 1973 130. Mund ML, Rodrigues MM, Fine BS: Light and electron microscopic observations on the pigmented layers of
the developing human eye. Am J Ophthalmol 73:167, 1972 131. Wulle KG: Frühentwicklung der Ciliarfortsatze im menschlichen Auge. Phasenkontrast
und electronenmikroskopische Untersuchungen. Z Zellforsch 71:545, 1966 132. Bard J, Ross A: The morphogenesis of the ciliary body of the avian eye. Dev Biol 92:87, 1982 133. Wulle KG: Elektronenmikroskopie der Kapillarentwick-lung in menschlichen Ziliarfortsatzen. Anat Anz 120(Suppl.):465, 1967 134. Takei Y: Development of the ciliary non-pigmented epithelium in monkey fetuses—A
light and electron microscopic study. Jpn J Ophthalmol 22:259, 1978 135. Ozanics V, Rayborn ME, Sagun D: Observations on the ultrastructure of the developing primate choroid coat. Exp Eye Res 26:25, 1978 136. Brihaye-Van Geertruyden M: Contribution l'étude des tumeurs mélaniques de l'uvée
et de leur origine. Doc Ophthalmol 17:163, 1963 137. Feeney L, Grieshaber JA, Hogan MJ. In Rohen J (ed): Eye Structure II: Symposium, p 535. Stuttgart: Schattauer Verlag, 1965 138. Heimann K: The development of the choroid in man: Choroidal vascular system. Ophthalmol Res 3:257, 1972 139. Spira AW, Hollenberg MJ: Human retinal development: Ultrastructure of the inner retinal layers. Dev Biol 31:1, 1973 140. Smelser GK, Ozanics V, Rayborn M et al: Retinal synaptogenesis in the primate. Invest Ophthalmol 13:340, 1974 141. Hollenberg MJ, Spira AW: Human retinal development: Ultrastructure of the outer retina. Am J Anat 137:357, 1973 142. Yamada E, Ishikawa T: Some Observations on the Submicroscopic Morphogenesis
of the Human Retina, pp 1–5. Stuttgart: Schattauer Verlag, 1965 143. Vrabec F: The temporal raphe of the human retina. Am J Ophthalmol 62:926, 1966 144. Blechschmidt E: Developmental movements of the human retina at the time of development
of the iris: Development of the optic ganglion as an instance of the development
of the cytoarchitecture amenable to ultramicroscopic study. Ophthalmologica 154:531, 1967 145. Fisher SK, Linberg KA: Intercellular junctions in the early human embryonic retina. J Ultrastruct Res 51:69, 1975 146. Hollenberg MJ, Spira AW: Early development of the human retina. Can J Ophthalmol 7:472, 1972 147. Breathnach AS, Wyllie LM: Ultrastructure of retinal pigment epithelium of the human fetus. J Ultrastruct Res 16:584, 1966 148. Streeten BW: Development of the human retinal pigment epithelium and the posterior segment. Arch Ophthalmol 81:383, 1969 149. Zhou G, Williams RW: Eye1 and eye2: Gene loci that modulate eye size, lens weight, and retinal
area in the mouse. Invest Ophthalmol Visual Sci 40:817, 1999 150. Bhattacharjee J, Sanyal S: Developmental origin and early differentiation of retinal Muller cells
in mice. J Anat 120:367, 1975 151. Graymore C: Metabolism of the developing retina. Exp Eye Res 3:5, 1964 152. Keefe JR, Ordy JM, Samorajski T: Prenatal development of the retina in a diurnal primate (Macaca Mulatta). Anat Rec 154:759, 1966 153. Kuwabara T, Weidman TA: Development of prenatal rat retina. Invest Ophthalmol 13:725, 1974 154. Olney JW: An electron microscopic study of synapse formation, receptor outer segment
development and other aspects of developing mouse retina. Invest Ophthalmol 7:250, 1968 155. Smelser GK, Ozanics V, Rayborn M et al: The fine structure of the retinal transient layer of Chiewitz. Invest Ophthalmol 12:504, 1973 156. Tsukahara I, Uehara M, Okuma H: Development of the retinal inner limiting membrane and Bruch's membrane. Nippon Ganka Gakkai Zasshi 76:783, 1972 157. Uga S, Smelser GK: Electron microscopic study of the development of retinal Mullerian cells. Invest Ophthalmol 12:295, 1973 158. Weidman TA: Fine structure of the developing retina. Int Ophthalmol Clin 15:65, 1975 159. Lopashov GV, Stroeva OG: In Ambercrombie M, Brachet J (eds): Advances in
Morphogenesis, p 331. San Diego: Academic Press, 1961 160. De Robertis E: Some observations on the ultrastructure and morphogenesis of photoreceptors. J Gen Physiol 43(Suppl 43):1, 1960 161. Hendrickson A, Kupfer C: The histogenesis of the fovea in the macaque monkey. Invest Ophthalmol 15:746, 1976 162. Wilmer HA, Scammon RE: Growth of the components of the human eyeball. Arch Ophthalmol 43:599, 1950 163. Ashton N: Studies on developing retinal vessels. VIII: Effect of oxygen on the retinal
vessels of the ratling. Br J Ophthalmol 45:321, 1961 164. Ashton N, Pedler C: Studies on developing vessels. IX: Reaction of endothelial cells to oxygen. Br J Ophthalmol 46:257, 1962 165. Ashton N: Oxygen and the growth and development of retinal vessels: In vivo and in
vitro studies. Am J Ophthalmol 62:412, 1966 166. Ashton N, Tripathi B, Knight G: Effect of oxygen on the developing retinal vessels of the rabbit. I: Anatomy
and development of the retinal vessels of the rabbit. Exp Eye Res 14:214, 1972 167. Ashton N, Tripathi B, Knight G: Effect of oxygen on the developing retinal vessels of othe rabbit. II. In
vivo experiments. Exp Eye Res 14:221, 1972 168. Braekevelt CR, Hollenberg MJ: Comparative electron microscopic study of development of hyaloid and retinal
capillaries in albino rats. Am J Ophthalmol 69:1032, 1970 169. Engerman RL, Meyer RK: Development of retinal vasculature in rats. Am J Ophthalmol 60:628, 1965 170. Oshima K, Ishikawa T: Effects of oxygen on developing vessels in the kitten retina. Nippon Ganka Gakkai Zasshi 79:813, 1975 171. Shakib M, De Oliveira F: Studies on developing retinal vessels. X: Formation of the basement membrane
and differentiation of intramural pericytes.Br J Ophthalmol 50:124, 1966 172. Shakib M, De Oliveira F, Henkind P: Development of retinal vessels. II: Earliest stages of vessel formation. Invest Ophthalmol 7:689, 1968 173. Tripathi B, Knight R, Ashton N: Effect of oxygen on the developing retinal vessels of the rabbit. IV. Exp Eye Res 19:449, 1974 174. Mutlu F, Leopold IH: The structure of the retinal vascular system of the human fetus eye. Arch Ophthalmol 71:531, 1964 175. Cogan DG: Development and senescence of the human retinal vasculature. Trans Ophthalmol Soc UK 83:465, 1963 176. Engerman RL: Development of macular circulation. Invest Ophthalmol 15:835, 1976 177. Henkind P, Bellhorn RW, Murphy ME et al: Development of macular vessels in monkey and cat. Br J Ophthalmol 59:703, 1975 178. Wise GN, Dollery CT, Henkind P. The Retinal Circulation. New York: Harper & Row, 1971 179. Ashton N: Retinal angiogenesis in the human embryo. Br Med Bull 26:103, 1970 180. Michaelson IC: The mode of development of the vascular system of the retina with some
observations on its significance for certain retinal diseases. Trans Ophthalmol Soc UK 68:137, 1948 181. Ashton N (ed): The William MacKenzie Centenary Symposium on the Ocular
Circulation in Health and Disease, p 7. St Louis: CV Mosby, 1969 182. Cook CS, Generoso WM, Hester D et al: RPE dysplasia with retinal duplication in a mutant mouse strain. Exp Eye Res 52:409, 1991 183. Moyer F (ed): The Retina: Morphology, Function and Clinical Characteristics, p 10. Berkeley: University of California Press, 1969 184. Tombran-Tink J, Shivaram SM, Chader GJ et al: Expression, secretion, and age-related downregulation of pigment epithelium-derived
factor, a serpin with neurotrophic activity. J Neurosci 15:4992, 1995 185. Kong Y, Nagata T: Nucleic acid synthesis in mouse retina and retinal pigment epithelium by
radioautography. Cell Molec Biol 41:171, 1995 186. Zhao S, Thornquist SC, Barnstable CJ: In vitro transdifferentiation of
embryonic rat retinal pigment epithelium. 1995 187. Robb RM: Regional changes in RPE cell density during ocular development. Invest Ophthalmol Vis Sci 26:614, 1985 188. Takei Y, Ozanics V: Origin and development of Bruch's membrane in monkey fetuses: An electron
microscopic study. Invest Ophthalmol 14:903, 1975 189. O'Rahilly R: The prenatal development of the human eye. Exp Eye Res 21:93, 1975 190. Geeraets R: An electron microscopic study of the closure of the optic fissure in the
golden hamster. Am J Anat 145:411, 1976 191. Spira AW, Price PG. In Yamada E, Mishima S (eds): Structure of the Eye, p 229. Tokyo: 1976 192. Silver J, Hughes AFW: The role of cell death during morphogenesis of the mammalian eye. J Morphol 140:159, 1973 193. Berman M, Pierro L: Lens detachment and choroid fissure closure in the embryonic mouse eye. Am Zool 9:365, 1969 194. Kuwabara T: Development of the optic nerve of the rat. Invest Ophthalmol 14:732, 1975 195. Sturrock RR: A light and electron microscopic study of proliferation and maturation
of fibrous astrocytes in the optic nerve of the human embryo. J Anat 119:223, 1975 196. Vaughn JE, Peters A: Electron microscopy of early postnatal development of fibrous astrocytes. Am J Anat 121:131, 1967 197. Hogan MJ, Alvarado JA, Weddell JE: Histology of the Human Eye. An Atlas
and Textbook. Philadelphia: WB Saunders, 1971 198. Blunt MG, Baldwin F, Wendell-Smith CP: Glyogenesis and myelination in kitten optic nerve. Z Zellsforsch 124:293, 1972 199. Vaughn J: An electron microscopic analysis of gliogenesis in rat optic nerves. Z Zellforsch 94:293, 1969 200. Rawlines FA: A quantitative electron microscopic analysis of myelination in the optic
nerve of suckling rats treated with an inhibitor of cholesterol biosynthesis. Z Zellforsch 140:9, 1973 201. Kanazawa S: Electron microscopic study of the human foetal optic nerve. Nippon Ganka Gakkai Zasshi 73:1330, 1969 202. Friede RL, Hu KH: Proximo-distal differences in myelin development in human optic fibers. Z Zellforsch 79:259, 1967 203. Balazs EA, Laurent TC, Laurent UBG: Studies on the structure of the vitreous body. VI: Biochemical changes
during development. Biol Chem 234:422, 1959 204. Balazs EA, Toth LZ, Jutheden G et al: Cytological and biochemical studies of the developing chicken vitreous. Exp Eye Res 4:237, 1965 205. Balazs EA: Fine structure of developing vitreous. Int Ophthalmol Clin 15:53, 1975 206. Balazs EA, Toth LZ, Ozanics V: Cytological studies on the developing vitreous as related to the hyaloid
vessel system. Graefes Arch Klin Exp Ophthalmol 213:71, 1980 207. Balazs EA, Bloom GD, Ozanics V: The fine structure of the hyaloid arteriole in bovine vitreous. Exp Eye Res 31:129, 1980 208. Falbe-Hansen I, Ehlers N, Degn JK: Development of the human foetal vitreous body. 1. Biochemical changes. Acta Ophthalmol 47:39, 1969 209. Jack RL: Ultrastructural aspects of hyaloid vessel development. Arch Ophthalmol 87:427, 1972 210. Jack RL: Ultrastructure of the hyaloid vascular system. Arch Ophthalmol 87:555, 1972 211. Hamming NA, Apple DJ, Gieser DK et al: Ultrastructure of the hyaloid vasculature in primates. Invest Ophthalmol Vis Sci 16:408, 1977 212. Mutlu F, Leopold IH: The structure of the fetal hyaloid system and tunica vasculosa lentis. Arch Ophthalmol 71:102, 1964 213. Porte A, Brini A, Stoeckel ME: In Rohen J (ed): The Structure of the Eye
II, Symposium, p 515. Stuttgart: Schattauer Verlag, 1965 214. Ozeki H, Shirai S, Ikeda K et al: Critical period for retinoic acid-induced developmental abnormalities of
the vitreous in mouse fetuses. Exp Eye Res 68:223, 1999 215. Bremer FM, Rasquin F: Histochemical localization of hyaluronic acid in vitreous during embryonic
development. Invest Ophthalmol Visual Sci 39:2466, 1998 216. Sang DN: Embryology of the vitreous. Congenital and developmental abnormalities. Bull Soc Belge Ophtalmol 223:11, 1987 217. Jack RL: Regression of the hyaloid vascular system: An ultrastructural analysis. Am J Ophthalmol 74:261, 1972 218. Stades F: Persistent hyperplastic tunica vasculosa lentis and persistent hyperplastic
primary vitreous (PHTVL/PHPV) in 90 closely related Doberman Pinschers: Clinical
aspects. J Am Anim Hosp Assoc 16:739, 1980 219. van der Linde-Sipman J, Stades F, de Wolff-Rouendaal D: Persistent hyperplastic tunica vasculosa lentis and persistent hyperplastic
primary vitreous in the Doberman Pinscher: Pathological aspects. J Am Anim Hosp Assoc 19:791, 1983 220. Takei Y: Electron microscopic studies on zonule. Jpn J Ophthalmol 19:376, 1975 221. Okita M: Embryological and structural studies on the chicken zonule (1): Embryonic
development. Nippon Ganka Gakkai Zasshi 75:432, 1971 222. Andersen H, Ehlers N, Matthiessen ME: Histochemistry and development of the human eyelids. Acta Ophthalmol 43:642, 1965 223. Andersen H, Ehlers N, Matthiessen ME et al: Histochemistry and development of the human eyelids. II. Acta Ophthalmol 45:288, 1967 224. Makino K: On the distribution and growth of nerves in the eyelid of human embryo. Igaku Kenkyu 30:124, 1960 225. Gilbert PW (ed): The origin and development of the human extrinsic ocular muscles. Carnegie Inst Publication, 246:59, 1957 226. Sevel D: Reappraisal of the origin of human extraocular muscles. Ophthalmology 88:1330, 1981 227. Sevel D: The origins and insertions of the extraocular muscles: Development, histologic
features, and clinical significance. Trans Am Ophthalmol Soc 84:488, 1986 228. Cassady JV. In Veirs E (ed): The Lacrimal System Clinical Application, p 20. Philadelphia: Grune & Stratton, 1955 | 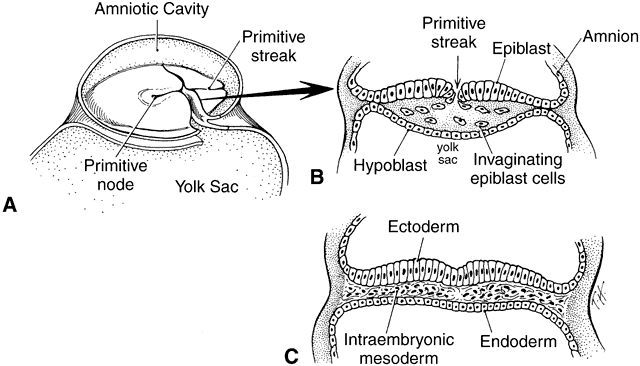
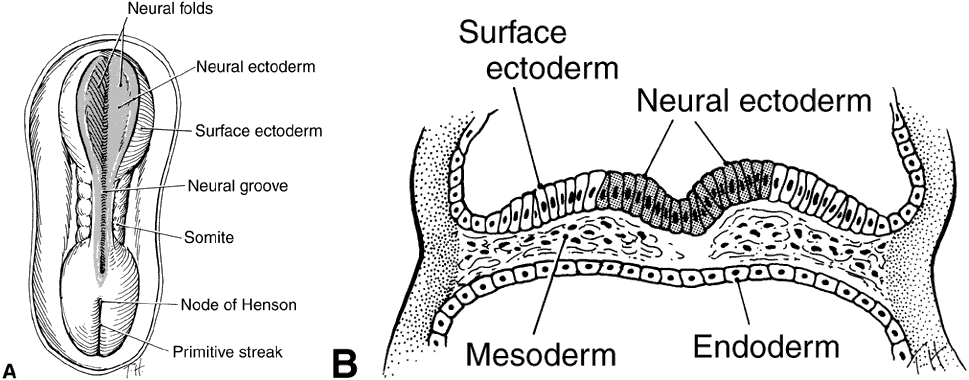
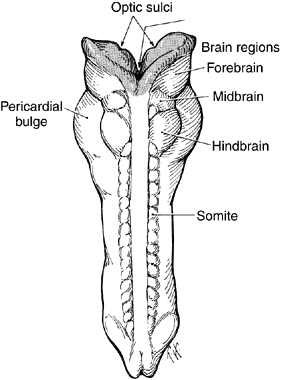
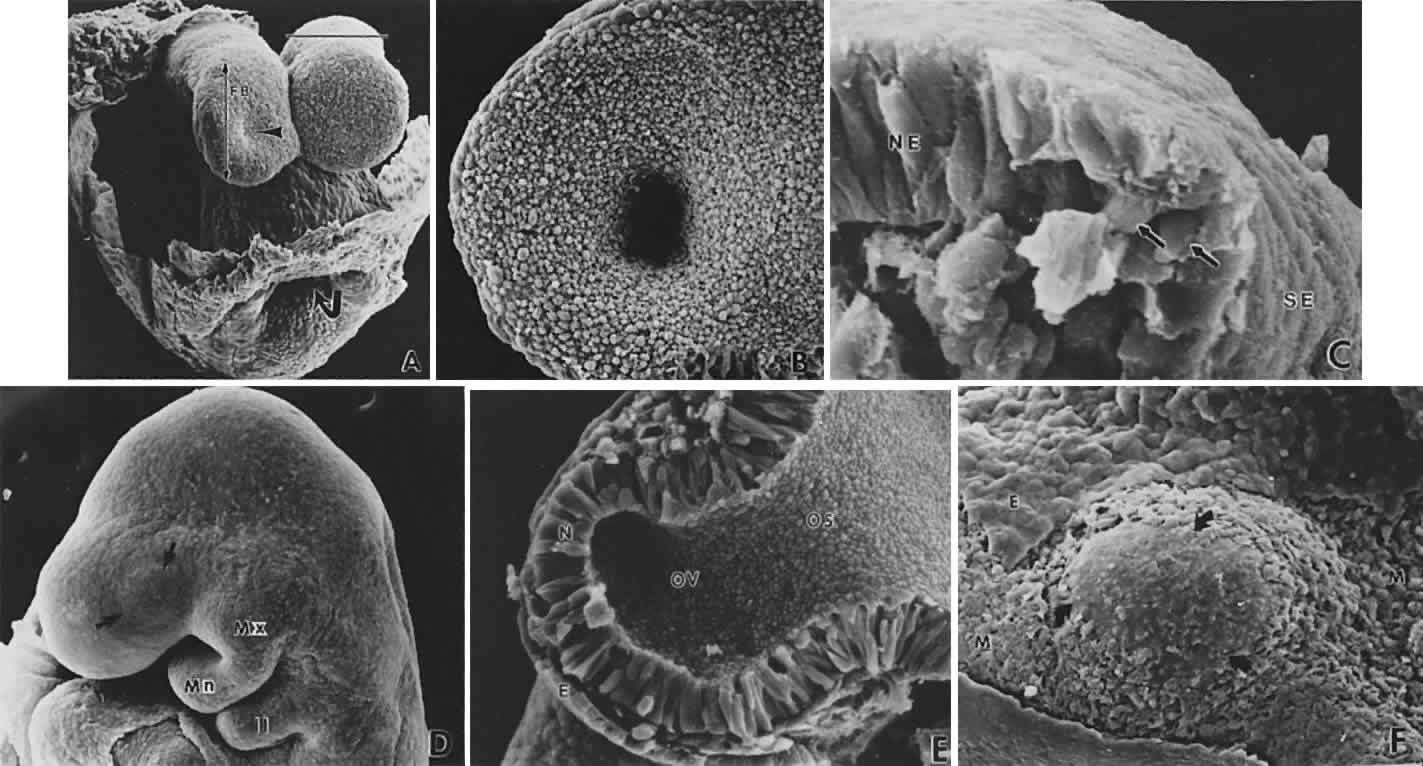
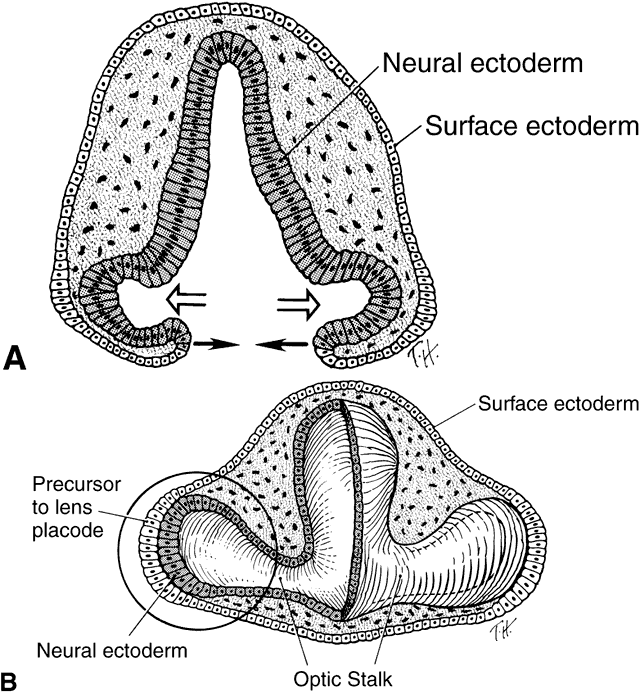
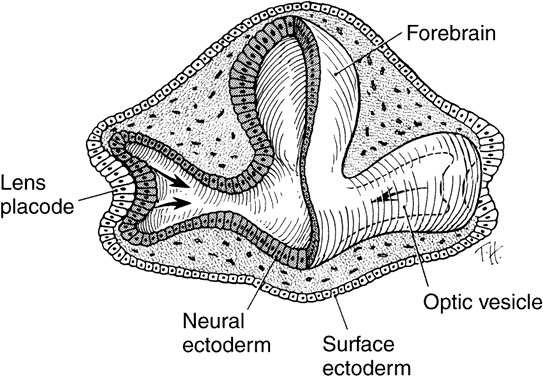
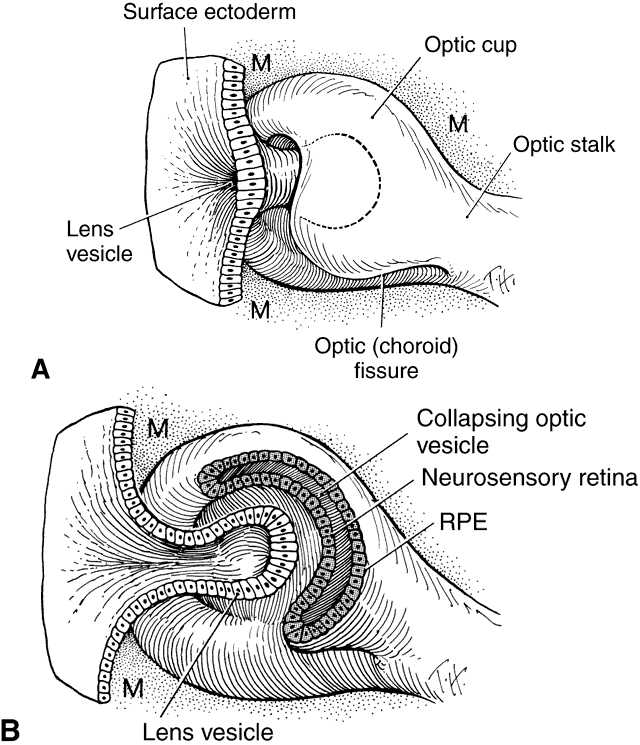
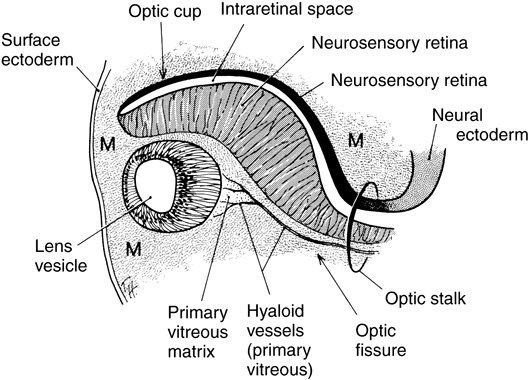
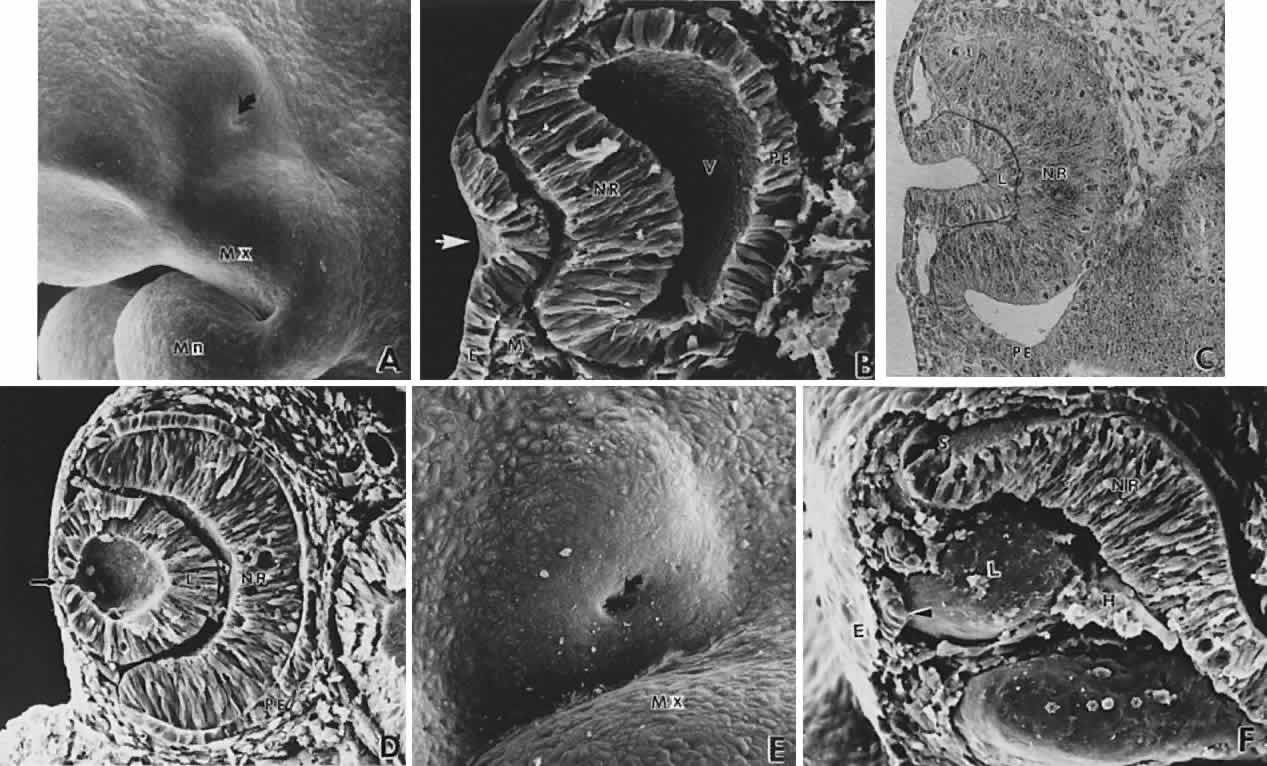
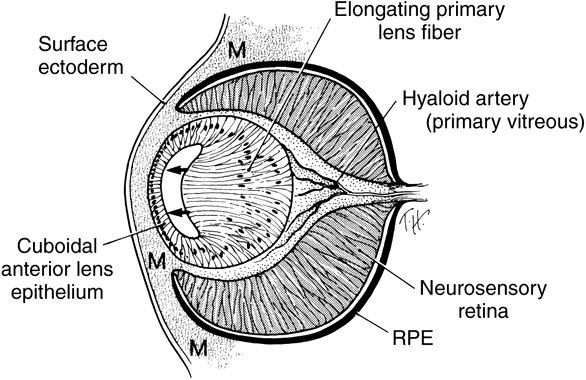
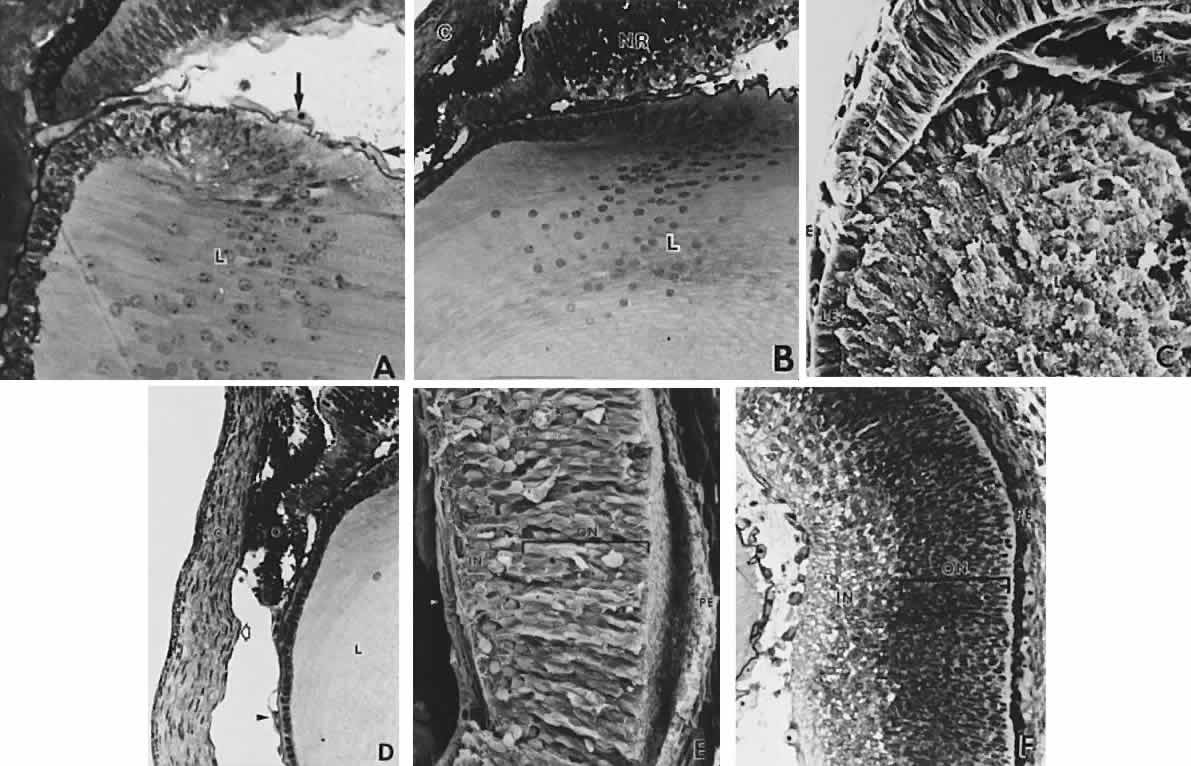
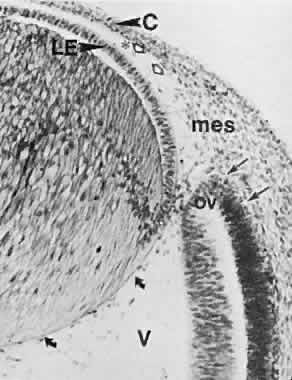
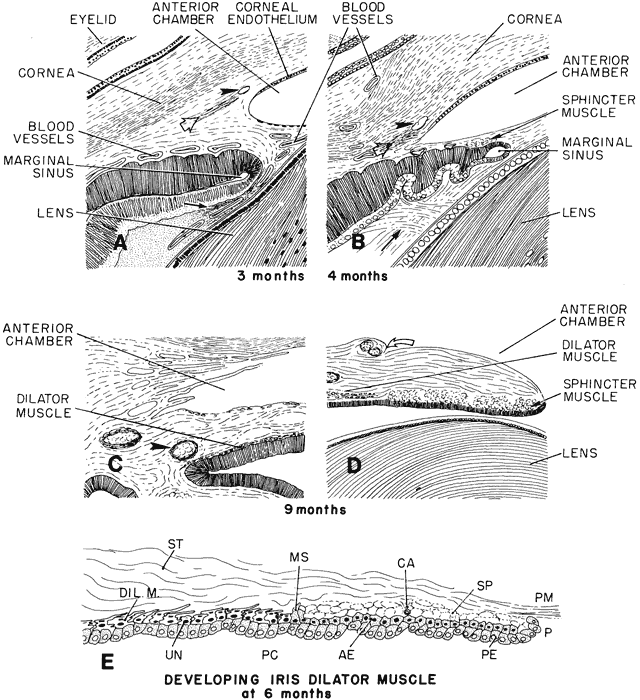
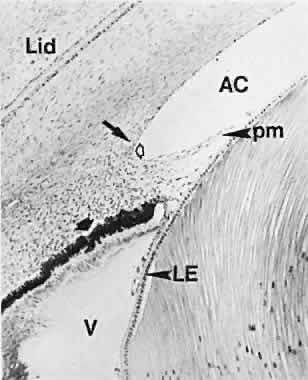
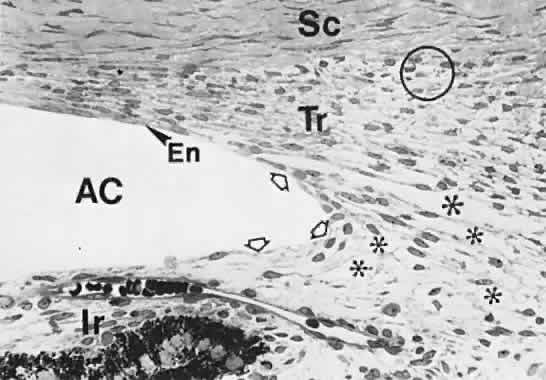
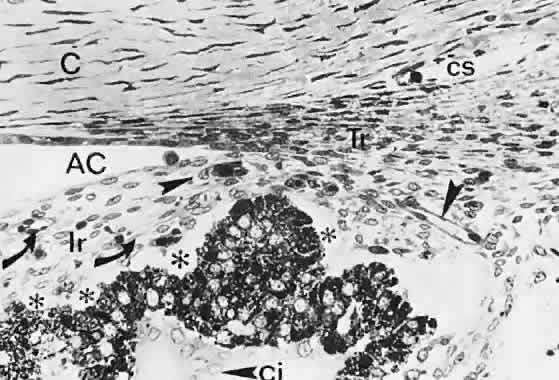
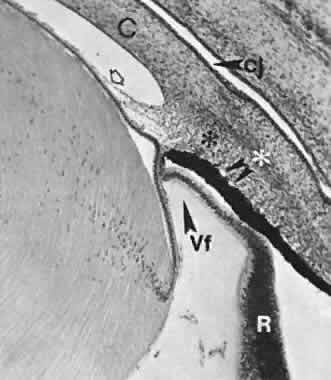
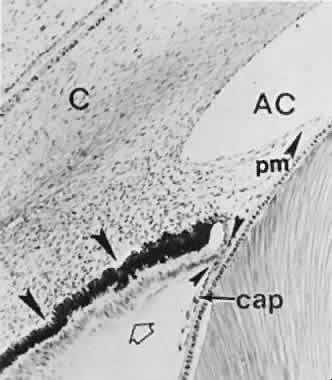
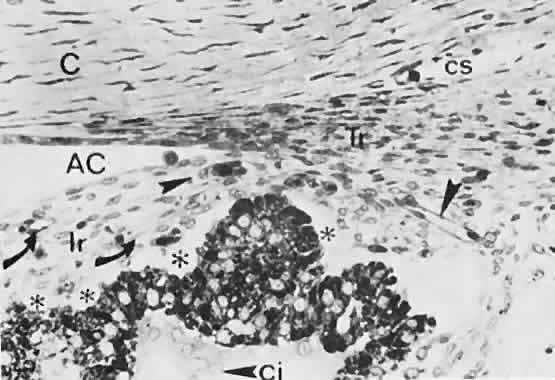
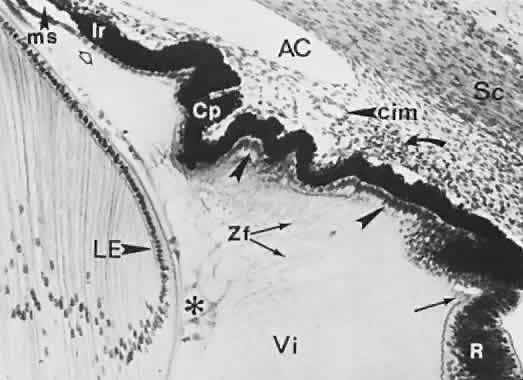

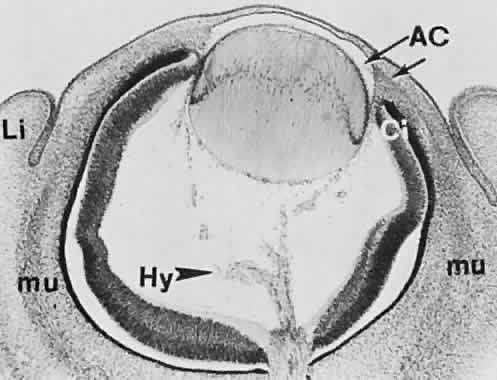
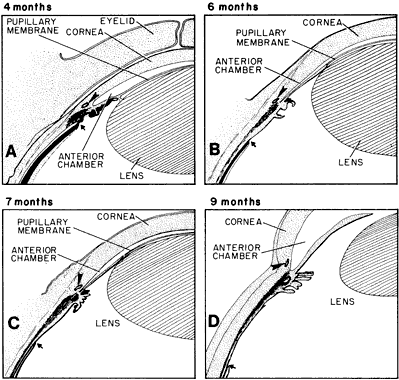
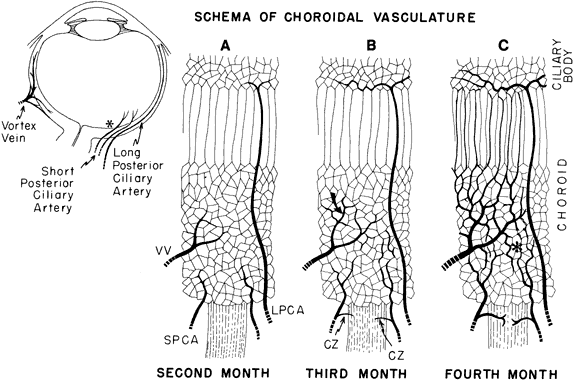
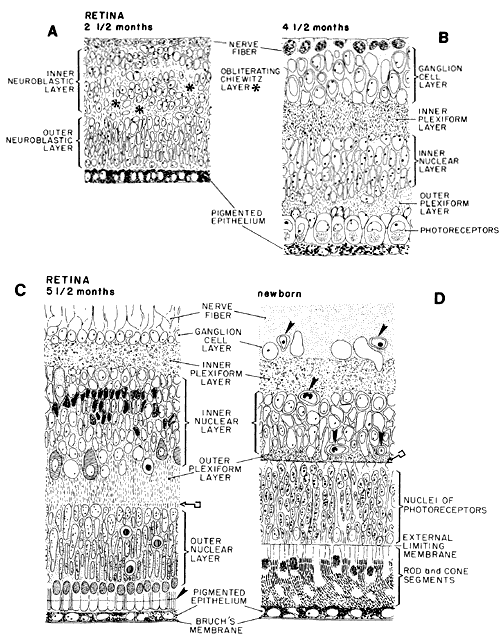
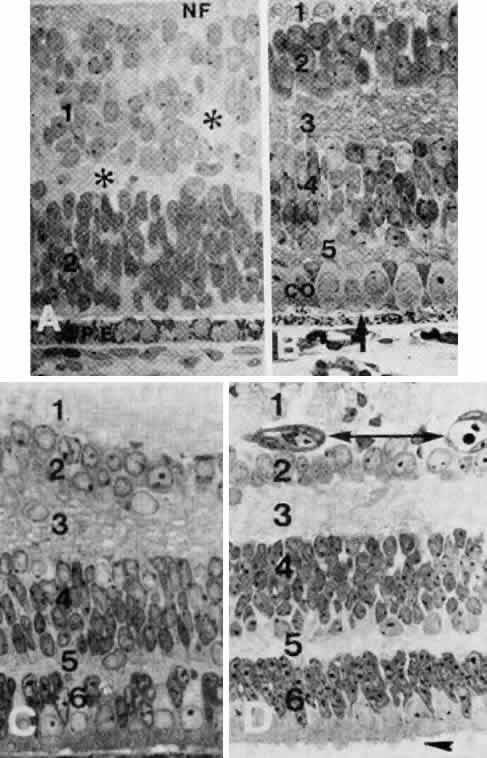
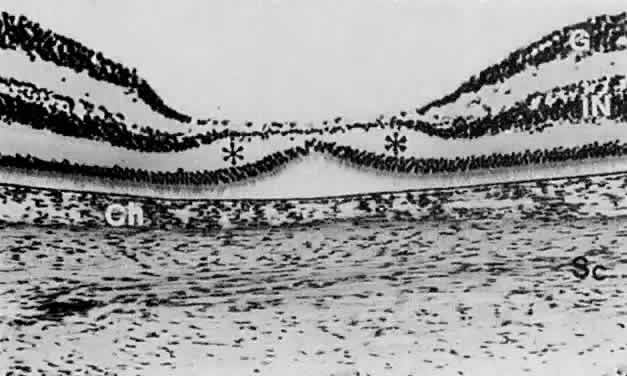
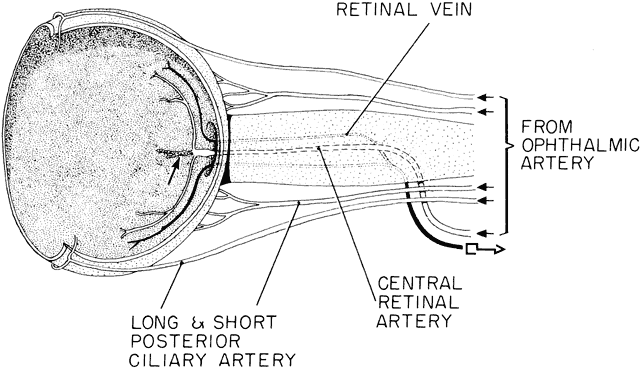
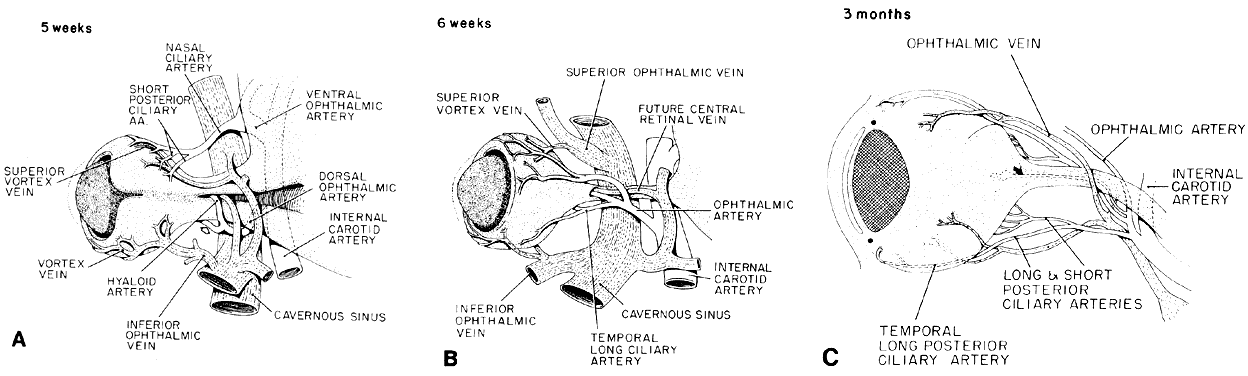
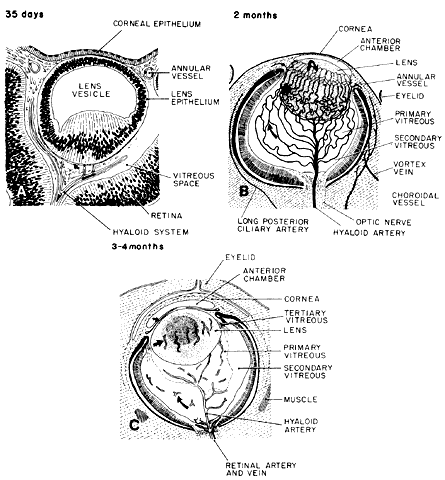
 geln and Saugern. Acta Anat 76:381, 1970
geln and Saugern. Acta Anat 76:381, 1970