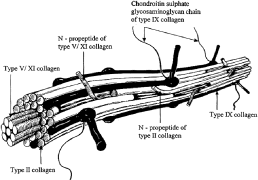
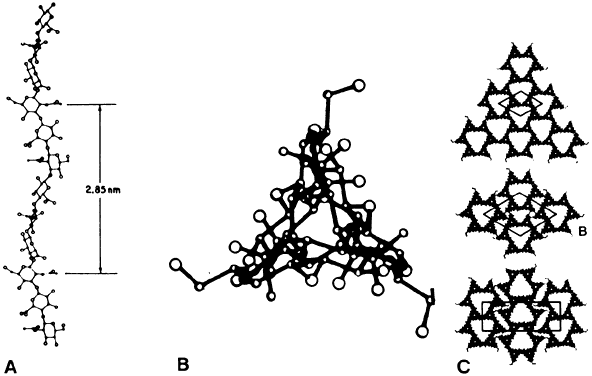
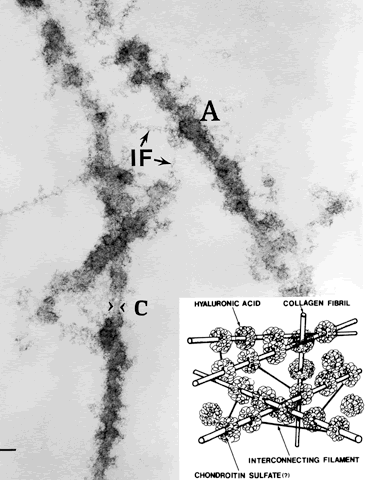
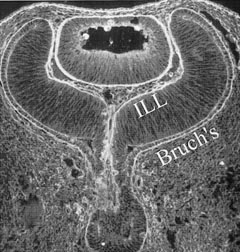
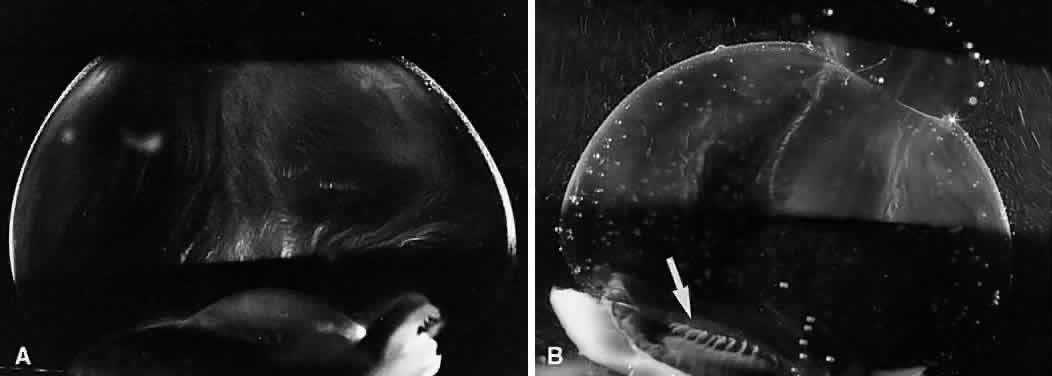
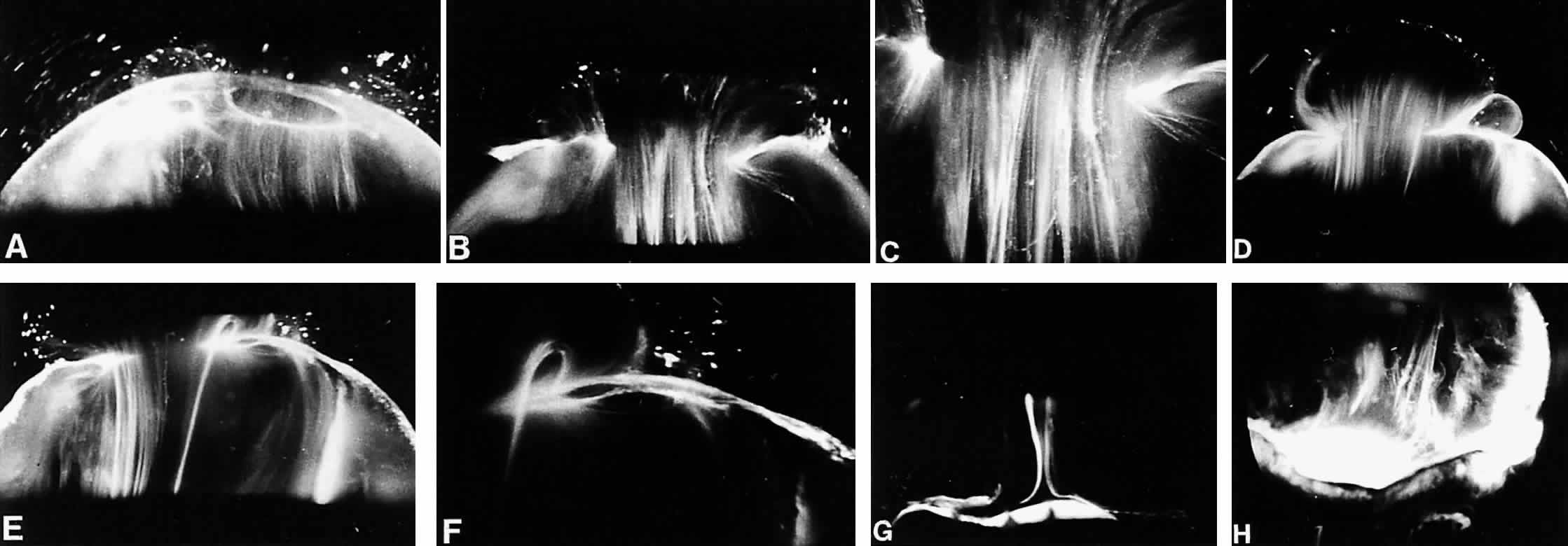
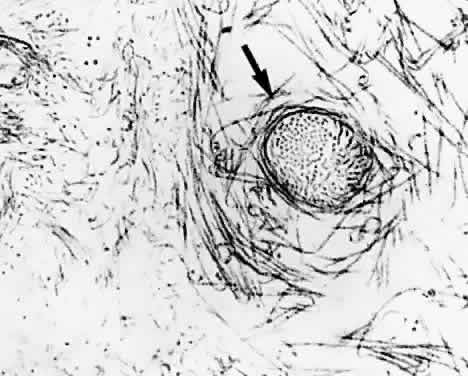
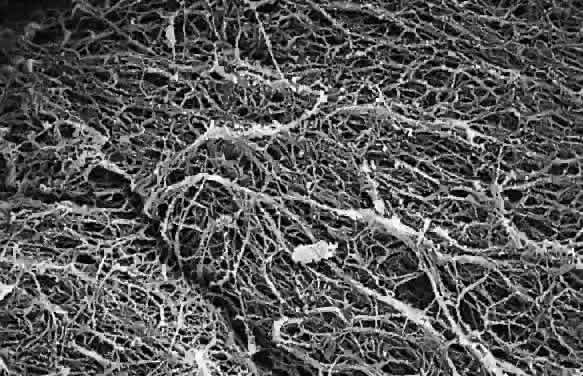
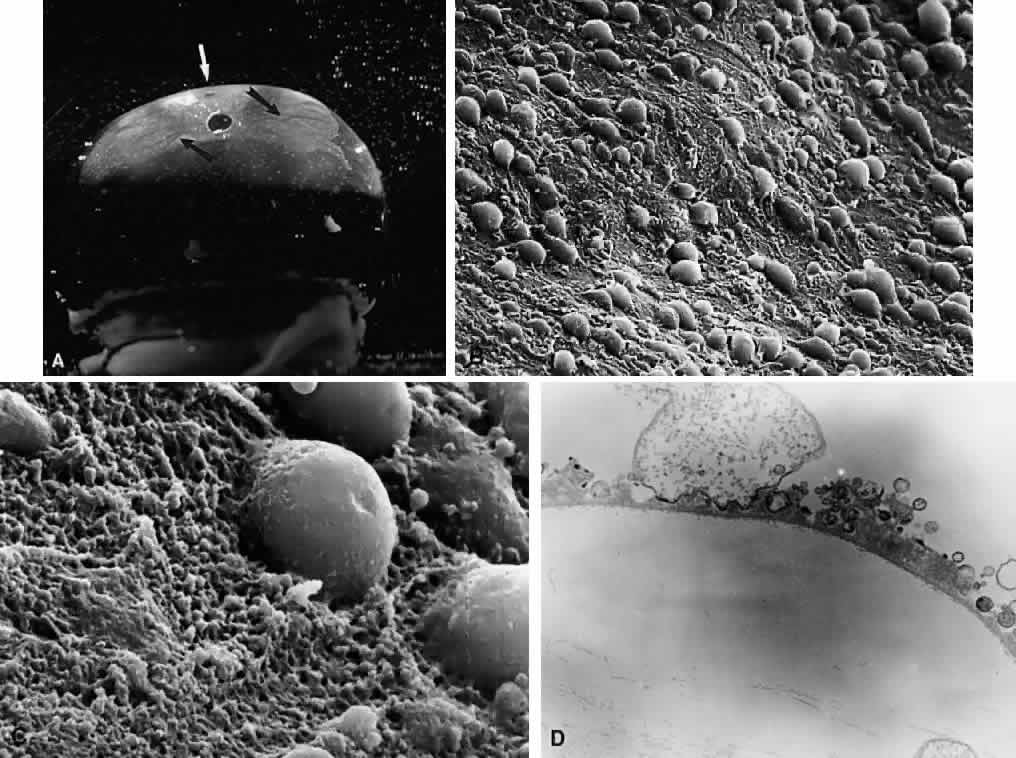
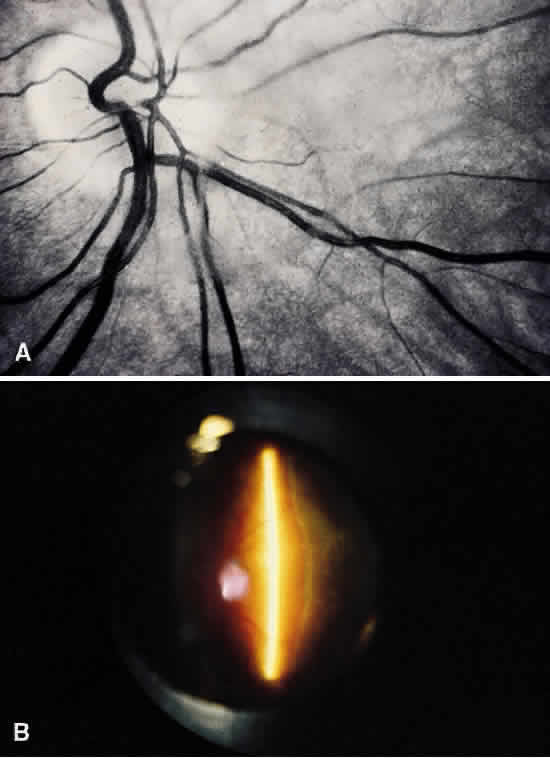
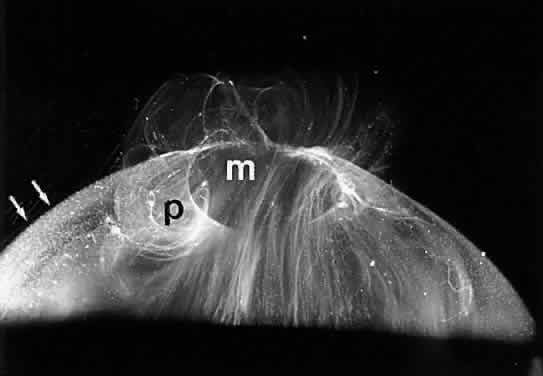
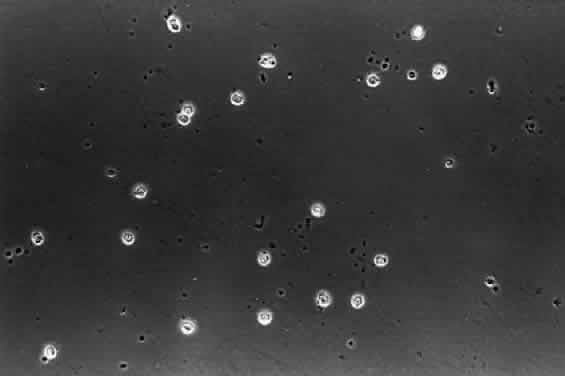
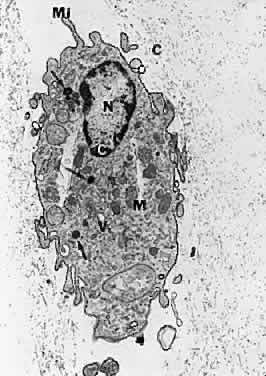
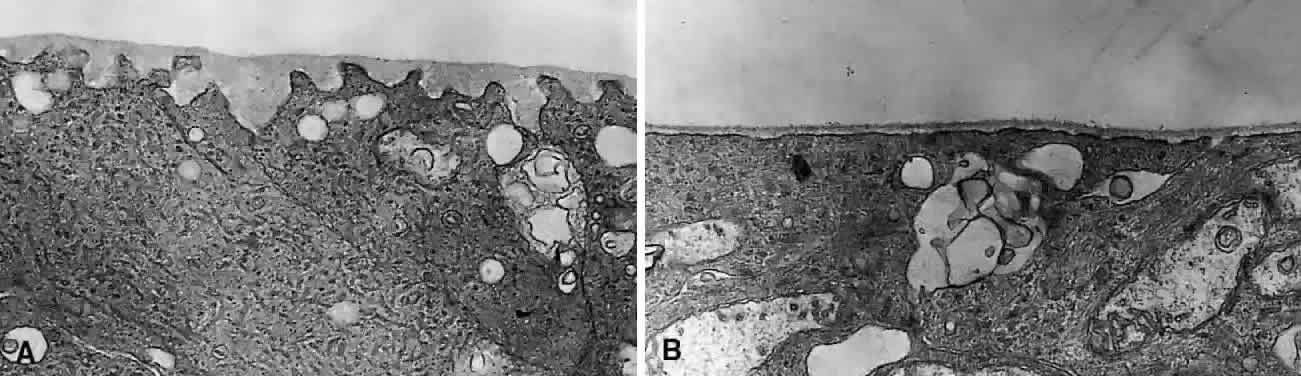
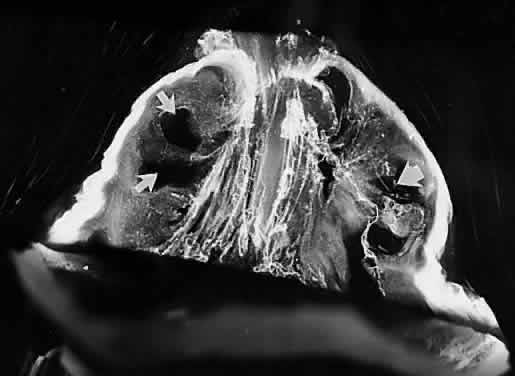
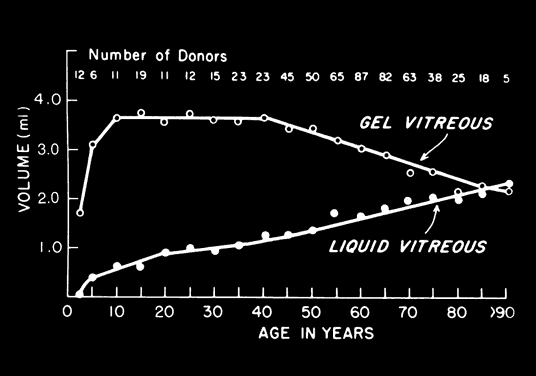
1. Sebag J. Classifying posterior vitreous detachment–a new way to look at the
invisible. Br J Ophthalmol 81:521, 1997 2. Baurmann M. Untersuchungen Uber die Sturktur des Glaskorpers bei Saugetieren. Albrecht Von Graefes Arch Ophthalmol 110:352, 1922 3. Redslob E. Le Corps Vitre. Societe Francaise d'Ophtalmologie Monogr, Paris: Masson, 1932, 174 4. Gullstrand A. Die Nernspaltlampe in der Ophthalmologischen Praxis. Klin Monatsbl Augenheilkd 50:48, 1912 5. Eisner G. Biomicroscopy of the Peripheral Fundus. New York: Springer-Verlag, 1973 6. Worst JGF. Cisternal systems of the fully developed vitreous body in the young adult. Trans Ophthalmol Soc UK 97:550, 1977 7. Sebag J, Balazs EA. Human vitreous fibres and vitreo-retinal disease. Trans Ophthalmol Soc UK 104:123, 1985 8. Sebag J, Balazs EA. Morphology and ultrastructure of human vitreous fibers. Invest Ophthalmol Vis Sci 30:187, 1989 9. Kishi S, Shimizu K. Posterior precortical vitreous pocket. Arch Ophthalmol 108:979, 1990 10. Sebag J. Letter to the editor. Arch Ophthalmol 109:1059, 1991 11. Kakehashi A. Age related changes in the premacular vitreous cortex. Invest Ophthalmol Vis Sci 37:2253, 1996 12. Sebag J. The Vitreous-Structure, Function, and Pathobiology. New York: Springer-Verlag, 1989 13. Sebag J. Anomalous posterior vitreous detachment—A unifying concept in vitreo-retinal
disease. Graef Arch Clin Exp Ophthalmol 242:690-698, 2004 14. Sebag J. Surgical anatomy of vitreous and the vitreo-retinal interface. In Tasman W, Jaeger EA, eds. Duane's Clinical Ophthalmology, Vol. 6. Philadelphia: JB Lippincott, 1994:1 15. Sebag J. Pharmacologic vitreolysis. Retina 18:1, 1998 16. Sebag J. Is pharmacologic vitreolysis brewing? Retina 22:1, 2002 17. Gloor BP. The vitreous. In Moses RA, ed. Adler's Physiology of the Eye. St. Louis: Mosby 1975:246–267 18. Gross J. Comparative biochemistry of collagen. In Florkin M, Mason HS, eds. Comparative Biochemistry. New York: Academic Press 1963:307–347 19. Swann DA, Constable IJ, Harper E. Vitreous structure. III. Composition of bovine vitreous collagen. Invest Ophthalmol 11:735, 1972 20. Swann DA, Caulfield JB, Broadhurst JB. The altered fibrous form of vitreous collagen following solubilization
with pepsin. Biochem Biophys Acta 427:365, 1976 21. Stuart JM, Cremer MA, Dixit SN et al Collagen-induced arthritis in rats: Comparison of vitreous and cartilage-derived
collagens. Arthritis Rheum 22:347, 1979 22. Swann DA. Chemistry and biology of vitreous body. Int Rev Exp Pathol 22:1, 1980 23. Schmut O, Mallinger R, Paschke E. Studies on a distinct fraction of bovine vitreous body collagen. Graefes Arch Clin Exp Ophthalmol 221:286, 1984 24. Linsenmeyer TF, Gibney E, Little CD. Type II collagen in the early embryonic chick cornea and vitreous-immunoradiochemical
evidence. Exp Eye Res 34:371, 1982 25. Snowden JM. The stabilization of in vivo assembled collagen fibrils by proteoglycans/glycosaminoglycans. Biochem Biophys Acta 703:21, 1982 26. Swann DA, Sotman SS. The chemical composition of bovine vitreous humour collagen fibres. Biochem J 185:545, 1980 27. Liang JN, Chakrabarti B. Stereoscopic studies on pepsin-solubilized vitreous and cartilage collagens. Curr Eye Res 1:175, 1981 28. Von der Mark K. Localization of collagen types in tissues. Int Rev Connect Tissue Res 9:265, 1981 29. Ayad S, Weiss JB. A new look at vitreous humour collagen. Biochem J 218:835, 1984 30. Van der Rest M. Type IX collagen. In Structure and Function of Collagen Types. New York: Academic Press 1987:195–221 31. Eyre DR, Apon S, Wu JJ et al Collagen type IX: Evidence for covalent linkages to type II collagen in
cartilage. Fed Eur Biochem Soc 220, 2:337, 1987 32. Wright DW, Mayne R. Vitreous humor of chicken contains two fibrillar systems: An analysis of
their structure. J Ultrastr Mol Struct Res 100:224, 1988 33. Hong BS, Davison DF. Identification of type II procollagen in rabbit vitreous. Ophthalm Res 17:162, 1985 34. Seery CM, Davison PF. Collagens of the bovine vitreous. Invest Ophthalmol Vis Sci 32:1540, 1990 35. Bishop PN, Crossman MV, McLeod D. Extraction and characterization of the tissue forms of collagen type II
and IX from bovine vitreous. Biochem J 299:497, 1994 36. Reardon A, Sandell L, Jones CJP, et al Localization of pN-type IIA procollagen on adult bovine vitreous collagen
fibrils. Matrix Biology 19:169, 2000 37. Zhu Y, Oganesian A, Keene DR, et al Type IIA procollagen containing the cysteine-rich amino propeptide is deposited
in the extracellular matrix of prechondrogenic tissue and binds
to TGFbeta-1 and BMP-2. J Cell Biol 144:1069, 1999 38. Bishop PN, Reardon AJ, McLeod D, et al Identification of alternatively spliced variants of type II procollagen
in vitreous. Biochem Biophys Res Commun 203:289, 1994 39. Brewton RG, Ouspenskaia MV, Van der Rest M, et al Cloning of the chicken alpha 3 (IX) collagen chain completes
the primary structure of type IX collagen. Eur J Biochem 205:443, 1992 40. Asakura A. Histochemistry of hyaluronic acid of the bovine vitreous body as studied
by electron microscopy. Acta Soc Ophthalmol Jpn 89:179, 1985 41. Scott JE. The chemical morphology of the vitreous. Eye 6:553, 1992 42. Zhidkova NI, Justice S, Mayne R. Alternative in RNA processing occurs in the variable region of the pro-peptide. J Biol Chem 270:9485, 1995 43. Knupp C, Munro PM, Luther PK, et al Structure of abnormal molecular assemblies (collagen VI) associated
with human full thickness macular holes. J Struct Biol 129:38, 2000 44. Toledo DMS, Dietrich CP. Tissue specific distribution of sulfated mucopolysaccharides in mammals. Biochem Biophys Acta 498:114, 1977 45. Balazs EA.The vitreous. In Davson H, ed. The Eye. Vol. la. London: Academic Press, 1984:533–589 46. Breen M, Bizzell JW, Weinstein MG. A galactosamine-containing proteoglycan in human vitreous. Exp Eye Res 24:409, 1977 47. Allen WS, Otterbein EC, Wardi AH. Isolation and characterization of the sulfated glycosaminoglycans of the
vitreous body. Biochem Biophys Acta 498:167, 1977 48. Kamei A, Totani A. Isolation and characterization of minor glycosaminoglycans in the rabbit
vitreous body. Biochem Biophys Res Comm 109:881, 1982 49. Meyer K, Palmer JW. The polysaccharide of the vitreous humor. J Biol Chem 107:629, 1934 50. Laurent UBG, Fraser JRE. Turnover of hyaluronate in aqueous humor and vitreous body of the rabbit. Exp Eye Res 36:493, 1983 51. Sheehan JK, Atkins EDT, Nieduszynski IA. X-Ray diffraction studies on the connective tissue polysaccharides. Two
dimensional packing scheme for threefold hyaluronic chains. J Mol Biol 91:153, 1975 52. Atkins EDT, Phelps CF, Sheehan JK. The conformation of the mucopolysaccharides—Hyaluronates. Biochem J 128:1255, 1972 53. Chakrabarti B, Park JW. Glycosaminoglycans structure and interaction. CRC Crit Rev Biochem 8:225, 1980 54. Brewton RG, Mayne R. Mammalian vitreous humor contains networks of hyaluronan molecules. Exp Eye Res 198:237, 1992 55. Reardon A, Heinegard D, McLeod D, et al The large chondroitin sulphate proteoglycans versican in mammalian vitreous. Matrix Biol 17:325, 1998 56. Comper WD, Laurent TC. Physiological functions of connective tissue polysaccharides. Physiol Rev 58:255, 1978 57. Sebag J. Diabetic vitreopathy. Ophthalmology 103:205, 1996 58. Laurent UBG, Granath KA. The molecular weight of hyaluronate in the aqueous humour and vitreous
body of rabbit and cattle eyes. Exp Eye Res 36:481, 1983 59. Laurent TC, Ryan M, Pietruszkiewiecz A. Fractionation of hyaluronic acid: The polydispersity of hyaluronic acid
from the vitreous body. Biochem Biophys Acta 42:476, 1960 60. Berman ER. Studies on mucopolysaccharides in ocular tissues. 1. Distribution and localization
of various molecular species of hyaluronic acid in the bovine
vitreous body. Exp Eye Res 2:1, 1963 61. Ogston AG, Phelps CF. The partition of solutes between buffer solutions containing hyaluronic
acid. Biochem J 78:827, 1961 62. Bishop PN. Structural macromolecules and supramolecular organization of the vitreous
gel. Prog Retinal Eye Res 19:323, 2000 63. Theocaris AD, Papageorgakopoulou N, Feretis E, et al Occurrence and structural characterization of versican-like proteoglycan
in human vitreous. Biochimie 84:1237, 2002 64. Allen WS, Ottenbein E, Wardi AH. Isolation and characterization of the sulphated glycosaminoglycans of the
vitreous body. Biochim Biophys Acta 498:167, 1977 65. Tsen G, Halfter W, Kroger S, et al Agrin is a heparan sulphate proteoglycans. J Biol Chem 270:3392, 1995 66. Kroger S. Differential distribution of agrin isoforms in the developing and adult
avian retina. Mol Cell Neurosci 10:149, 1997 67. Reardon AJ, LeGoff M, Briggs MD, et al Identification in vitreous and molecular cloning of opticin, a novel member
of the family of leucine-rich repeat proteins of the extracellular
matrix. J Biol Chem 275:2123, 2000 68. LeGoff MM, Hindson VJ, Jowitt TA, et al Characterization of opticin and evidence of stable dimerization in solution. J Biol Chem 278:45280, 2003 69. Ramesh S, Bonshek RE, Bishop PN. Immunolocalisation of opticin in the human eye. Br J Ophthalmol 88:697, 2004 70. Sebag J. Ageing of the vitreous. Eye 1:254-262, 1987 71. Mayne R, Ren Z-X, Liu J, Cook T, et al VIT1—The second member of a new branch of the von willebrand A domain
superfamily. Biochem Soc Trans 27:832, 1999 72. Balazs EA. Functional anatomy of the vitreus. In Duane TD, Jaeger EA, eds. Biomedical Foundation of Ophthalmology, Vol. 1. Philadelphia: Harper & Row, 1984:14 73. Haddad A, Laicine EM, Almeida JC, et al Partial characterization, origin and turnover of glycoproteins of the rabbit
vitreous body. Exp Eye Res 51:139, 1990 74. Haddad A, Laicine EM. Studies on the origin of the glycoproteins of the rabbit vitreous body
using a protein synthesis inhibitor and radioactive fucose and amino acids. Ger J Ophthalmol 2:127, 1993 75. Rhodes RH, Mandelbaum SH, Minckler DS, et al Tritiated fucose incorporation in the vitreous body, lens, and zonules
of the pigmented rabbit. Exp Eye Res 39:373, 1984 76. Hageman GS, Johnson LV. Lectin binding glycoporteins in the vertebrate vitreous body and inner
limiting membrane–tissue localization and biochemical characterization. J Cell Biol 99:179a, 1984 77. Nguyen BO, Fife RS. Vitreous contains a cartilage-related protein. Exp Eye Res 43:375, 1986 78. Bishop PN, Takanosu M, LeGoff M, et al The role of the posterior ciliary body in the biosynthesis of vitreous
humour. Eye 16:454, 2002 79. McGahan MC. Ascorbic acid levels in aqueous and vitreous humors of the rabbit-effects
of inflammation and cerruloplasmin. Exp Eye Res 41:291, 1985 80. Ringvold A. Aqueous humor and ultraviolet radiation. Acta Ophthalmol 58:69, 1980 81. Balazs EA. Studies on structure of vitreous body—Absorption of ultraviolet light. Am J Ophthalmol 38:21, 1954 82. Ueno N, Sebag J, Hirokawa H, et al Effects of visible-light irradiation on vitreous structure in the presence
of a photosensitizer. Exp Eye Res 44:863, 1987 83. Williams RN, Patterson CA, Eakins KE, et al Ascorbic acid inhibits the acitivity of polymorphonuclear leukocytes in
inflamed ocular tissue. Exp Eye Res 39:261, 1984 84. Mc Gahan MC, Fleisher LN. Antioxidant activity of aqueous and vitreous humor from the inflamed rabbit
eye. Curr Eye Res 5:641, 1986 85. Mayne R. The eye. In Connective Tissue and Its Heritable Disorders. Wiley-Liss, Inc., 2001:131–141 86. Jackson DS. Chondroitin sulphuric acid as a factor in the stability of tendon. Biochem J 54:638, 1953 87. Gelman RA, Blackwell J, Kefalides NA, et al Thermal stability of basement membrane collagen. Biochem Biophys Acta 427:492, 1976 88. Snowden JM. The stabilization of in vivo assembled collagen fibrils by proteoglycans/glycosaminoglycans. Biochem Biophys Acta 703:21, 1982 89. Tokita M, Fujiya Y, Hikichi K. Dynamic viscoelasticity of bovine vitreous body. Biorheology 21:751, 1984 90. Mathews MB. The interaction of collagen and acid mucopolysaccharides: A model for connective
tissue. Biochem J 96:710, 1965 91. Podrazky V, Stevens FS, Jackson DS, et al Interaction of tropocollagen with protein-polysaccharide complexes. An
analysis of the ionic groups responsible for interaction. Biochem Biophys Acta 229:690, 1971 92. Stitt AW, Moore JE, Sharkey JA, et al Advanced glycation end products in vitreous: Structural and functional
implications for diabetic vitreopathy. Invest Ophthalmol Vis Sci 39:2517, 1998 93. Sebag J. Abnormalities of human vitreous structure in diabetes. Graef Arch Clin Exp Ophthalmol 231:257-260, 1993 94. Sebag J. Age-related changes in human vitreous structure. Graefes Arch Clin Exp Ophthalmol 225:89, 1987 95. Sebag J. Aging of the vitreous. Eye 1:254, 1987 96. Sebag J, The vitreous. In: Hart WM, ed: Adler's physiology of the eye. St. Louis: Mosby, 1992, 268 97. Hong BS, Davison DF. Identification of type II procollagen in rabbit vitreous. Ophthalmic Res 17:162, 1985 98. Tokita M, Fujiya Y, Hikichi K. Dynamic viscoelasticity of bovine vitreous body. Biorheology 21:751, 1984 99. Swann DA, Constable IJ, Caulfield JB. Vitreous structure IV. Chemical composition of the insoluble residual protein
fraction from the rabbit vitreous. Invest Ophthalmol 14:613, 1975 100. Delpech B, Halavent C. Characterization and purification from human brain of a hyaluronic acid-binding
glycoprotein, hyaluronectin. J Neurochem 36:855, 1981 101. Hardingham TE. The role of link-protein in the structure of cartilage proteoglycan aggregates. Biochem J 177:237, 1979 102. Scott JE. Proteoglycan-collagen interactions and corneal ultrastructure. Biochem Soc Trans 19:1991, 1992 103. Scott JE, Chen Y, Brass A. Secondary and tertiary structures involving chondroitin and chondroitin
sulphate in solution, investigated by rotary shadowing electron microscopy
and computer simulation. Eur J Biochem 209:675, 1992 104. Mayne R, Brewton RG, Ren Z-H. Vitreous body and zonular apparatus. In Harding JJ, ed. Biochemistry of the Eye. London: Chapman and Hall, 1997:135–143 105. Bishop PN, McLeod D, Reardon A. The role of glycosaminoglycans in the structural organization of mammalian
vitreous. Invest Ophthalmol Vis Sci 40:2173, 1999 106. Armand G, Balazs EA. Physical and chemical characterization of icthyosanpolysaccharides of fish
eyes. Proc Intl Soc Eye Res 1:68, 1980 107. Gherezghiher T, Koss MC, Nordquist RE, et al Analysis of vitreous and aqueous levels of hyaluronic acid-application
of high performance liquid chromatography. Exp Eye Res 45:347, 1987 108. Denlinger JL, Eisner G, Balazs EA. Age-related changes in the vitreous and lens of rhesus monkeys (macaca
mulatta). Exp Eye Res 31:67, 1980 109. Balazs EA. Physiology of the vitreous body in retinal surgery with special emphasis
on re-operation. In Schepens CL, ed. Proceedings of the 11th Conference of the Retina Foundation. St. Louis: CV Mosby, 1960:29–48 110. Snowden JM, Swann DA. Vitreous structure. V. The morphology and thermal stability of vitreous
collagen fibers and comparison to articular cartilage (type II) collagen. Invest Ophthalmol Vis Sci 19:610, 1980 111. Hogan MJ, Alvarado JA, Weddel JE. Histology of the Human Eye: An Atlas and Textbook. Philadelphia: WB Saunders, 1971 112. Chakrabarti B, Hultsch E. Owl monkey vitreous–a novel model for hyaluronic acid structural
studies. Biochem Biophys Res Commun 71:1189, 1976 113. Sebag J, Hageman GS. Interfaces. Eur J Ophthalmol 10:1-3, 2000 114. Mann I. The vitreous and suspensory ligament of the lens. In The Development of the Human Eye. New York: Grune & Stratton, 1964:150 115. Jack RL. Regression of the hyaloid artery system: An ultrastructural analysis. Am J Ophthalmol 74:261, 1972 116. Balazs EA. Fine structure of the developing vitreous. Int Ophthalmol Clin 15:53, 1975 117. Gloor BP. Zur entwicklung des glaskorpers und der Zonula. III. Henkunft, Lebenszeit
und ersatz der glaskorpezellen beim kaninchen. Graefes Arch Clin Exp Ophthalmol 187:21, 1973 118. Balazs EA, Toth LZ, Ozanics V. Cytological studies on the developing vitreous as related to the hyaloid
vessel system. Graefes Arch Clin Exp Ophthalmol 213:71, 1980 119. Raymond L, Jacobson B. Isolation and identification of stimulatory and inhibiting growth factors
in bovine vitreous. Exp Eye Res 34:267, 1982 120. Lutty GA, Mello RJ, Chandler C, et al Regulation of cell growth by vitreous humour. J Cell Sci 76:53, 1985 121. Jacobson B, Dorfman T, Basu PK, et al Inhibition of vascular endothelial cell growth and trypsin activity by
vitreous. Exp Eye Res 41:581, 1985 122. Feeney SA, Simpson DA, Gardiner TA, et al Role of vascular endothelial growth factor and placental growth factors
during retinal vascular development and hyaloid regression. Invest Ophthalmol Vis Sci 44:839, 2003 123. Mitchell CA, Risau W, Drexler HC. Regression of vessels in the tunica vasculosa lentis is initiated by coordinated
endothelial apoptosis: A role for vascular endothelial growth
factor as a survival factor for endothelium. Dev Dyn 213:322, 1998 124. Ito M, Yoshioka M. Regression of the hyaloid vessels and papillary membrane of the mouse. Anat Embryol 200:403, 1999 125. McMenamin PG, Djano J, Wealthall R, et al Characterization of the macrophages associated with the tunica vasculosa
lentis of the rat eye. Invest Ophthalmol Vis Sci 43:2076, 2002 126. Meeson A, Palmer M, Calfon M, et al A relationship between apoptosis and flow during programmed capillary regression
is revealed by vital analysis. Development 122:3929, 1996 127. Terry TL. Extreme prematurity and fibroblastic overgrowth of persistent vascular
sheath behind each crystalline lens. Am J Ophthalmol 25:203, 1942 128. Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Arch Ophthalmol 102:1130, 1984 129. Patz A. Clinical and experimental studies on retinal neovascularization. Am J Ophthalmol 94:715, 1984 130. Kretzer FL, Hittner M. Spindle cells and retinopathy of prematurity. Birth Defects 24:147, 1988 131. Sebag J, McMeel JW. Diabetic retinopathy: Pathogenesis and role of retina-derived growth factor
in angiogenesis. Surv Ophthalmol 30:377, 1986 132. Alon T, Hemo I, Itin A, et al Vascular endothelial growth factor acts as a survival factor for newly
formed retinal vessels and has implications for retinopathy of prematurity. Nat Med 1:1024, 1995 133. Sebag J. Imaging vitreous. Eye 16:429, 2002 134. Machemer R. Description and pathogenesis of late stages of retinopathy of prematurity. Ophthalmology 92:1000, 1985 135. Foos RY. Chronic retinopathy of prematurity. Ophthalmology 92:563, 1985 136. Hirose T, Sang DA. Vitreous changes in retinopathy of prematurity. In Schepens CL, Neetens A, eds. The Vitreous and Vitreo-Retinal Interface. New York: Springer-Verlag, 1987:165–177 137. Brockhurst RJ, Albert DM, Zakov N. Pathologic findings in familial exudative vitreoretinopathy. Arch Ophthalmol 99:2143, 1981 138. Campo RV. Similarities of familial exudative vitreoretinopathy and retinopathy of
prematurity [letter]. Arch Ophthalmol 101:821, 1983 139. van Nouhuys CE. Congenital retinal fold as a sign of dominant exudative vitreoretinopathy. Graefes Arch Ophthalmol 217:55, 1981 140. van Nouhuys CE. Juvenile retinal detachment as a complication of familial exudative vitreoretinopathy. Fortschr Ophthalmol 86:221, 1989 141. Ikeda T, Fujikado T, Tano Y, et al Vitrectomy for rhegmatogenous or tractional retinal detachment with familial
exudative vitreoretinopathy. Ophthalmology 106:1081, 1999 142. Larsen JS. The sagittal growth of the eye. III. Ultrasonic measurement of the posterior
segment from birth to puberty. Acta Ophthalmol (Copenh) 49:441, 1971 143. Tigges M, Tigges J, Fernandez A, et al Postnatal axial eye elongation in normal and visually-deprived rhesus monkeys. Invest Ophthamol Vis Sci 31:1035, 1990 144. Fledelius HC. Ophthalmic changes from age of 10 to 18 years—A longitudinal study
of sequels to low birth weight IV. Ultrasound oculometry of vitreous
and axial length. Acta Ophthalmol 60:403, 1982 145. Newsome DA, Linsemayer TF, Trelstad RJ. Vitreous body collagen. Evidence for a dual origin from the neural retina
and hyalocytes. J Cell Biol 71:59, 1976 146. Chaine G, Sebag J, Coscas G. The induction of retinal detachment. Trans Ophthalmol Soc UK 103:480, 1983 147. Sebag J. Age-related differences in the human vitreoretinal interface. Arch Ophthalmol 109:966, 1991 148. Sebag J. Anatomy and pathology of the vitreo-retinal interface. Eye 6:541, 1992 149. Sebag J, Wendell R, DeBustros S. Disorders of the vitreo-macular interface. In Margo C, Hamed L, Mames R, eds. Diagnostic Problems in Clinical Ophthalmology. Philadelphia: WB Saunders, 1994:556 150. Gass JDM. Reappraisal of biomicroscopic classification of stages of development of
a macular hole. Am J Ophthalmol 119:752, 1995 151. Qiao H, Hisatomi T, Sonoda KH, et al: The characterisation of hyalocytes: the origin, phenotype, and turnover. Br J Ophthalmol 89:513,2005 152. Foos RY. Vitreoretinal juncture—Topographical variations. Invest Ophthalmol 11:801, 1972 153. Eisner G, Bachmann E. Vergleichende morphologische—Spaltlampenuntersuchun des Glaskörpers. Alb v Graef Arch Kil Exp Ophthalmol 192:1, 1974 154. Balazs EA, Denlinger JL: Aging changes in the vitreous. In Sekular R, Kline D, Dismukes N, eds: Aging and the Human Visual Function. New York Alan R Liss, 1982, 45 155. Morner CT. Untersuchung der Proteinsubstanz in den lichtbrechenden Medien des Auges. Z Physiol Chem 18:233, 1894 156. Friedenwald JS, Stiehler RD. Structure of the vitreous. Arch Ophthalmol 14:789, 1935 157. Pirie A, Scmidt G, Waters JW. Ox vitreous humor. I. The residual protein. Br J Ophthalmol 32:3211, 1948 158. Aguayo J, Glaser B, Mildvan A, et al Study of vitreous liquefaction by NMR spectroscopy and imaging. Invest Ophthalmol Vis Sci 26:692, 1985 159. Armand G, Chakrabarti B. Conformational differences between hyaluronates of gel and liquid human
vitreous—Fractionation and circular dichroism studies. Curr Eye Res 6:445, 1987 160. Andley UP, Chapman SF. Effect of oxidation on the conformation of hyaluronic acid. Invest Ophthalmol Vis Sci 25:318, 1984 161. Harooni M, McMillan T, Refojo M. Enzymatic PVD by intravitreal injection of hyaluronidase. Invest Ophthalmol Vis Sci 38:S286, 1997 162. Kamei A, Totani A. Isolation and characterization of minor glycosaminoglycans in the rabbit
vitreous body. Biochem Biophys Res Commun 109:881, 1982 163. Hageman GS, Russell SR. Chondroitinase-mediated disinsertion of the primate vitreous body. Invest Ophthalmol Vis Sci 35:1260, 1994 164. Gartner J. Electron microscopic study on the cilio-zonular border of the human eye
with particular reference to the aging changes. Anat Entwicklungsgesch 131:263, 1970 165. Tokita M, Fujiya Y, Hikichi K. Dynamic viscoelasticity of bovine vitreous body. Biorheology 21:751, 1984 166. Constable J II, Horne R, Slater DH, et al Regeneration of retinal limiting membranes after chorioretinal biopsy in
dogs. Invest Ophthalmol Vis Sci 20:246, 1981 167. Teng CC, Chi HH. Vitreous changes and the mechanism of retinal detachment. Am J Ophthalmol 44:335, 1957 168. Wang J, McLeod D, Henson DB, et al Age-dependent changes in the basal retinovitreous adhesion. Invest Ophthalmol Vis Sci 44:1793, 2003 169. Gartner J. Electron microscopic study on the fibrillar network and fibrocyte-collagen
interactions in the vitreous cortex at the ora serrata of human eyes
with special regard to the role of disintegrating cells. Exp Eye Res 42:21, 1986 170. Foos RY, Wheeler NC. Vitreoretinal juncture, synchisis senilis and posterior vitreous detachment. Ophthalmology 89:1502, 1982 171. Lindner B. Acute posterior vitreous detachment and its retinal complications. Acta Ophthalmologica 87:1, 1966 172. Hyams SW, Neumann E, Friedman Z. Myopia and aphakia II. Vitreous and peripheral retina. Br J Ophthalmol 59:483, 1975 173. Maumenee IH. Vitreoretinal degenerations as a sign of generalized connective tissue
diseases. Am J Ophthalmol 88:432-449, 1979 174. Wise GN. Relationship of idiopathic preretinal macular fibrosis to posterior vitreous
detachment. Am J Ophthalmol 79:358, 1975 175. Voerhoeff FH. Are Moore's lightning streaks of serious portent. Am J Ophthalmol 41:837, 1956 176. Dotrelova D, Karel I, Clupkova E. Retinal detachment in Marfan's syndrome. Characteristics and surgical
results. Retina 17:390, 1997 177. Christiansson J. Changes in mucopolysaccharides during alloxan diabetes in the rabbit. Acta Ophthalmol 36:141, 1958 178. Xiong H, Cheng HM. Change of vitreous tonicity in “sugar” cataracts. Invest Ophthalmol Vis Sci 29:149, 1988 179. Lundquist O, Osterlin S. Glucose concentration in the vitreous of nondiabetic and diabetic human
eyes. Graefes Arch Clin Exp Ophthalmol 232:71, 1994 180. Sebag J, Buckingham B, Charles MA, et al Biochemical abnormalities in vitreous of humans with proliferative diabetic
retinopathy. Arch Ophthalmol 110:1472, 1992 181. Sebag J, Nie S, Reiser K, et al Raman spectroscopy of human vitreous in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci 35:2976, 1994 182. Stitt AW, Moore JE, Sharkey JA, et al Advanced glycation end products in vitreous: Structural and functional
implications for diabetic vitreopathy. Invest Ophthalmol Vis Sci 39:2517, 1998 183. Katsumura C, Sugiyama T, Nakamura K, et al Effects of advanced glycation end products on hyaluronan photolysis: A
new mechanism of diabetic vitreopathy. Ophthalmol Res 36:327, 2004 184. Faulborn J, Bowald S. Microproliferations in proliferative diabetic retinopathy and their relation
to the vitreous. Graefes Arch Clin Exp Ophthalmol 223:130, 1985 185. Tasman WS. Diabetic vitreous hemorrhage and its relationship to hypoglycemia. Mod Prob Ophthalmol 20:413, 1979 186. Snead MP, Yates JRW. Clinical and molecular genetics of Stickler syndrome. J Med Genet 36:353, 1999 187. Sebag J, Ansari RR, Dunker S, Suh SI: Dynamic light scattering of diabetic vitreopathy. Diabetes Technol Ther 1:169, 1999 188. Sebag J. Seeing the invisible—The challenge of imaging vitreous. J Biomed Optics 9:38, 2004 189. Sebag J. Myopia effects upon vitreous—Significance in retinal detachments. In Stirpe M, ed. Anterior and Posterior Segment Surgery: Mutual Problems and Common Interests. Acta
of the 5th International Congress on Vitreo-Retinal Surgery. New York: Ophthalmic Communications Society, Inc., 1998:366–372 190. Verbraeken H, Van Egmond J. Non-diabetic and non-oculotraumatic vitreous haemorrhage treated by pars
plana vitrectomy. Bull Soc Belge Ophthalmol 272:83, 1999 191. Sarrafizadeh R, Hassan TS, Ruby AJ, et al Incidence of retinal detachment and visual outcome in eyes presenting with
posterior vitreous separation and dense fundus-obscuring vitreous
hemorrhage. Ophthalmology 108:2273, 2001 192. Byer NE. Posterior vitreous detachment as a risk factor for retinal detachment. Ophthalmology 102:528, 1995 193. Kakehashi A, Schepens CL, de Sousa-Neto A, et al Biomicroscopic findings of posterior vitreoschisis. Ophthalmic Surg 24:846, 1993 194. Green RL, Byrne SF. Diagnostic ophthalmic ultrasound. In Ryan SJ, ed. Retina. St. Louis: CV Mosby, 1989:268 195. Chu T, Lopez PF, Cano MR, et al Posterior vitreoschisis—An echographic finding in proliferative diabetic
retinopathy. Ophthalmology 103:315, 1996 196. Rodman HI, Johnson FB, Zimmerman LE. New histopathological and histochemical observations concerning asteroid
hyalitis. Arch Ophthalmol 66:552, 1961 197. Wasano T, Hirokuwa H, Tagawa H, et al Asteroid hyalosis–posterior vitreous detachment and diabetic retinopathy. Am J Ophthalmol 19:255, 1987 198. Moss SE, Klein R, Klein BE. Asteroid hyalosis in a population- the Beaver Dam eye study. Am J Ophthalmol 132:70, 2001 199. Mitchell P, Wang MY, Wang JJ. Asteroid hyalosis in an older population: The Blue Mountains Eye Study. Ophthalmic Epidemiol 10:331, 2003 200. Sebag J, Albert DM, Craft JL. The Alstrom syndrome–ocular histopathology and retinal ultrastructure. Br J Ophthalmol 68:494, 1984 201. Rodman HI, Johnson FB, Zimmerman LE. New histopathological and histochemical observations concerning asteroid
hyalitis. Arch Ophthalmol 66:552, 1961 202. Streeten BA. Disorders of the vitreous. In Garner A, Klintworth GK, eds. Pathobiology of Ocular Disease—A Dynamic Approach.. New York: Marcel Dekker, 1982:1381 203. Yu SY, Blumenthal HT. The calcification of elastic tissue. In Wagner BM, Smith DE, eds. The Connective Tissue. Baltimore: Williams & Williams, 1967:17 204. Lamba PA, Shukla KM. Experimental asteroid hyalopathy. Br J Ophthalmol 55:279, 1971 205. Winkler J, Lunsdorf H. Ultrastructure and composition of asteroid bodies. Invest Ophthalmol Vis Sci 42:902, 2001 206. Komatsu H, Kamura Y, Ishi K, et al Fine structure and morphogenesis of asteroid hyalosis. Med Electron Microsc 36:112, 2003 207. Yazar Z, Hanioglu S, Karakoc G, et al Asteroid hyalosis. Eur J Ophthalmol 11:57, 2001 208. Hitchings RA, Triparthi RC. Vitreous opacities in primary amyloid disease—A clinical, histochemical, and
ultrastructural report. Br J Ophthalmol 60:41, 1976 209. Koga T, Ando E, Hirata A, et al Vitreous opacities and outcome of vitreous surgery in patients with familial
amyloidotic polyneuropathy. Am J Ophthalmol 135:188, 2003 210. Doft BH, Rubinow A, Cohen AS. Immunocytochemical demonstration of prealbumin in the vitreous in heredofamilial
amyloidosis. Am J Ophthalmol 97:296, 1984 211. Jaffe NS. Retinal detachment in aphakia and pseudophakia. In Klein EA, ed. Cataract Surgery and its Complications, 5th ed. St. Louis: CV Mosby, 1990:635 212. Ninn-Pedersen K, Bauer B. Cataract patients in a defined Swedish population, 1986 to 1990. V. Postoperative
retinal detachments. Arch Ophthalmol 114:382, 1996 213. Osterlin S. Macromolecular composition of the vitreous in the aphakic owl monkey eye. Exp Eye Res 26:77, 1978 214. Osterlin S. On the molecular biology of the vitreous in the aphakic eye. Acta Ophthalmol 55:353, 1977 215. Bradford JD, Wilkinson CP, Fransen SR. Pseudophakic retinal detachments. Retina 9:181, 1989 216. Kangro M, Osterlin S. Hyaluronate concentration in the vitreous of the pseudophakic eye. Invest Ophthalmol Vis Sci 26:28, 1985 217. Javitt JC, Vitale S, Canner JK, et al National outcomes of cataract extraction. I. Retinal detachment after inpatient
surgery. Ophthalmology 98:895, 1991 218. Thompson JT, Glaser BM. Role of lensectomy and posterior capsule in movement of tracers from vitreous
to aqueous. Arch Ophthalmol 103:420, 1985 219. Smith RT, Moscoso WE, Trokel S, et al The barrier function in Neodymium-YAG laser capsulotomy. Arch Ophthalmol 113:645, 1995 220. Schubert HD, Morris WJ, Trokel SL, et al The role of vitreous in the intraocular pressure rise after Nd:YAG laser
capsulotomy. Arch Ophthalmol 103:1538, 1985 221. McDonnell PJ, Patel A, Green WR. Comparison of intracapsular and extracapsular surgery. Histopathologic
study of eyes obtained postmortem. Ophthalmology 92:1208, 1985 222. Kraff MC, Sanders DR. Incidence of retinal detachment following posterior chamber intraocular
lens surgery. J Cataract Refract Surg 16:477, 1990 223. Javitt JC, Tielsch JM, Canner JK, et al National outcomes of cataract extraction—Increased risk of retinal
complications associated with Nd:YAG laser capsulotomy. Ophthalmology 99:1487, 1992 224. Tielsch JM, Legro MW, Cassard SD, et al Risk factors for retinal detachment after cataract surgery—A population-based
case-control study. Ophthalmology 103:1537, 1996 225. Coscas G, Soubrane G. Severe myopia or myopia-disease? Rev Prat 43:1768, 1993 226. The Eye Disease Case-Control Study Group. Risk factors for idiopathic rhegmatogenous retinal detachment. Am J Epidemiol 137:749, 1993 227. Percival SP. Redefinition of high myopia—The relationship of axial length measurement
to myopic pathology and its relevance to cataract surgery. Dev Ophthalmol 14:42, 1987 228. Curtin BJ. The Myopias—Basic Science and Clinical Management. Philadelphia: Harper & Row, 1985 229. Berman ER, Michaelson IC. The chemical composition of the human vitreous body as related to age and
myopia. Exp Eye Res 3:9, 1964 230. Balazs EA, Toth LZJ, Jutheden GM, et al Cytological and biochemical studies of the developing chicken vitreous. Exp Eye Res 4:237, 1965 231. Pickett-Seltner RL, Doughty MJ, Pasternak JJ, et al Proteins of the vitreous humor during experimentally-induced myopia. Invest Ophthalmol Vis Sci 33:3424, 1992 232. Van Alphen GWHM. Emmetropization in the primate eye. In Boch G, Widdows K, eds. Myopia and the Control of Eye Growth. New York: John Wiley & Sons, 1990:115 233. Grossniklaus HE, Green WR. Pathologic findings in pathologic myopia. Retina 12:127, 1992 234. Stirpe M, Heimann K. Vitreous changes and retinal detachment in highly myopic eyes. Eur J Ophthalmol 6:50, 1996 235. Singh A, Paul SD, Singh K. A clinical study of the vitreous body in emmetropia and refractive errors. Orient Arch Ophthalmol 8:11, 1970 236. Akiba J. Prevalence of posterior vitreous detachment in high myopia. Ophthalmology 100:1384, 1993 237. Barraquer C, Cavelier C, Mejia LF. Incidence of retinal detachment following clear-lens extraction in myopic
patients. Arch Ophthalmol 112:336, 1994 238. Ogawa A, Tanaka M. The relationship between refractive errors and retinal detachment—Analysis
of 1,166 retinal detachment cases. Jpn J Ophthalmol 32:310, 1988 239. Younan C, Mitchell P, Cumming RG, et al Myopia and incident cataract and cataract surgery: The Blue Mountains Eye
Study. Invest Ophthalmol Vis Sci 43:3625, 2002 240. Sebag J. Vitreous effects of cataract surgery and YAG capsulotomy—Role in
postoperative retinal detachments. In Stirpe M, ed. Anterior and Posterior Segment Surgery: Mutual Problems and Common Interests. Acta
of the 5th International Congress on Vitreo-Retinal Surgery. New York: Ophthalmic Communications Society, Inc., 1998:85–88 241. Jaffe NS, Clayman HM, Jaffe MS. Retinal detachment in myopic eyes after intracapsular and extracapsular
cataract extraction. Am J Ophthalmol 97:48, 1984 242. Badr IA, Hussain HM, Jabak M, et al Extracapsular cataract extraction with or without posterior chamber intraocular
lenses in eyes with cataract and high myopia. Ophthalmology 102:1139, 1995 243. Colin J, Robinet A. Clear lensectomy and implantation of a low-power posterior chamber intraocular
lens for correction of high myopia: A four-year follow-up. Ophthalmology 10:73, 1997 244. Fernandez-Vega L, Alfonso JF, Villacampa T. Clear lens extraction for the correction of high myopia. Ophthalmology 110:2349, 2003 245. Colin J, Robinet A, Cochener B. Retinal detachment after clear lens extraction for high myopia: Seven-year
follow-up. Ophthalmology 106:2281, 1999 246. Ripandelli G, Billi B, Fedeli R, et al Retinal detachment after clear lens extraction in 41 eyes with high axial
myopia. Retina 17:78, 1997 247. Sebag J. Myopia effects upon vitreous—Significance in retinal detachments. In Stirpe M, ed. Anterior and Posterior Segment Surgery: Mutual Problems and Common Interests. Acta
of the 5th International Congress on Vitreo-Retinal Surgery. New York: Ophthalmic Communications Society, Inc. 1998:366–372 248. Pirie A. The effect of hyaluronidase injection on the vitreous humour of the rabbit. Br
J Ophthalmol. 33:678, 1949 249. O'Neill R, Shea M. The effects of bacterial collagenase in rabbit vitreous.Can J Ophthalmol 8:366, 1973 250. Moorhead LC, Chu HH, Garcia CA. Enzyme-assisted vitrectomy with bacterial collagenase—Time course
and toxicity studies. Arch Ophthalmol 101:265, 1983 251. Verstraeten T, Chapman C, Hartzer M, et al Pharmacologic induction of PVD in the rabbit. Arch Ophthalmol 111:849, 1993 252. Chow DR, Williams GA, Trese MT, et al Successfulclosure of traumatic macular holes. Retina 19:405, 1999 253. Trese MT, Williams GA, Hartzer MK. A new approach to stage 3 macular holes.Ophthalmology 107:1607, 2000 254. Williams JG, Trese MT, Williams GA, et al Autologous plasmin enzyme in thesurgical management of diabetic retinopathy. Ophthalmology 108:1902, 2001 255. Hesse L, Nebeling B, Schroeder B, et al Induction of posterior vitreousdetachment in rabbits by intravitreal injection
of tissue plasminogen activator following cryopexy. Exp Eye Res 70:31, 2000 256. Unal M, Peyman GA. The efficacy of plasminogen-urokinase combination in inducing posterior
vitreous detachment. Retina 20:69, 2000 257. Valmaggia C, Willekens B, de Smet M. Microplasmin induced vitreolysis in porcine eyes. Invest Ophthalmol Vis Sci 44:3050, 2003 258. Gandorfer A, Rohleder M, Sethi C, et al Posterior vitreous detachment induced by microplasmin. Invest Ophthalmol Vis Sci 45:641, 2004 259. Tezel TH, Del Priore LV, Kaplan HJ. Posterior vitreous detachment with dispase. Retina 18:7, 1998 260. Oliviera LB, Tatebayashi M, Mahmoud TH, et al Dispase facilitates posterior vitreousdetachment during vitrectomy in young
pigs. Retina 21:324, 2001 261. Jorge R, Oyamaguchi EK, Cardillo JA, et al Intravitreal injection of dispase causes retinal hemorrhages in rabbit
and human eyes. Curr Eye Res 26:107, 2003 262. Hikichi T, Masanori K, Yoshida A. Intravitreal injection of hyaluronidase cannot induce posterior vitreous
detachment in the rabbit. Retina 20:195, 2000 263. Wang ZL, Zhang X, Xu X, Sun XD, Wang F: PVD following plasmin but not hyaluronidase: implications for combination
pharmacologic vitreolysis therapy. Retina 25:38, 2005 264. Hikichi T, Masanori K, Yoshida A. Posterior vitreous detachment induced by injection of plasmin and sulfur
hexafluoride in the rabbit vitreous. Retina 19:55, 1999 265. Thresher RJ, Ehrenberg M, Machemer R. Gas-mediated vitreous compression: an experimental alternative to mechanized
vitrectomy Graefes Arch Clin Exp Ophthalmol 221:192, 1984 266. Hageman GS, Russell SR. Chondroitinase-mediated disinsertion of the primate vitreous body. Invest Ophthalmol Vis Sci 35:1260, 1994 |