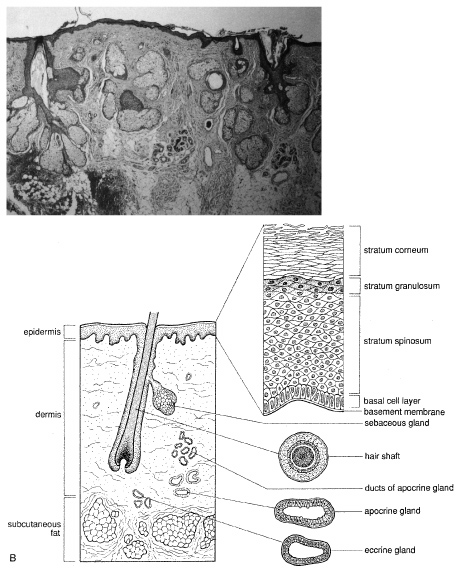
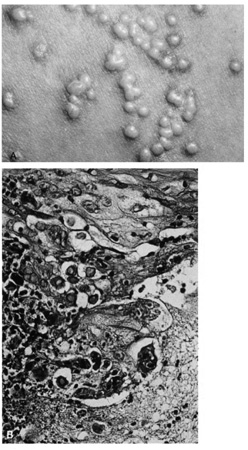
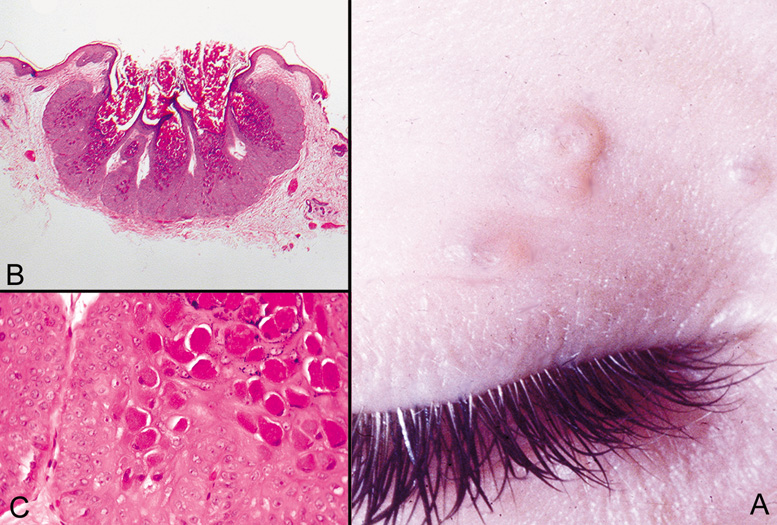
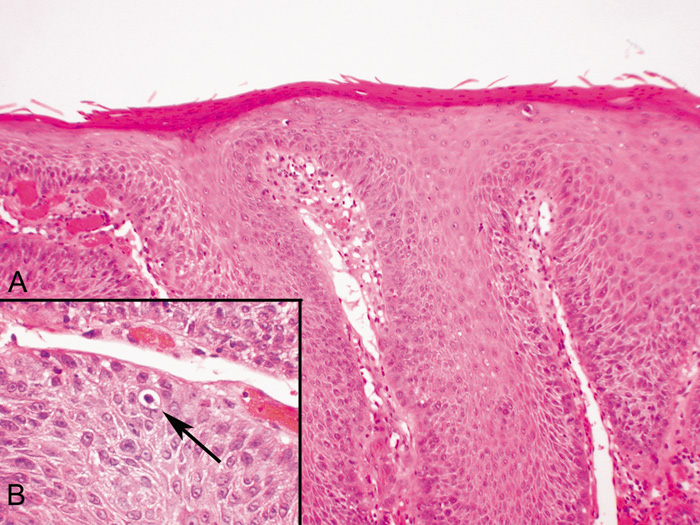
CYSTS Epidermal Inclusion Cysts and Milia Epidermal inclusion cysts are follicular cysts of the hair follicle infundibulum, which
most often occur on the outer upper portion of the eyelid
as firm, round, slow growing lesions. Cystic dilatation of the follicle
occurs from occlusion of the orifice, which may be precipitated
by trauma. Milia are identical to epidermal cysts but are smaller. Multiple
epidermal cysts, especially of the face and scalp, may occur in Gardner's syndrome. Histologically, epidermal cysts have a wall composed of epidermis with
a granular cell layer that is essentially identical to the surface epidermis. Within
the cyst there is loose, laminated keratin, much of which
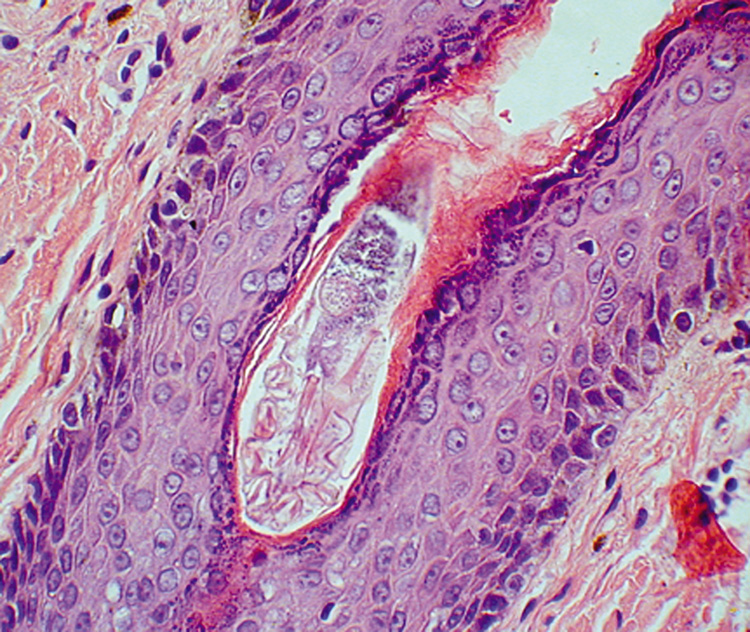
may be lost during processing (Fig. 18). Occasionally, the cyst will rupture and produce a foreign body
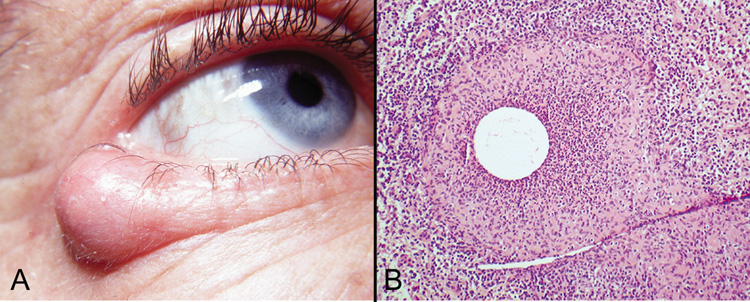
reaction with multinucleated giant cells in the adjacent tissue. 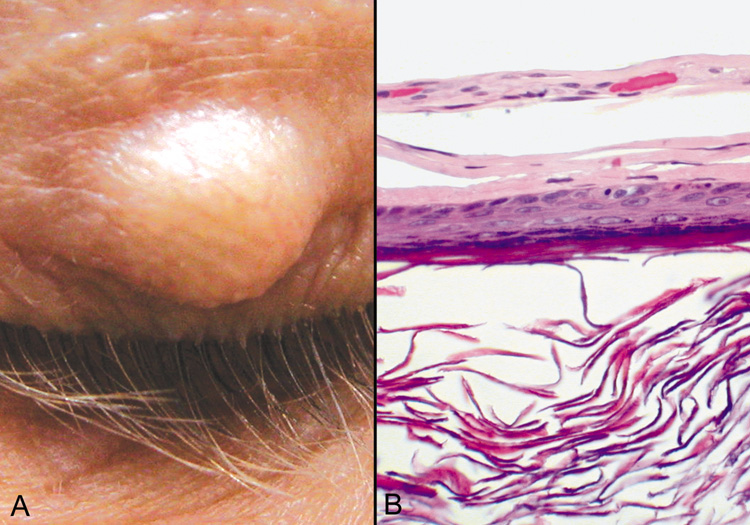 Fig. 18. Epidermal Inclusion Cyst—A. Clinically this cystic lesion usually has a smooth dome-shape and
is light yellow to white. B. Histopathologically, this cystic lesion is lined with stratified squamous
epithelium that includes the granular cell layer. The lumen is filled
with keratin produced by the epithelium (hematoxylin and eosin
stain). (Photos courtesy of William Morris, M.D.) Fig. 18. Epidermal Inclusion Cyst—A. Clinically this cystic lesion usually has a smooth dome-shape and
is light yellow to white. B. Histopathologically, this cystic lesion is lined with stratified squamous
epithelium that includes the granular cell layer. The lumen is filled
with keratin produced by the epithelium (hematoxylin and eosin
stain). (Photos courtesy of William Morris, M.D.)
|
Hidrocystomas Cysts resulting from occlusion of the eccrine or apocrine duct are referred
to as hidrocystomas. Apocrine hidrocystomas are usually solitary
and translucent and are often found near the eye. Eccrine hidrocystomas
may be solitary or multiple and are indistinguishable from apocrine
hidrocystomas clinically. Histologically, apocrine hidrocystomas are irregularly shaped cysts and
are lined by a double layer of epithelium: the outer layer is myoepithelium, and
the luminal layer demonstrates decapitation secretion (Fig. 19). Eccrine hidrocystomas are more rounded and show a flattened wall
with one or two layers of cuboidal cells (Fig. 20). 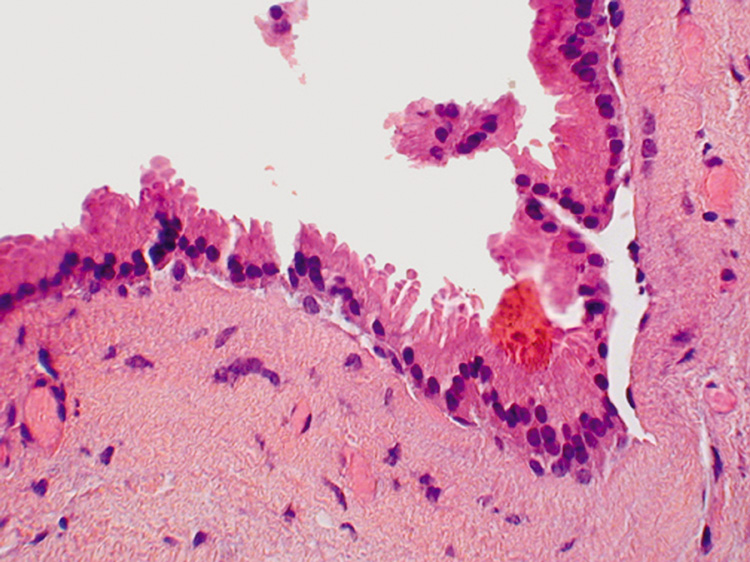 Fig. 19. Apocrine Hidrocystoma—Apocrine cells line the hidrocystoma wall. Notice
the “decapitation snouts“ at the apex of the cells, indicating
that the cytoplasm has been pinched off to form the glandular
secretion (hematoxylin and eosin stain). (Photo courtesy
of William Morris, M.D.) Fig. 19. Apocrine Hidrocystoma—Apocrine cells line the hidrocystoma wall. Notice
the “decapitation snouts“ at the apex of the cells, indicating
that the cytoplasm has been pinched off to form the glandular
secretion (hematoxylin and eosin stain). (Photo courtesy
of William Morris, M.D.)
|
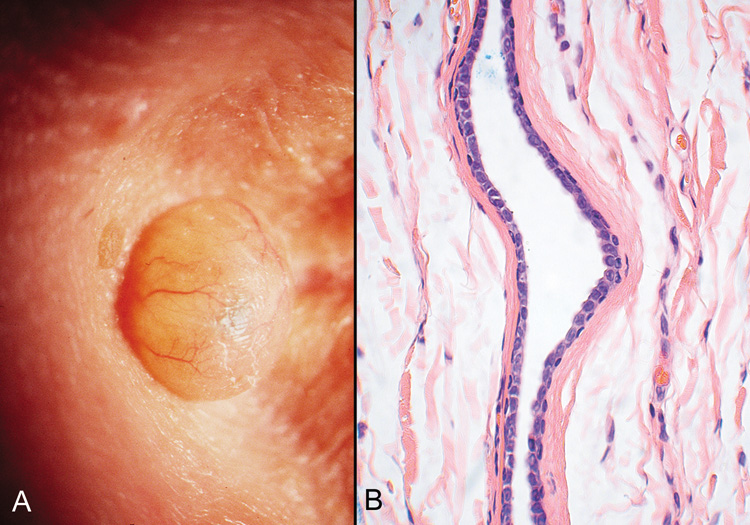 Fig. 20. Eccrine Hidrocystoma—A. Clinically, this appears as a softly cystic lesion that transilluminates
brightly. B. Photomicrograph showing collapsed cyst lined by a double row of cuboidal
cells. The lumen appears empty (hematoxylin and eosin stain). (Photos
courtesy of William Morris, M.D.) Fig. 20. Eccrine Hidrocystoma—A. Clinically, this appears as a softly cystic lesion that transilluminates
brightly. B. Photomicrograph showing collapsed cyst lined by a double row of cuboidal
cells. The lumen appears empty (hematoxylin and eosin stain). (Photos
courtesy of William Morris, M.D.)
|
Pilar (Trichilemmal) Cysts Pilar, or trichilemmal, cysts were previously referred to as sebaceous
cysts. They are less common than epidermal cysts and may arise from hair
follicles on the lid surface or the brow. These often occur in an autosomal
dominant pattern of inheritance. In contrast to epidermal cysts, these
cysts are easily enucleated and appear as firm, smooth, white
cysts. The name, trichilemmal cyst, came about because these cysts are
derived from the isthmus portion of the hair follicle from which keratinization
analogous to the outer root sheath of the hair, or trichilemma, occurs. Histologically, pilar cysts possess an epithelial wall without clearly
visible intercellular bridges. There is palisading of the peripheral cell
layer, and the cells closest to the cyst cavity appear swollen and
have a pale cytoplasm (Fig. 21). Within the cyst cavity, there is homogeneous compact eosinophilic
material which also may show focal calcification. Cyst rupture is accompanied
by a foreign-body granulomatous response. 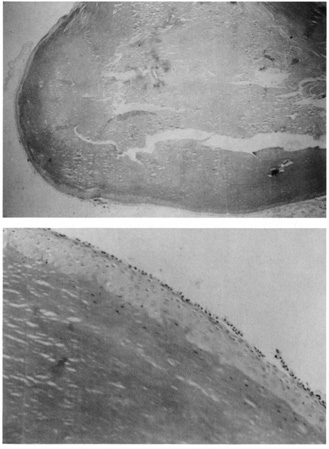 Fig. 21. Pilar cyst—A. Scanning magnification shows a cyst containing compact keratin. B. Higher magnification shows absent granular layer of the cyst wall and
compact keratin in the lumen. Fig. 21. Pilar cyst—A. Scanning magnification shows a cyst containing compact keratin. B. Higher magnification shows absent granular layer of the cyst wall and
compact keratin in the lumen.
|
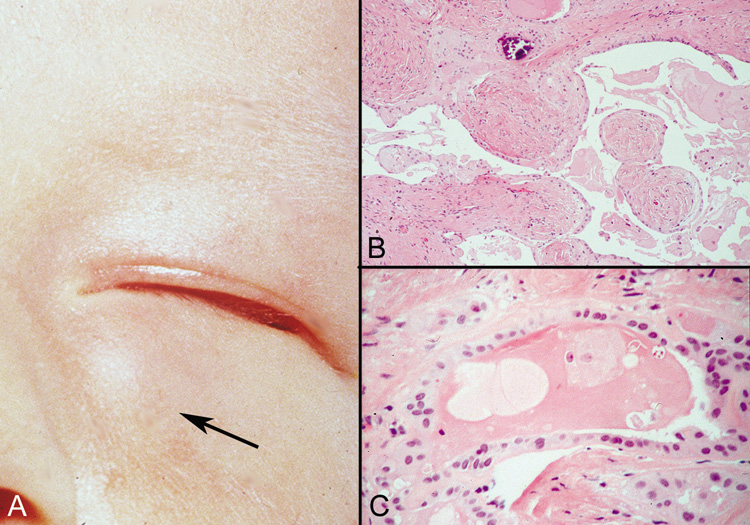
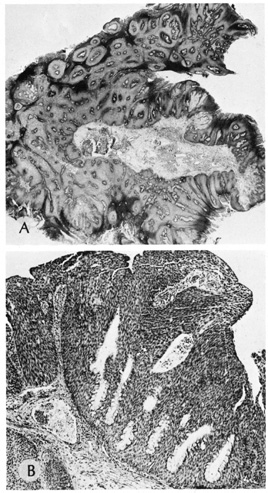
Dermoid Cysts Dermoid cysts are usually present at birth and commonly occur around the
eyes. They may be adherent to the periosteum. Dermoid cysts are believed
to result from sequestration of skin and its appendages along embryonic
lines of closure and, thus, are commonly found at the frontozygomatic
suture. Histologically, dermoid cysts are lined by epidermis possessing various
mature appendageal structures (Fig. 22). These include hair follicles with terminal hairs, sebaceous glands, eccrine
glands, and, occasionally, apocrine glands. 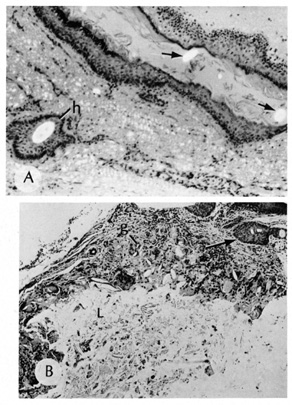 Fig. 22. A. Dermoid cyst shows hair follicle (h) and hair shaft (arrows) in cyst lumen. B. Dermoid cyst has skin appendages in cyst wall (arrow), is lined by stratified squamous epithelium, and contains desquamated
keratin in its lumen (L). There is a granulomatous inflammatory infiltrate (g) due to rupture of cyst. (From Yanoff M, Fine BS: Ocular Pathology, 3rd
ed. Philadelphia, JB Lippincott, 1989, 186.) Fig. 22. A. Dermoid cyst shows hair follicle (h) and hair shaft (arrows) in cyst lumen. B. Dermoid cyst has skin appendages in cyst wall (arrow), is lined by stratified squamous epithelium, and contains desquamated
keratin in its lumen (L). There is a granulomatous inflammatory infiltrate (g) due to rupture of cyst. (From Yanoff M, Fine BS: Ocular Pathology, 3rd
ed. Philadelphia, JB Lippincott, 1989, 186.)
|
Steatocystoma Steatocystoma may occur as solitary cysts (simplex) or multiple
cysts (multiplex). The latter is often inherited as an autosomal
dominant trait. These cysts are small and firm and, when punctured, exude
an oily or creamy fluid. Steatocystoma is derived from cystic
dilatation of the sebaceous duct, which empties into the hair follicle. Histologically, the cyst wall is folded and shows several layers of epithelial
cells with a palisading of the peripheral cell layer. The cells
lining the cystic cavity are covered by a thick, eosinophilic cuticle. Characteristically, flattened sebaceous lobules are present either
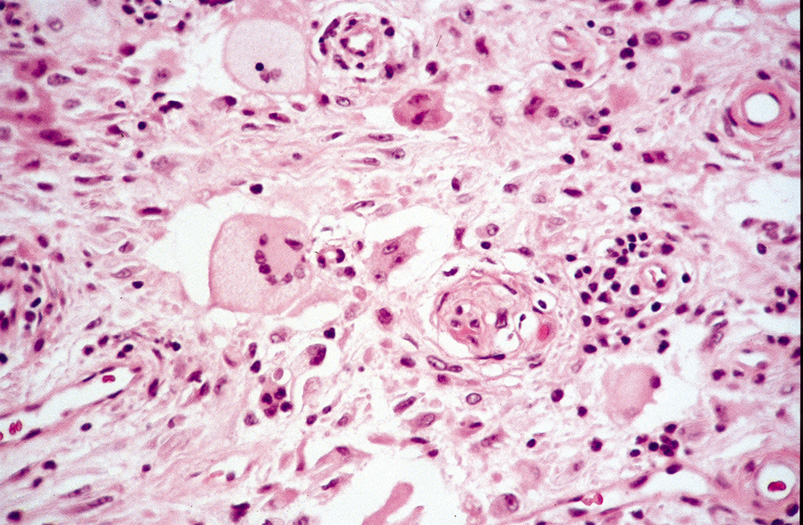
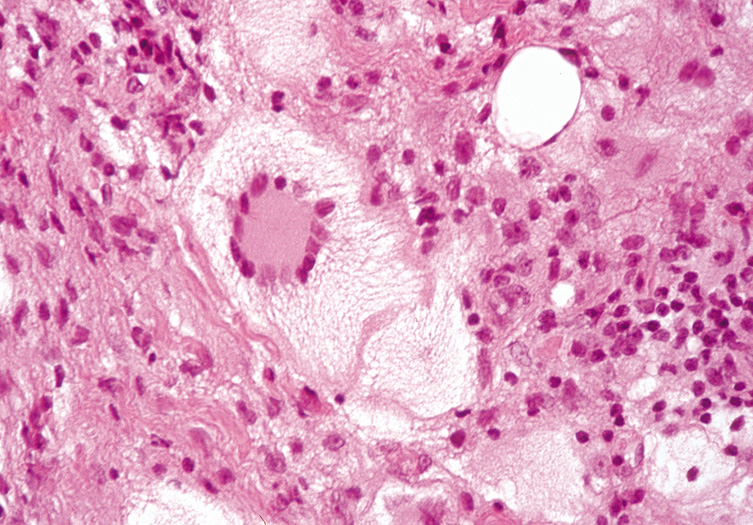
within or close to the cyst wall. Pilomatrixoma Pilomatrixoma, also referred to as Malherbe's calcifying epithelioma, is
a cyst derived from the hair matrix that forms the hair. It is
often a solitary lesion and most commonly occurs on the face. Most pilomatrixomas
occur in the first two decades of life and, if superficially
located, produce a blue-red skin discoloration. Excision is
curative. Histologically, pilomatrixomas show two types of cells in variable proportions: a
basophilic cell with a dark basophilic nucleus and scanty cytoplasm
and a “shadow cell,“ which has an unstained central
nucleus and faintly eosinophilic cytoplasm (Fig. 23). There may be an abrupt or gradual transition between the two cells, and
few or no basophilic cells may be seen in “old“ lesions. Calcification
of pilomatrixoma is frequent and may occur within
the shadow cells or in the stroma. The stroma usually is fibrotic and
contains a foreign body reaction. Ossification also can occur occasionally. 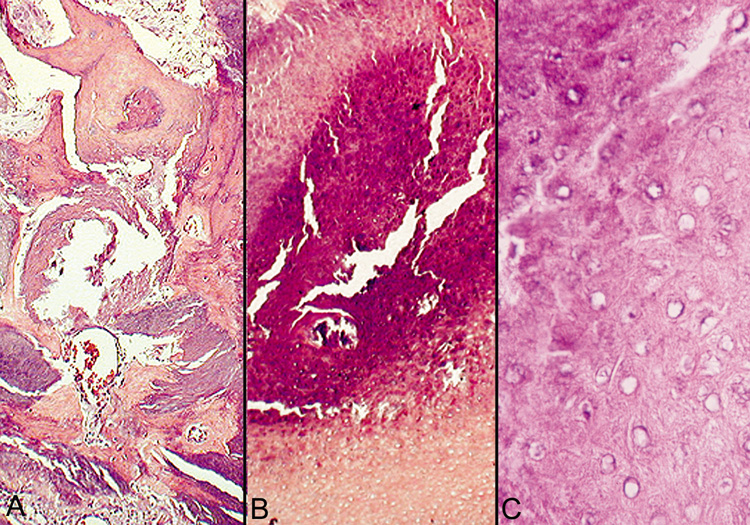 Fig. 23. Pilomatrixoma—A. Low-power photomicrograph demonstrating immature bone formation
and proliferation of epithelial cells (hematoxylin and eosin stain). B. Higher-power view showing an area of calcification within the tumor (ematoxylin
and eosin stain). C. High-power photomicrograph showing the transition from basophilic
epithelial cells (upper left) to “shadow cells“ in
the lower right (hematoxylin and eosin stain). (Photos
courtesy of William Morris, M.D.) Fig. 23. Pilomatrixoma—A. Low-power photomicrograph demonstrating immature bone formation
and proliferation of epithelial cells (hematoxylin and eosin stain). B. Higher-power view showing an area of calcification within the tumor (ematoxylin
and eosin stain). C. High-power photomicrograph showing the transition from basophilic
epithelial cells (upper left) to “shadow cells“ in
the lower right (hematoxylin and eosin stain). (Photos
courtesy of William Morris, M.D.)
|
“Hybrid“ Cysts Follicular cysts with differentiation toward two or more of the previously
mentioned cysts are referred to as hybrid cysts. Although originally
described as a cyst of infundibular and trichilemmal keratinization, hybrid
cysts may show any permutation of follicular keratinization. BENIGN TUMORS Epithelial Derivation FIBROEPITHELIAL PAPILLOMA (ACROCHORDON) The fibroepithelial papilloma, also known as a squamous papilloma, acrochordon
or skin tag, is a polyp of skin that occurs commonly on or around
the eyelids. Histologically, fingerlike projections of papillary dermis
are covered by epidermis, which is of normal thickness and shows
elongation of the rete ridges and hyperkeratosis. Dilated capillaries
are seen in the dermis with a variable chronic inflammatory infiltrate (Fig. 24). If traumatized, there may be necrosis of the epidermis and dermis
with ulceration and crust. 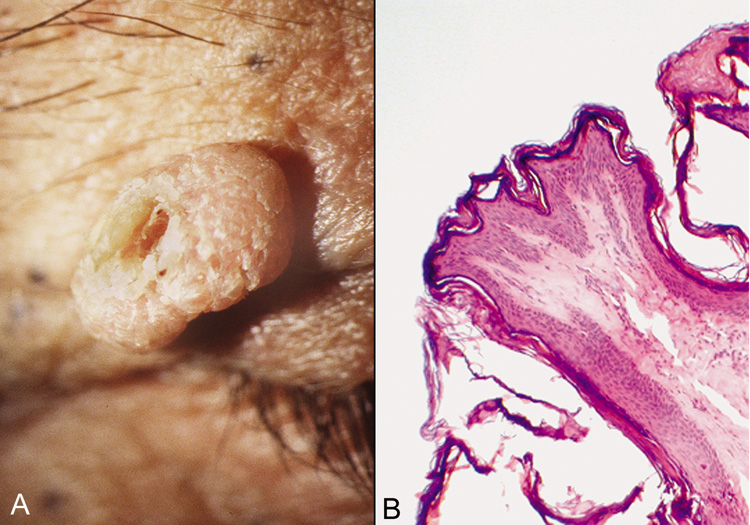 Fig. 24. Achrocordon (skin tag)—A. Clinical photograph of typical achrocordon. B. Normal skin epithelium covers a stalk of loose connective tissue and blood
vessels (hematoxylin and eosin stain). (Courtesy of
William Morris, M.D.) Fig. 24. Achrocordon (skin tag)—A. Clinical photograph of typical achrocordon. B. Normal skin epithelium covers a stalk of loose connective tissue and blood
vessels (hematoxylin and eosin stain). (Courtesy of
William Morris, M.D.)
|
SEBORRHEIC KERATOSIS. Seborrheic keratoses are the most common benign skin lesions in the geriatric
population. They typically increase in size and number with age. Clinically, the
lesions are well-demarcated, tan-to-brown
papules or plaques with a rough, almost warty, “stuck
on“ appearance. Due to their pigmentation, distinguishing the lesions
from malignant melanoma is sometimes difficult. In some cases, seborrheic
keratoses may be polypoid, resembling papillomas, or shiny
and glistening, resembling basal cell carcinomas. Although clinically
confused with both melanoma and basal cell carcinoma, they are not thought
to be a precursor to malignancy. Histologically, seborrheic keratoses are composed of a proliferation of
basaloid cells resembling the basal cell layer of the epidermis (Fig. 25). Six subtypes are recognized: acanthotic, hyperkeratotic, reticulated, clonal, irritated, and melanoacanthoma. All types show acanthosis, hyperkeratosis, and
papillomatosis. Because the acanthosis produces
an upward extension, the lower border of seborrheic keratoses is even, and
a straight line can be drawn from one end of the tumor to the other. A
characteristic feature of seborrheic keratoses are the horn pseudocysts, which
are horny invaginations cut on cross section. 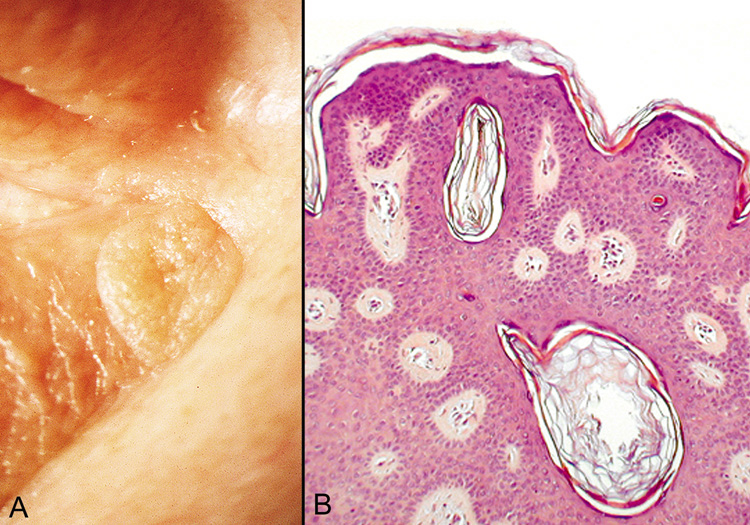 Fig. 25. Seborrheic Keratosis—A. Clinical photograph of seborrheic keratosis illustrating its “stuck
on“ appearance. B. Low-power photomicrograph showing the proliferating cords of basal
cells, the vascular islands, and the “horn cysts“ within
the thickened epithelium (hematoxylin and eosin stain). (Photos
courtesy of William Morris, M.D.) Fig. 25. Seborrheic Keratosis—A. Clinical photograph of seborrheic keratosis illustrating its “stuck
on“ appearance. B. Low-power photomicrograph showing the proliferating cords of basal
cells, the vascular islands, and the “horn cysts“ within
the thickened epithelium (hematoxylin and eosin stain). (Photos
courtesy of William Morris, M.D.)
|
Dermatosis papulosa nigra is a small, pigmented, polypoid seborrheic keratosis
seen around the eyes and on the cheeks of Black people. The sudden
appearance of numerous seborrheic keratoses, called the Leser-Trélat
sign, may herald an internal malignancy. EPIDERMAL NEVUS. Epidermal nevi (nevus verrucosus) are linear verrucous plaques
usually present at birth. The lesions may be localized or, rarely, generalized. The
latter type may be associated with skeletal or central
nervous system abnormalities (epidermal nevus syndrome). There
are two major classifications of epidermal nevi: nonorganoid (keratinocytic) and
organoid (sebaceous, follicular, and sweat
gland). The type of epidermal nevus is determined by its predominant
components, keratinocytes, or epidermal appendages. Histologically, there is considerable hyperkeratosis, papillomatosis, and
acanthosis with fusion of the rete ridges. Epidermolytic hyperkeratosis
may be seen in the localized or, more frequently, the generalized
type. INVERTED FOLLICULAR KERATOSIS. Inverted follicular keratosis is a benign epithelial lesion occurring exclusively
on hair-bearing surfaces and, most frequently, the face. Middle-aged
or older individuals are usually affected. Clinically, the
lesion presents as an asymptomatic, pink or flesh-colored
papule or plaque. Rapidly growing lesions may be confused with
keratoacanthomas. Histologically, inverted follicular keratoses are exoendophytic and symmetric. There
is a bulbous proliferation of keratinocytes showing abundant
eosinophilic cytoplasms into the dermis. Often, the stratum corneum
is parakeratotic and contains neutrophils, serum, and red blood cells. A
characteristic feature, which is also shared with irritated seborrheic
keratoses, is the presence of squamous eddies, which are whorls
of eosinophilic keratinocytes arranged in an onion-peel fashion (Fig. 26). Some authors believe that inverted follicular keratoses are really
irritated seborrheic keratoses or verrucae with squamous eddies. 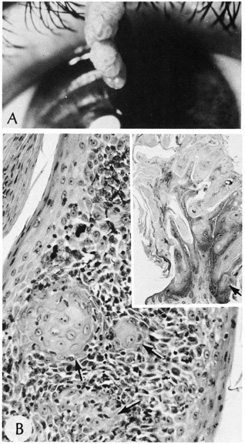 Fig. 26. Inverted follicular keratosis. A. Clinical appearance. B. Inset shows hyperkeratotic papillomatous lesion shown under high magnification, (arrow main figure). Note: acantholytic squamous cells surrounding squamous
eddies (arrows). (Modified from Sassani JW, Yanoff M: Inverted follicular keratosis. Am
J Ophthalmol 87:810, 1979; and Scheie HG, Yanoff M, Sassani
JW: Inverted follicular keratosis mimicking malignant melanoma. Ann
Ophthalmol 9:949, 1977.) Fig. 26. Inverted follicular keratosis. A. Clinical appearance. B. Inset shows hyperkeratotic papillomatous lesion shown under high magnification, (arrow main figure). Note: acantholytic squamous cells surrounding squamous
eddies (arrows). (Modified from Sassani JW, Yanoff M: Inverted follicular keratosis. Am
J Ophthalmol 87:810, 1979; and Scheie HG, Yanoff M, Sassani
JW: Inverted follicular keratosis mimicking malignant melanoma. Ann
Ophthalmol 9:949, 1977.)
|
WARTY DYSKERATOMA Warty dyskeratoma presents as an umbilicated keratotic papule resembling
a keratoacanthoma, but can also be confused with a squamous cell carcinoma. It
occurs primarily on the scalp, face, or neck. The characteristic histologic features are a cupshaped invagination filled
with keratinous material and acantholytic, dyskeratotic cells. Villi
of dermal papillae project into the base of the crater and are lined
by a single layer of basal cells. Corps ronds, which are dyskeratotic
cells containing a pyknotic nucleus surrounded by a clear halo, are
seen in the granular layer at the entrance of the invagination (Fig. 27). The presence of acantholytic dyskeratosis with corps ronds is reminiscent
of Darier's disease, but warty dyskeratoma is believed
to represent a distinct cutaneous tumor with histologic resemblance to
Darier's disease. 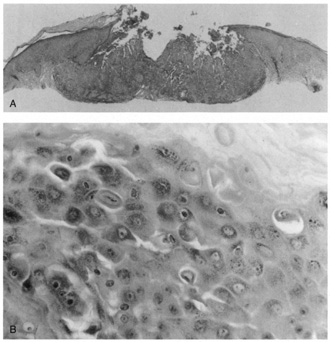 Fig. 27. Warty dyskeratoma. A. Scanning magnification shows a bulbous endophytic proliferation with acantholysis. B. High magnification shows dyskeratotic keratinocytes with a pyknotic nucleus (corp
ronds). Fig. 27. Warty dyskeratoma. A. Scanning magnification shows a bulbous endophytic proliferation with acantholysis. B. High magnification shows dyskeratotic keratinocytes with a pyknotic nucleus (corp
ronds).
|
Melanocytic Derivation MELANOCYTIC NEVUS. Nevi can be classified as ordinary nevi, spindle-cell nevi (Spitz), blue
nevi, cellular blue nevi, plexiform spindle-cell
nevi, or a combination of any of the above based on cell type and
location (Fig. 28). Melanocytic nevi first appear as small, tan, flat macules around 6–12 months
of age; they enlarge radially with body growth, and
regress in later life. Clinically, they are distinguished from melanoma
by their characteristic homogenous pigmentation, symmetric and well-defined
borders, and smaller diameter (<5 mm). Pertaining
to the periocular region, kissing nevi are congenital nevi that appear symmetrically on adjacent aspects of the
upper and lower eyelids and are formed secondary to melanocytic migration
to this aspect of the lids prior to separation of the embryonic
eyelids. Histologically, nevi are composed of benign melanocytes with
little pleomorphism or cellular atypia. The overall distribution of melanocytes
is symmetrical and they tend to form nests. Melanocytes become
smaller with less cytoplasm as they descend deeper into the dermis. Acquired
melanocytic nevi can be classified histologically as junctional
nevi (cells at dermoepidermal junction), intradermal nevi (cells
found only in dermis, not at junction), and compound
nevi (combined features of junctional and intradermal nevi) (Fig. 29). Increased risk of malignant transformation occurs as nests of nevus
cells migrate from the epidermal to dermal region; thus, junctional
and compound nevi more commonly have malignant transformation. 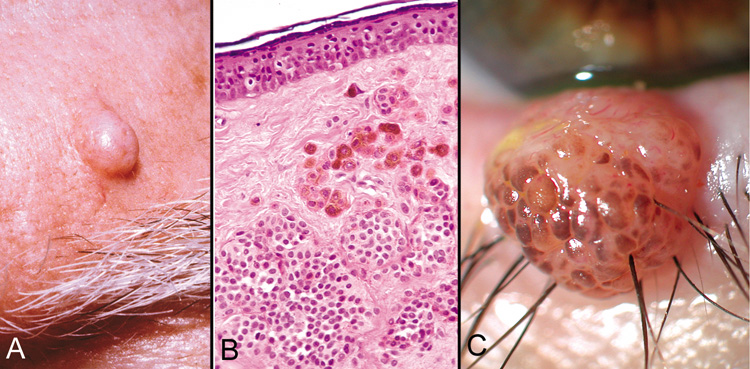 Fig. 28. Blue Nevus—Proliferation of elongated dermal melanocytes are noted
within the dermis of skin. The epithelium is normal (hematoxylin
and eosin stain). (Photo courtesy of William Morris, M.D.) Fig. 28. Blue Nevus—Proliferation of elongated dermal melanocytes are noted
within the dermis of skin. The epithelium is normal (hematoxylin
and eosin stain). (Photo courtesy of William Morris, M.D.)
|
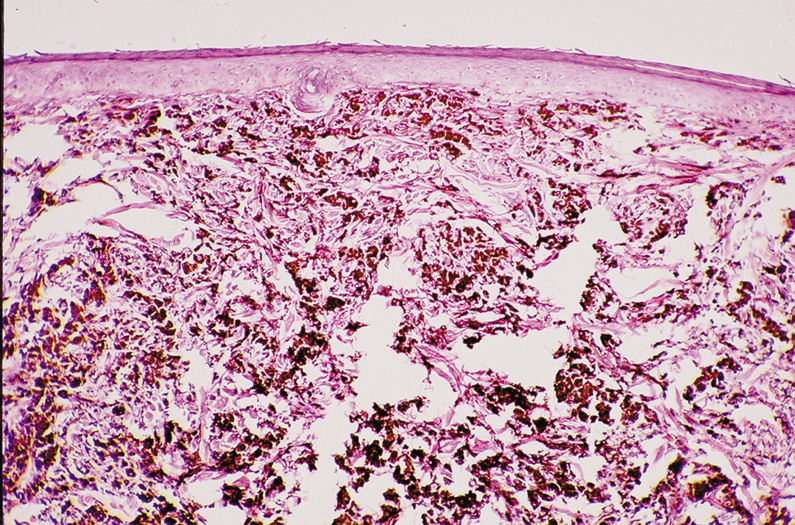 Fig. 29. Dermal (Intradermal) Nevus—A, C. Clinical photographs of two different dermal nevi. A. Smooth, dome-shaped appearance. C. Papillary appearance. B. Proliferation of nests of nevus cells in dermis of skin with the larger
more heavily pigmented cells more superficial than the smaller less
pigmented nevus cells. No nevus cells are present within the overlying
epithelium (hematoxylin and eosin stain). (Photos courtesy
of William Morris, M.D.) Fig. 29. Dermal (Intradermal) Nevus—A, C. Clinical photographs of two different dermal nevi. A. Smooth, dome-shaped appearance. C. Papillary appearance. B. Proliferation of nests of nevus cells in dermis of skin with the larger
more heavily pigmented cells more superficial than the smaller less
pigmented nevus cells. No nevus cells are present within the overlying
epithelium (hematoxylin and eosin stain). (Photos courtesy
of William Morris, M.D.)
|
SPITZ NEVUS. As mentioned previously, the Spitz nevus or spindle-cell nevus is
a benign, acquired melanocytic proliferation often involving the head
and neck that shares many features with melanoma; thus the two are often
confused. They are typically smaller, more symmetric, and more circumscribed
than their malignant counterpart, ending with junctional nests
at the periphery. Generally, appearance is uniform, with a wedge-shaped
pattern in the dermis, and, as with ordinary nevi, nuclear
and cellular sizes diminish with depth. There are few mitoses in the
dermal component and none in the deep dermis. There is no consensus
regarding histologic criteria for the Spitz nevus; it appears to be more
of a continuum from a benign spitz tumor to malignant melanoma. Those
lesions with a greater degree of atypical characteristics are considered
malignant melanoma. DYSPLASTIC NEVUS. Much debate surrounds the terminology for this melanocytic lesion, with
dysplastic nevus, atypical melanocytic nevus or Clark's nevus, falling
out of favor to the increasingly popular nevus with architectural
disorder and cytologic atypia (ARDCA). As with the name, there
is also considerable controversy surrounding the histologic criteria
distinguishing these lesions from ordinary nevi and melanoma. Clinically, these
nevi are larger (5–6 mm), have more asymmetric
borders that are less well circumscribed, and have more heterogeneous
pigmentation than ordinary nevi. Histologically, there is variable cytologic atypia, retraction of cytoplasm
with high nuclear-to-cytoplasm ratios, variable nuclear
enlargement, with nuclei approximately the size of nearby keratinocytic
nuclei, nuclear pleomorphism and hyperchromatism, and nucleoli
with moderate-to-severe atypia. Appendageal (Adnexal) Derivation SEBACEOUS. Nevus Sebaceus. Nevus sebaceus is a congenital lesion most often located on the scalp or
face. Being a sebaceous neoplasm, the lesion appears yellowish at birth
but, during childhood, loses its distinctive color and appears as
a hairless patch. During puberty, the sebaceous derivation of the lesion
again becomes apparent, and the lesion appears verrucous and nodular. A
number of benign and malignant tumors (see syringocystadenoma
papilliferum; basal cell carcinoma) may arise within the nevus
sebaceus during adulthood. Histologically, the features depend on the stage of the lesion. The most
distinctive changes are present in the verrucous stage and consist of
mature sebaceous glands in the dermis with overlying papillomatosis
and acanthosis of the epidermis (Fig. 30). Often, mature ectopic apocrine glands are seen in the deep dermis. 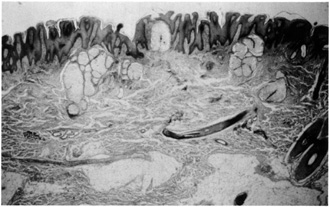 Fig. 30. Nevus sebaceous. Papillomatosis, acanthosis, and basalar hyperpigmentation
overlying mature sebaceous lobules in the dermis. Note ectopic apocrine
glands in the mid-dermis. Fig. 30. Nevus sebaceous. Papillomatosis, acanthosis, and basalar hyperpigmentation
overlying mature sebaceous lobules in the dermis. Note ectopic apocrine
glands in the mid-dermis.
|
Sebaceous Hyperplasia. Sebaceous hyperplasia occurs on the face of middle-aged people. Lesions
are often multiple and appear as small, yellowish, slightly umbilicated
papules with telangiectasia. Clinically, these can be confused
with basal cell carcinoma or sebaceous cell carcinoma. Histologically, multiple
mature sebaceous lobules are oriented around a central sebaceous
duct (Fig. 31). 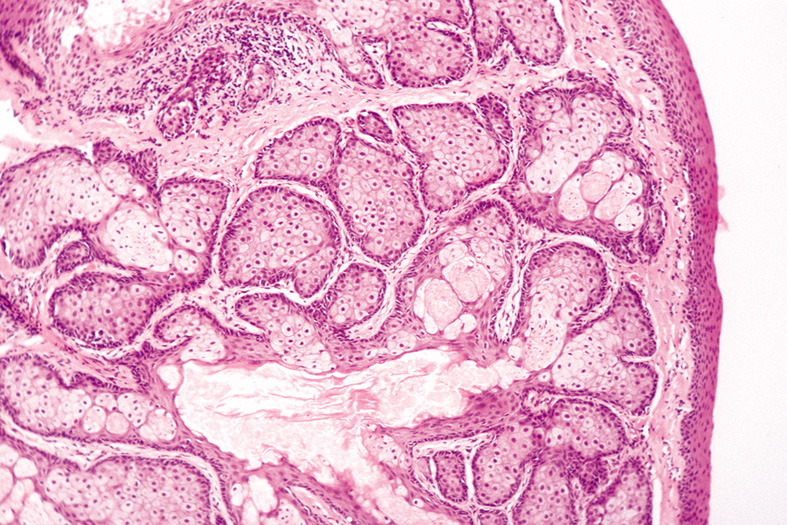 Fig. 31. Sebaceous Hyperplasia—Low-power photomicrograph showing an
enlarged sebaceous gland with many lobules and a dilated central duct (hematoxylin
and eosin stain). (Photo courtesy of William
Morris, M.D.) Fig. 31. Sebaceous Hyperplasia—Low-power photomicrograph showing an
enlarged sebaceous gland with many lobules and a dilated central duct (hematoxylin
and eosin stain). (Photo courtesy of William
Morris, M.D.)
|
Sebaceous Adenoma. Sebaceous adenoma is a rare tumor appearing as a circumscribed yellow nodule. The
appearance of a solitary sebaceous adenoma may herald an underlying
gastrointestinal malignancy (Muir-Torre syndrome). Histologically, lobules vary in size and shape and are composed of an approximately
equal ratio of mature and immature sebocytes (Fig. 32). 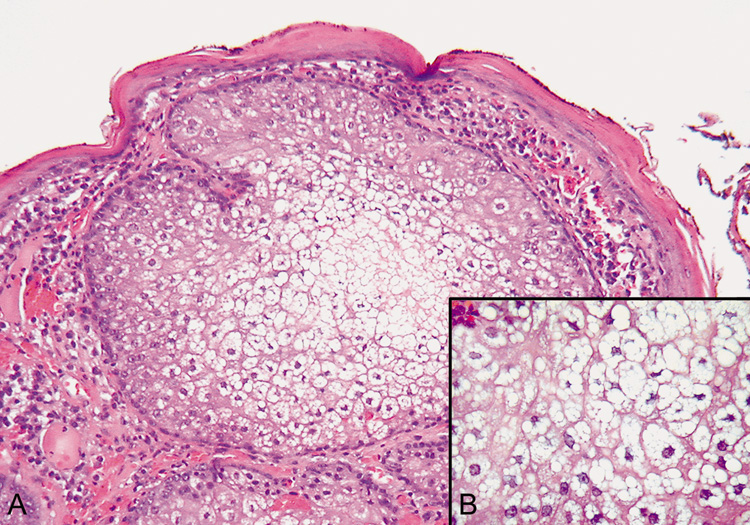 Fig. 32. Sebaceous Adenoma—A. Low-power photomicrograph showing sebaceous nodule containing undifferentiated
sebaceous cells in the periphery and mature sebaceous
cells in the center (hematoxylin and eosin stain). B. High-power view of the central mature sebaceous cells (hematoxylin
and eosin stain). (Photos courtesy of William Morris, M.D.) Fig. 32. Sebaceous Adenoma—A. Low-power photomicrograph showing sebaceous nodule containing undifferentiated
sebaceous cells in the periphery and mature sebaceous
cells in the center (hematoxylin and eosin stain). B. High-power view of the central mature sebaceous cells (hematoxylin
and eosin stain). (Photos courtesy of William Morris, M.D.)
|
FOLLICULAR TUMORS Trichoepithelioma Trichoepitheliomas may be solitary or multiple and occur most commonly
on the face. Multiple lesions are inherited in an autosomal-dominant
fashion. Clinically, the lesions are glistening, flesh-colored
papules or nodules. They can be confused with basal-cell
carcinoma. Trichoepitheliomas and cylindromas have coexisted in the same
patient. Histologically, numerous horn cysts surrounded by layers of flattened squamous
epithelium are the featured characteristics. There are also islands
of basaloid cells in variable numbers (Fig. 33). Often, a foreign-body, giant-cell reaction in the
stroma occurs due to the rupture of horn cysts. Desmoplastic trichoepithelioma, a
variant of trichoepithelioma, appears as an indurated plaque
with a raised annular border. Histologically, the lesion contains
narrow strands of tumor cells in a densely collagenous and acellular stroma. 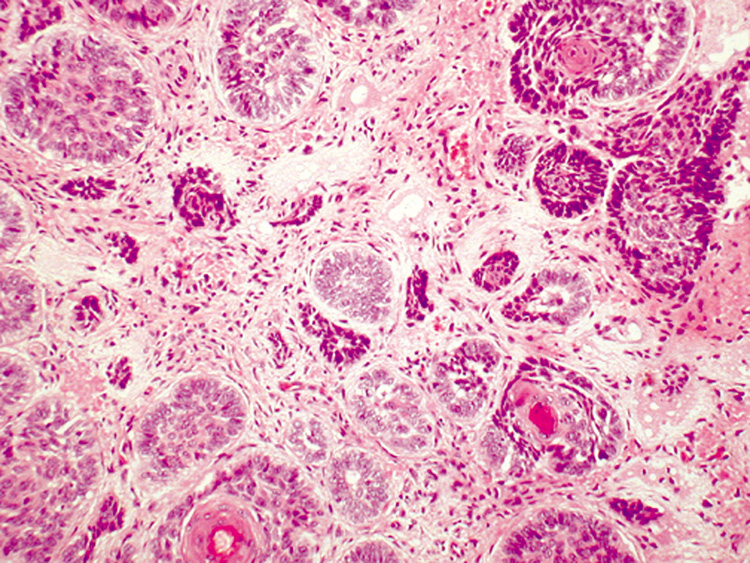 Fig. 33. Trichoepithelioma—Nodules of tumor demonstrating peripheral palisading
around central immature hair follicles (hematoxylin and eosin
stain). (Photo courtesy of William Morris, M.D.) Fig. 33. Trichoepithelioma—Nodules of tumor demonstrating peripheral palisading
around central immature hair follicles (hematoxylin and eosin
stain). (Photo courtesy of William Morris, M.D.)
|
Trichilemmoma Trichilemmomas are fairly common solitary tumors appearing as smooth or
keratotic papules on the head or neck, which should alert the clinician
to systemic malignancies such as breast, thyroid, and gastrointestinal
carcinomas. Multiple trichilemmomas are seen in Cowde's syndrome (multiple hamartoma syndrome). Histologically, one or several lobules of pale-staining cells extend
into the dermis. The cells stain pale due to their content of glycogen, which
can be demonstrated with PAS staining. The peripheral cells
composing the lobules palisade and are surrounded by a thickened basement
membrane (Fig. 34). 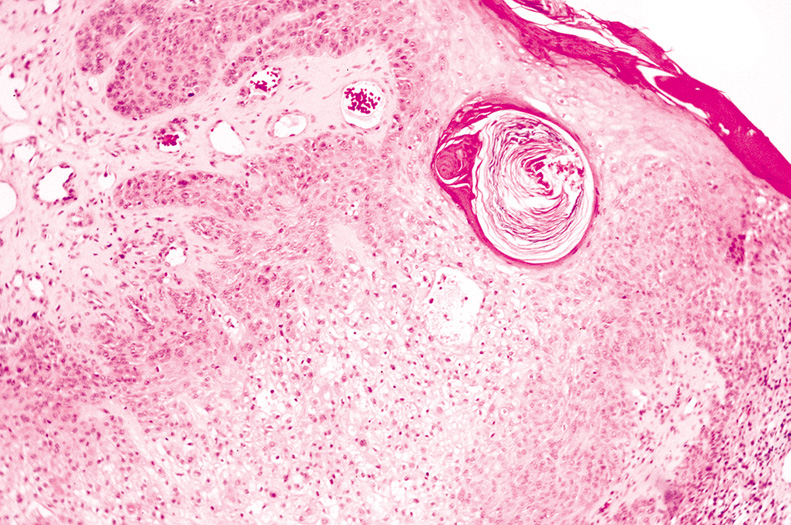 Fig. 34. Trichilemmoma—Low-power photomicrograph of tumor showing proliferation
of large clear cells in dermis (hematoxylin and eosin
stain). (Photo courtesy of William Morris, M.D.) Fig. 34. Trichilemmoma—Low-power photomicrograph of tumor showing proliferation
of large clear cells in dermis (hematoxylin and eosin
stain). (Photo courtesy of William Morris, M.D.)
|
Trichofolliculoma Trichofolliculomas are unique tumors clinically and histologically. They
often show a central pore that contains a tuft of wool-like white
hairs that are characteristic of this tumor. Histologically, a central cystic space represents an enlarged hair follicle. Surrounding
the central follicle, numerous small follicular structures
differentiate toward germinative pilar epithelium. The stroma of
these “secondary“ hair follicles contains numerous fibroblasts
and is oriented in parallel bundles. ECCRINE TUMORS Syringoma Syringomas are common, benign eccrine tumors most often occurring on the
lower eyelids of young women. They appear as small, flesh-colored
or yellowish papules. A large number of syringomas that develop in
crops on the trunk and extremities has been referred to as eruptive
syringomas. Histologically, numerous small ducts are lined by two rows of flattened
epithelial cells. Some ducts possess commalike extensions of the epithelial
cells, giving the appearance of tadpoles. The lumina of the ducts
contains an amorphous material, and the stroma is fibrous (Fig. 35). A variant of syringoma is the chondroid syringoma (mixed tumor
of the skin). These tumors are solitary, firm dermal or subcutaneous
nodules most often appearing on the head or neck. Histologically, chondroid
syringomas show tubular lumina that vary in size and shape
and may be cystically dilated and branching. A distinctive feature
is the stroma, which is faintly basophilic and contains fibroblasts with
surrounding halos reminiscent of normal cartilage. 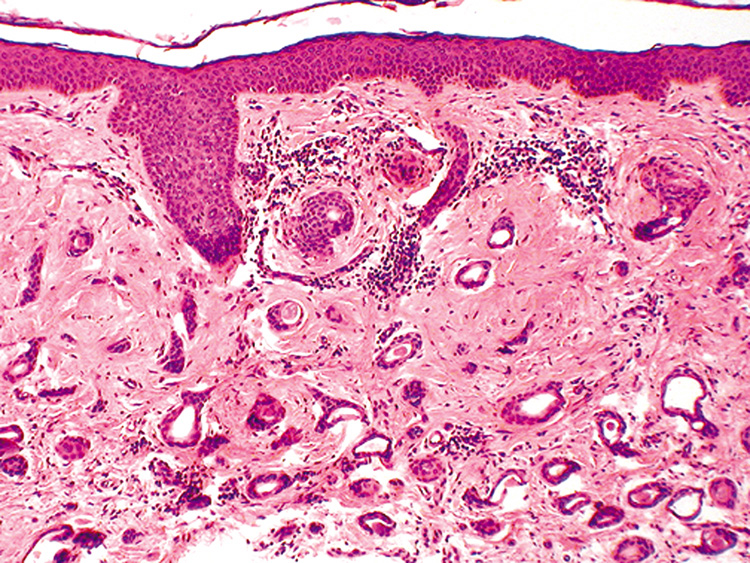 Fig. 35. Syringoma—Low-power photomicrograph showing proliferation
of multiple various-shaped ducts within a fibrous stroma. Some
of the ducts contain nonformed debris (hematoxylin and eosin stain). (Photo
courtesy of William Morris, M.D.) Fig. 35. Syringoma—Low-power photomicrograph showing proliferation
of multiple various-shaped ducts within a fibrous stroma. Some
of the ducts contain nonformed debris (hematoxylin and eosin stain). (Photo
courtesy of William Morris, M.D.)
|
Eccrine Spiradenoma These tumors are characteristically painful dermal nodules that are solitary
and, occasionally, multiple. They arise in adulthood and have no
characteristic location. Histologically, one or more large basophilic islands are present in the
dermis. The islands are composed of two cell types: small, dark cells
and large, pale cells. The latter cell may cluster into rosettes. There
is often hyaline material in the stroma surrounding the cells. Clear Cell Hidradenoma This tumor is known by several synonyms, including eccrine acrospiroma, nodular
hidradenoma, and solid cystic hidradenoma. It presents as an
intradermal nodule that may ulcerate or enlarge rapidly from internal
hemorrhage. Histologically, there is a well-circumscribed nodular or cystic
epithelial proliferation in the dermis. Within the tumor nodules, tubular
lumina lined by cuboidal or columnar secretory cells are found. Two
cell types of varying proportions are seen. One cell type is polyhedral
to fusiform and shows slightly basophilic cytoplasm. The other cell
type is round and contains a clear cytoplasm composed primarily of glycogen (Fig. 36). Focally, there may be squamous differentiation with horn pearl
formation. 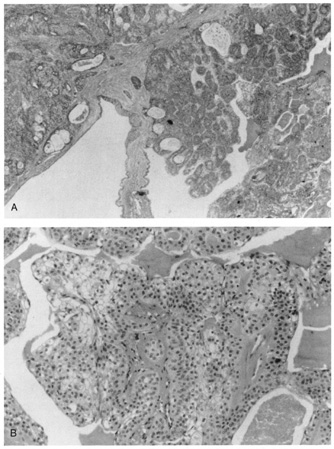 Fig. 36. Clear-cell hidradenoma. A. Solid islands and cystic spaces with duct formation in the dermis. B. High magnification shows clear cells. Fig. 36. Clear-cell hidradenoma. A. Solid islands and cystic spaces with duct formation in the dermis. B. High magnification shows clear cells.
|
Eccrine Poroma Eccrine poromas occur primarily on the sole of the foot but have been observed
on other areas of the skin (Fig. 37). They are firm, dome-shaped, slightly pedunculated tumors
that may appear pinkish-red. 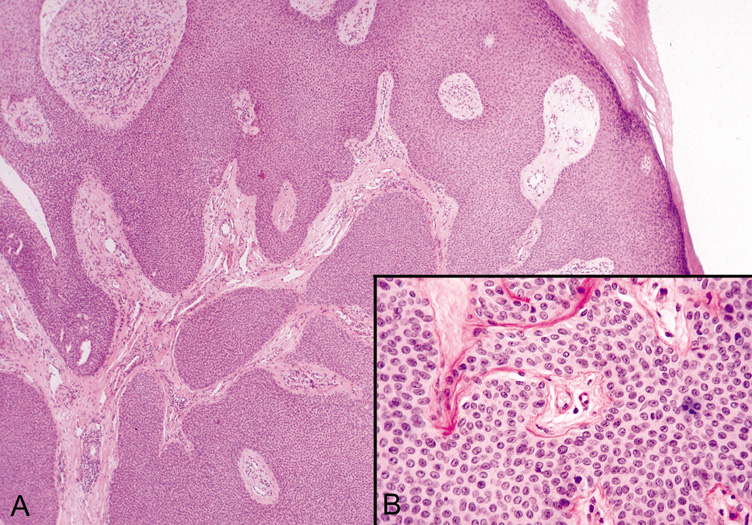 Fig. 37. Eccrine Poroma—A. Low-power and (B) high-power photomicrographs showing broad bands of epithelial cells
extending deep into the dermis. (Photos courtesy of William
Morris, M.D.) Fig. 37. Eccrine Poroma—A. Low-power and (B) high-power photomicrographs showing broad bands of epithelial cells
extending deep into the dermis. (Photos courtesy of William
Morris, M.D.)
|
Because eccrine poromas arise from the eccrine duct coursing through the
epidermis, the tumor shows a downward proliferation of broad anastomosing
cell masses into the dermis. The tumor cells are readily distinguished
from the surrounding keratinocytes because they are small and cuboidal
and possess a round basophilic nucleus. Glycogen is found in their
cytoplasm and can be readily demonstrated with PAS stain. In most
cases, narrow ductal lumina lined by an eosinophilic cuticle are seen. APOCRINE TUMORS Cylindroma This tumor typically occurs on the scalp and appears in early adulthood. Multiple
cylindromas are dominantly inherited and are frequently associated
with trichoepitheliomas. Lesions on the scalp may occur in such
large numbers that they cover the whole scalp like a turban, hence the
synonym “turban tumor.“ Histologically, numerous islands of epithelial cells vary in size and shape
and are surrounded by a hyaline sheath. Distinctively, the islands
of cells fit together like the pieces of a jigsaw puzzle (Fig. 38). There are two cell types: cells with small, dark nuclei and scant
cytoplasm that are found at the periphery of the islands and cells
with large pale nuclei that are present in the center of the islands. Usually, tubular
lumina are found, and may be lined by cells demonstrating
decapitation secretion similar to the secretory cells seen in normal
apocrine glands. 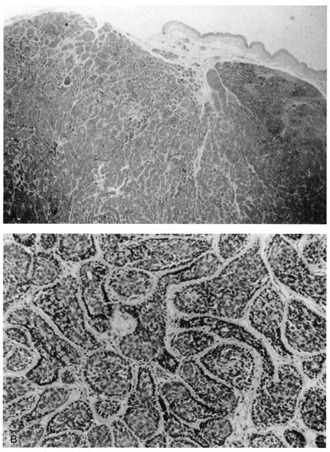 Fig. 38. Cylindroma. A. Basaloid islands fill the dermis. B. Islands are surrounded by a hyaline sheath and fit together in a jigsaw
puzzle arrangement. Fig. 38. Cylindroma. A. Basaloid islands fill the dermis. B. Islands are surrounded by a hyaline sheath and fit together in a jigsaw
puzzle arrangement.
|
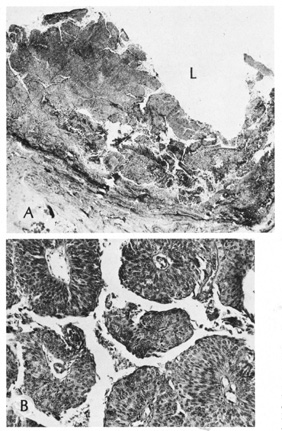
Syringocystadenoma Papilliferum This tumor occurs primarily on the scalp or face and usually is noted at
birth or early childhood. Clinically, the lesion consists of a papule
or plaque that increases in size and becomes verrucous at puberty. Most
syringocystadenomas develop within a preexisting nevus sebaceus. Histologically, the epidermis shows papillomatosis and cystic invaginations
into the dermis. Papillary projections lined by two rows of glandular
epithelium extend into the lower portion of the cystic spaces (Fig. 39). The luminal row of cells are columnar and may show decapitation
secretion, whereas the inner row of cells are small and cuboidal. A characteristic
feature of this tumor is a dense plasmacytic infiltrate
in the stroma, especially the papillary projections. 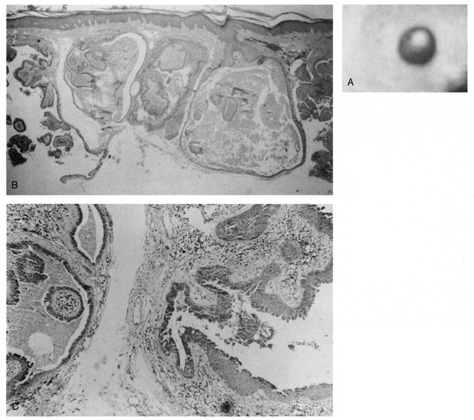 Fig. 39. Syringocystadenoma papilliferum. A. Clinical appearance of lesion. B. Numerous cystic spaces in the dermis contain papillary projections. C. Apical (decapitation) secretion from the papillary projection
with a dense plasmacytic infiltrate in the stroma. Fig. 39. Syringocystadenoma papilliferum. A. Clinical appearance of lesion. B. Numerous cystic spaces in the dermis contain papillary projections. C. Apical (decapitation) secretion from the papillary projection
with a dense plasmacytic infiltrate in the stroma.
|
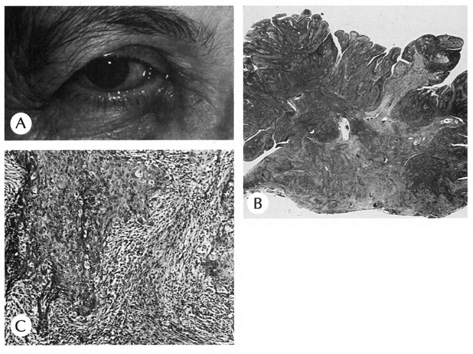
OTHER TUMORS Neurofibroma Neurofibromas are common benign tumors of the skin, which may be solitary
or multiple. Multiple neurofibromas are associated with café au
lait macules in the skin and, in von Recklinghausen's disease, Lisch
nodules in the iris. Clinically, the lesions are soft, flesh colored, and
pedunculated. Plexiform neurofibromas are typically found
on the upper lids and are clinically described as having a “bag
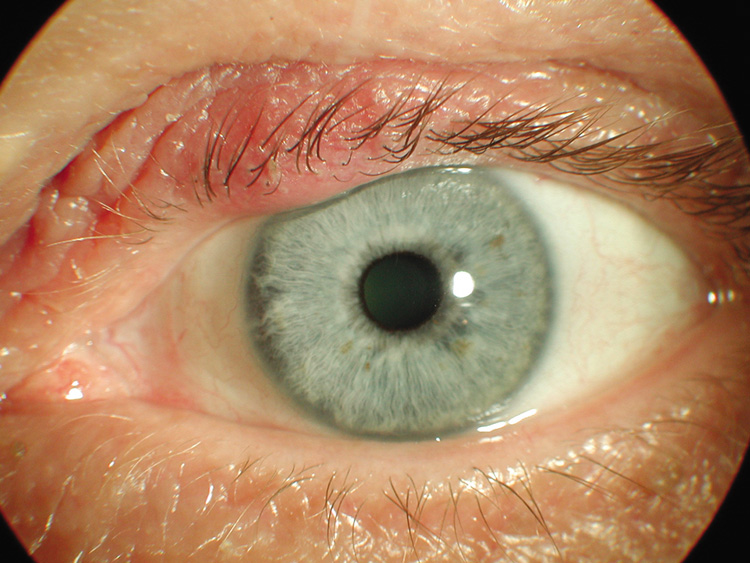
of worms“ consistency (Fig. 40).  Fig. 40. Plexiform neurofibroma—A. Clinical photograph of patient with plexiform neurofibroma involving the
upper lid. B. Low-power photomicrograph showing enlarged abnormal nerves composed
of endoneural fibroblasts, Schwann cells, and axons (hematoxylin
and eosin stain). (Photos courtesy of William Morris, M.D.) Fig. 40. Plexiform neurofibroma—A. Clinical photograph of patient with plexiform neurofibroma involving the
upper lid. B. Low-power photomicrograph showing enlarged abnormal nerves composed
of endoneural fibroblasts, Schwann cells, and axons (hematoxylin
and eosin stain). (Photos courtesy of William Morris, M.D.)
|
Histologically, the tumors are well circumscribed but not encapsulated
and may extend into the subcutaneous fat. The nuclei are elongated and
wavy and show tapering at their ends. They are embedded in loose, delicate
collagen fibers. The stroma is pale and may show significant mucoid
degeneration. Mast cells are present in considerable numbers. Dermatofibroma (Histiocytoma) Dermatofibromas are common fibrous tumors that most commonly occur on the
extremities. They are firm, red-brown papules or nodules that
are dome shaped or centrally depressed. Histologically, the lesions are divided into two types, although they tend
to overlap in many instances. The fibrous type is composed of cells
resembling fibroblasts embedded in intertwining and anastomosing bands
of collagen that blend imperceptibly into the surrounding dermis. The
cellular type consists predominantly of cells with round-to-oval
nuclei and abundant cytoplasm resembling histiocytes. In both
types, scattered small capillaries are present but may be numerous
and associated with hemorrhage and hemosiderin deposition. Vascular Tumors Several vascular tumors may occur on the skin around the eye, including
benign lesions, such as hemangiomas (Fig. 41), pyogenic granulomas, spider nevi, venous lakes, and port wine stains, as
well as malignant lesions, such as Kaposi's sarcoma and
angiosarcoma. 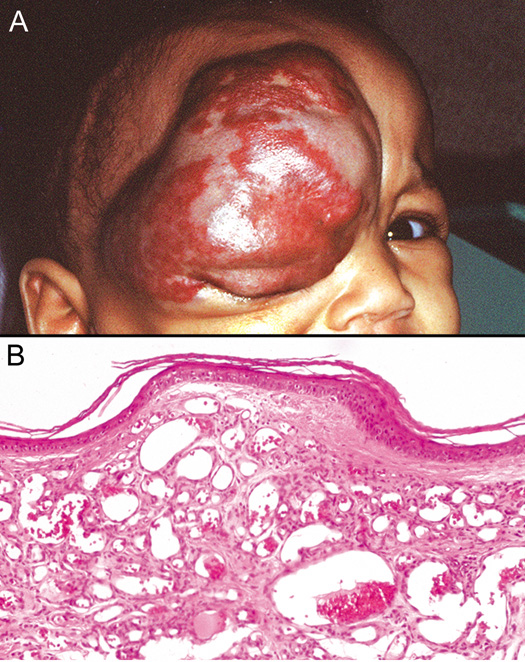 Fig. 41. Capillary Hemangioma—A. Clinical photograph of child with large capillary hemangioma involving
the face and upper eyelid. B. The capillary hemangioma is composed of multiple small endothelial-lined, blood-containing capillaries (hematoxylin and eosin
stain). (Photos courtesy of William Morris, M.D.) Fig. 41. Capillary Hemangioma—A. Clinical photograph of child with large capillary hemangioma involving
the face and upper eyelid. B. The capillary hemangioma is composed of multiple small endothelial-lined, blood-containing capillaries (hematoxylin and eosin
stain). (Photos courtesy of William Morris, M.D.)
|
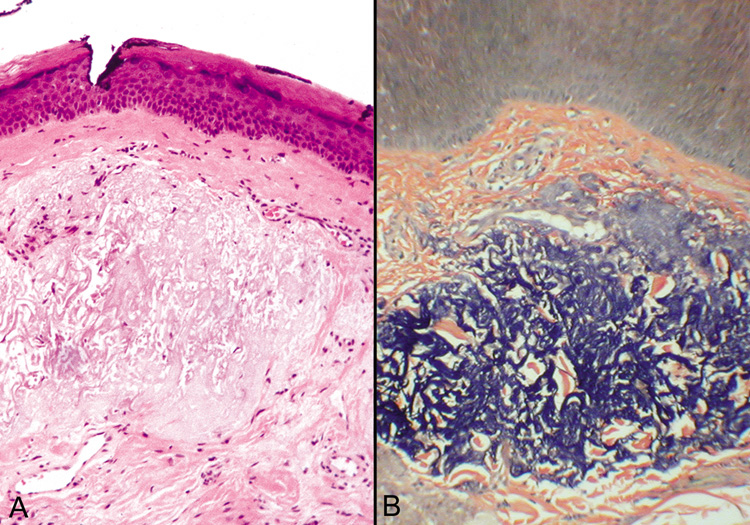
PREMALIGNANT TUMORS Actinic Keratosis Actinic keratoses occur on areas of chronically sun-exposed skin
and are more likely to develop in individuals with a fair complexion. They
are the most common premalignant skin lesion in adults. The risk
of malignant transformation is thought to be 0.25% per lesion
per year. Clinically, lesions are minimally elevated, slightly scaly, and
flesh colored to pink. A cutaneous horn may overlie an actinic keratosis
but also may overlie other lesions, such as verrucae and seborrheic
keratoses. Histologically, the characteristic findings are focal-to-confluent
parakeratosis overlying an epidermis of variable thickness. There
is atypia and mitoses of the lower epidermal layers, with the formation
of buds extending into the superficial dermis. The dermis shows
solar elastosis and a patchy inflammatory infiltrate (Fig. 42). 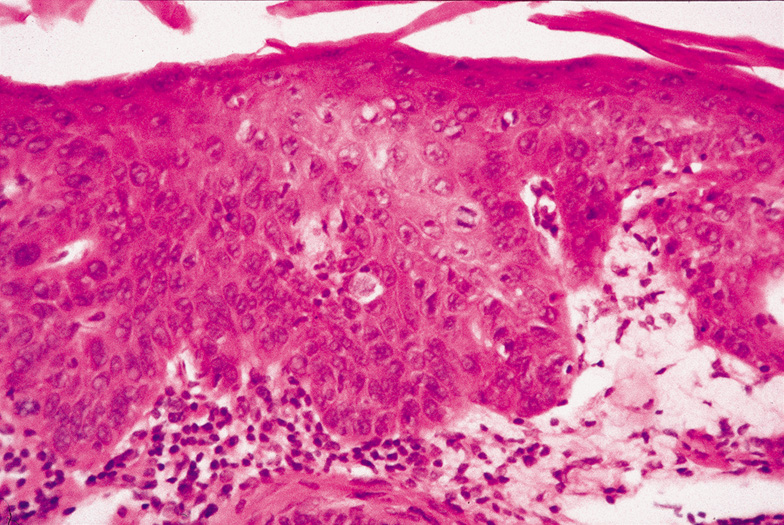 Fig. 42. Actinic (Solar) Keratosis—The epithelium is thickened and
the epithelial cells are dysplastic. No invasion of the dermis is present. A
mitotic figure is located just to the right of center in this
photomicrograph (hematoxylin and eosin stain). (Courtesy
of William Morris, M.D.) Fig. 42. Actinic (Solar) Keratosis—The epithelium is thickened and
the epithelial cells are dysplastic. No invasion of the dermis is present. A
mitotic figure is located just to the right of center in this
photomicrograph (hematoxylin and eosin stain). (Courtesy
of William Morris, M.D.)
|
Squamous Cell Carcinoma In Situ When epidermal atypia becomes full thickness, the clinical lesion is more
indurated than actinic keratosis and becomes a plaque. Squamous cell
carcinoma in situ may arise on sun-exposed skin from an actinic
keratosis or de novo on sun-protected skin. The latter lesion
is referred to as Bowen's disease. Squamous cell carcinoma in situ
may enlarge slowly for many years or may invade the dermis. Histologically, the epidermis is replaced by an atypical proliferation
of keratinocytes showing nuclear hyperchromatism and pleomorphism. There
are many dyskeratotic cells and mitotic figures, some of which may
be atypical (Fig. 43). The overlying stratum corneum is parakeratotic. If occurring on
sun-exposed skin, there will be solar elastosis. 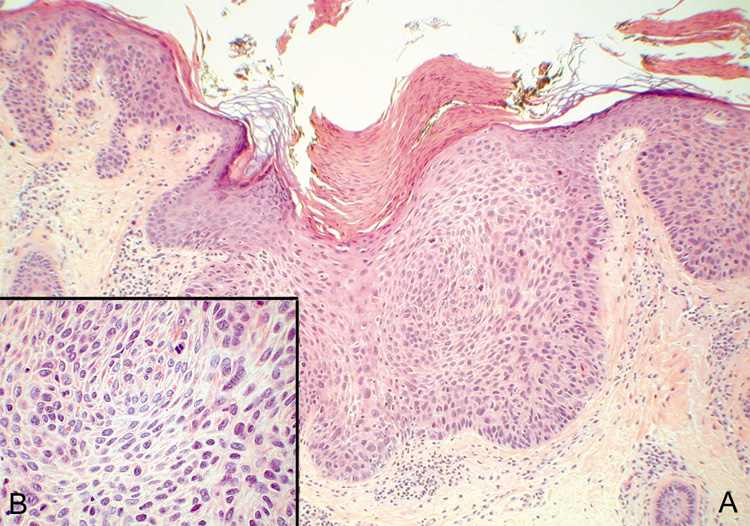 Fig. 43. Carcinoma In Situ—A. Low-power photomicrograph demonstrating parakeratosis, hyperkeratosis, and
epithelial dysplasia confined to the epithelium. No invasion
is present. B. High-power photomicrograph revealing pleomorphic cells and active
mitosis (hematoxylin and eosin stain). (Photos courtesy
of William Morris, M.D.) Fig. 43. Carcinoma In Situ—A. Low-power photomicrograph demonstrating parakeratosis, hyperkeratosis, and
epithelial dysplasia confined to the epithelium. No invasion
is present. B. High-power photomicrograph revealing pleomorphic cells and active
mitosis (hematoxylin and eosin stain). (Photos courtesy
of William Morris, M.D.)
|
MALIGNANT TUMORS Epithelial Tumors BASAL CELL CARCINOMA. There are over a million new cases of nonmelanoma skin cancer diagnosed
each year, of which more than 75% are basal cell carcinomas. Basal
cell carcinoma is the most common malignant tumor of the eyelids (85–95% of all malignant eyelid tumors), occurring
most frequently on the inner portion of the lower eyelid, followed
by the medial canthus, upper eyelid, and lateral canthus. There is an
equal sex distribution; most tumors occur in the elderly White patient
population. The clinical appearance varies, but the most common clinical
presentation is a shiny, waxy papule with a rolled border and telangiectasia. Ulceration
and pigmentation may or may not occur. Basal cell
carcinomas are slow growing, locally invasive, and, rarely, metastasize (Fig. 44). 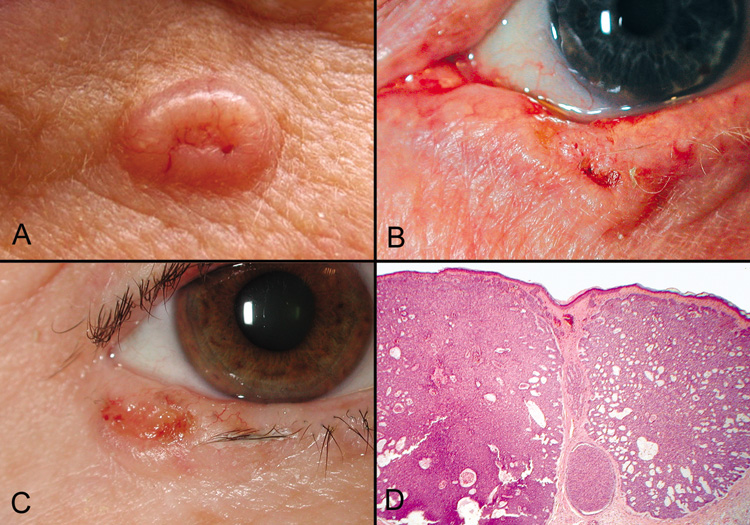 Fig. 44. Basal Cell Carcinoma—A, B, C. Clinical photographs of various appearances of basal cell carcinomas, the
typical “rodent ulcer.” Notice the loss of lashes at the
tumor site and in adjacent areas. D. Low-power photomicrograph of nodular variant of basal cell carcinoma (hematoxylin
and eosin stain). (Photos courtesy of
William Morris, M.D.) Fig. 44. Basal Cell Carcinoma—A, B, C. Clinical photographs of various appearances of basal cell carcinomas, the
typical “rodent ulcer.” Notice the loss of lashes at the
tumor site and in adjacent areas. D. Low-power photomicrograph of nodular variant of basal cell carcinoma (hematoxylin
and eosin stain). (Photos courtesy of
William Morris, M.D.)
|
Histologically, tumors can be classified into one of three types: nodular, superficial, and
morpheaform. Nodular basal cell carcinoma is the
most common type and consists of small, medium, or large cell islands
that resemble the basal cell of the epidermis. The tumor cells are characterized
by large, oval, or elongated nuclei and contain little cytoplasm. The
nuclei, as a rule, have a rather uniform appearance, do not
show a pronounced variation in size or staining intensity, and do not
demonstrate atypical mitoses (Fig. 45A-C). A helpful diagnostic feature is that nuclei of the peripheral cell
layer of the tumor masses have a palisading arrangement. The stroma
surrounding the tumor islands is mucinous, and spaces or lucunae form
between the tumor and its stroma. In some cases, central necrosis may
develop within the tumor islands forming cystic spaces. Other variants
of nodular basal cell carcinoma include keratotic, adenoidal, and pigmented
tumors. 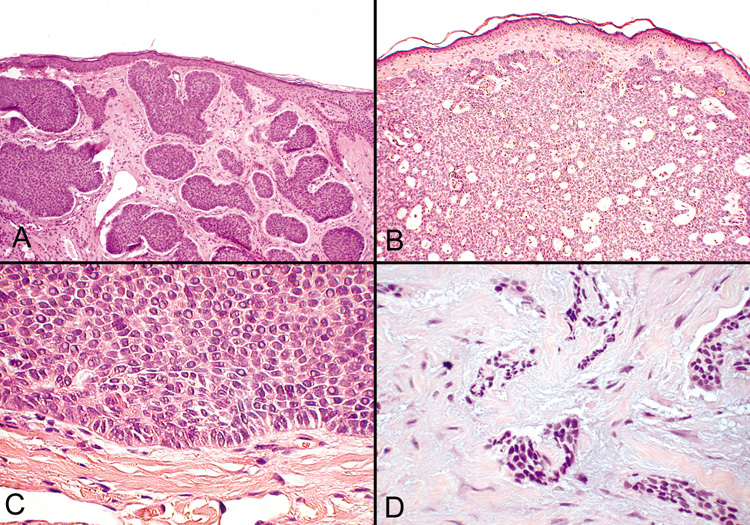 Fig. 45. Basal Cell Carcinoma—A, B. Photomicrographs of nodular basal cell carcinomas. C. Typical palisading of tumor cells along the periphery of the tumor nodule. D. The morpheaform variant shows longs strands or clumps of tumor cells invading
a dense fibrous stroma (hematoxylin and eosin stain). (Photos
courtesy of William Morris, M.D.) Fig. 45. Basal Cell Carcinoma—A, B. Photomicrographs of nodular basal cell carcinomas. C. Typical palisading of tumor cells along the periphery of the tumor nodule. D. The morpheaform variant shows longs strands or clumps of tumor cells invading
a dense fibrous stroma (hematoxylin and eosin stain). (Photos
courtesy of William Morris, M.D.)
|
Superficial basal cell carcinoma shows irregular buds of basaloid cells
arising from multiple foci of the undersurface of the epidermis. The
peripheral cell layer of the tumor islands shows palisading, and, often, there
are retraction spaces from the stroma. Due to this tumor's
superficial location, these are the most readily curable types of basal
cell carcinoma. In contrast, the morpheaform basal cell carcinoma is more likely to recur
after routine surgical excision because this tumor shows numerous small
groups of closely packed tumor cells arranged in elongated strands. The
tumor is embedded in a dense fibrous stroma reminiscent of the
fibrosis seen in scleroderma or morphea (hence the name morpheaform). Most
of the tumor strands will show retraction spaces from the
stroma. The tumor may extend deep into the dermis and, therefore, is
more clinically aggressive and more difficult to cure surgically (Fig. 45D). Squamous Cell Carcinoma Squamous cell carcinoma is the second most common malignancy of the skin, but
only rarely involves the eyelid (approximately 5% of
malignant eyelid tumors). The opposite is true of the conjunctiva, in
which squamous cell carcinoma is the most common epithelial malignancy; basal
cell carcinoma almost never occurs. Clinically, the lesions
commonly show a central ulceration surrounded by a raised, indurated
border (Fig. 46). The histology of squamous cell carcinoma has been discussed in
previous chapters. 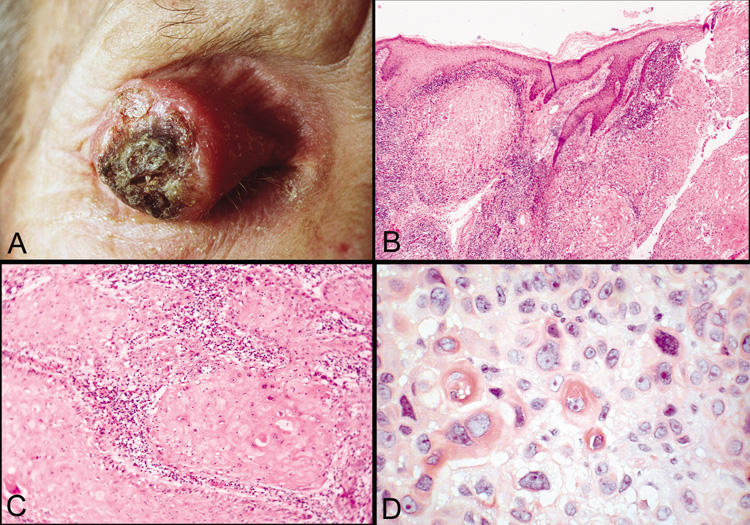 Fig. 46. Squamous cell carcinoma—A. Clinical photograph of large squamous cell carcinoma on the upper eyelid. B. Low-power photomicrograph of tumor demonstrating eosinophilic squamous
cells that have invaded the dermis and subcutaneous tissue. (hematoxylin
and eosin stain). C. Higher-power photomicrograph showing lobules of invading tumor
cells (hematoxylin and eosin stain). D. High-power photomicrograph illustrating dyskeratotic and atypical
cells within tumor (hematoxylin and eosin stain). (Photos
courtesy of William Morris, M.D.) Fig. 46. Squamous cell carcinoma—A. Clinical photograph of large squamous cell carcinoma on the upper eyelid. B. Low-power photomicrograph of tumor demonstrating eosinophilic squamous
cells that have invaded the dermis and subcutaneous tissue. (hematoxylin
and eosin stain). C. Higher-power photomicrograph showing lobules of invading tumor
cells (hematoxylin and eosin stain). D. High-power photomicrograph illustrating dyskeratotic and atypical
cells within tumor (hematoxylin and eosin stain). (Photos
courtesy of William Morris, M.D.)
|
Squamous cell carcinomas should be differentiated from pseudocarcinomatous
or pseudoepitheliomatous hyperplasia, which represents a reactive
downward proliferation of the epidermis that occurs with chronic proliferative
inflammatory processes, at the edge of chronic ulcers, or overlying
some tumors. Pseudoepitheliomatous hyperplasia resembles well-differentiated
squamous cell carcinoma histologically and shows
irregular invasion of the dermis by uneven, jagged, sharply pointed epidermal
masses and strands with horn pearl formation and often numerous
mitoses. Differentiation from squamous cell carcinoma cannot always
be made histologically, but the minimal or absent individual cell keratinization
and nuclear atypia in the appropriate clinical setting would
favor a diagnosis of pseudoepitheliomatous hyperplasia. Keratoacanthoma Keratoacanthomas are rapidly growing lesions that are most often solitary
but may be multiple. Solitary keratoacanthomas occur in middle-aged
or elderly people and appear as dome-shaped nodules with
horn-filled central craters. They are found primarily on sun-exposed
cutaneous surfaces and may be confused clinically and histologically
for squamous cell carcinoma. Although once thought to be
a benign entity, most now classify them as well-differentiated
squamous cell carcinomas that are capable of spontaneous regression. Keratoacanthomas
attain their full size within 6 to 8 weeks and slowly
involute, leaving depressed scars. Multiple keratoacanthomas may begin
in childhood and affect any surface of the skin, including the palms
and soles. Muir-Torre syndrome is a condition characterized by
the association of multiple keratoacanthomas and sebaceous adenomas, as
well as internal malignancies. The architectural appearance is the most distinctive feature of the lesion
for the histologic diagnosis of keratoacanthoma, which is made on
low power or scanning magnification. There is a cup-shaped invagination
of the epidermis filled with horny material. At the base of the
crater, there is a bulbous proliferation of squamous epithelium showing
abundant eosinophilic, glassy cytoplasm with little nuclear atypia. A
few dyskeratotic cells may be seen, and mitotic figures may be present. There
is often an inflammatory infiltrate containing lymphoid cells
and eosinophils surrounding the epithelial proliferation (Fig. 47). 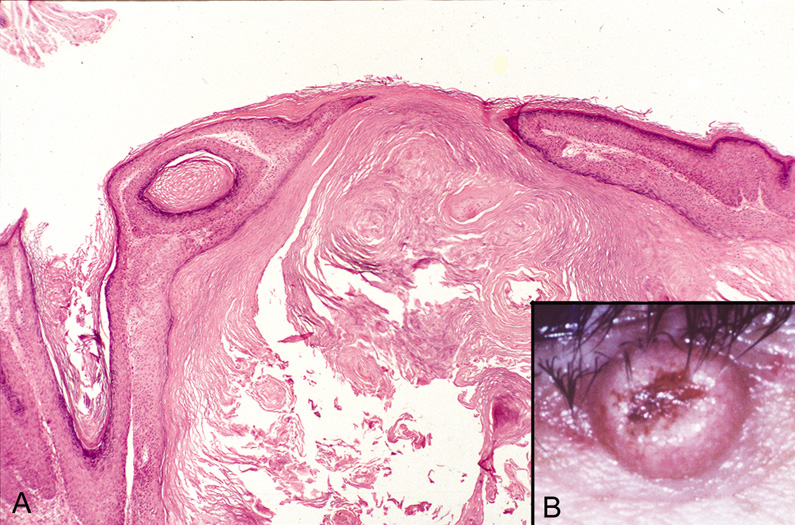 Fig. 47. Keratoacanthoma—A. Crater-shaped skin lesion filled with keratin. Parakeratosis and
inflammation near the base of the lesion are common. These tumors may
grow rapidly and may be confused microscopically with squamous cell
carcinoma (hematoxylin and eosin stain). B. Clinical photograph of keratoacanthoma. (Photos courtesy of William
Morris, M.D.) Fig. 47. Keratoacanthoma—A. Crater-shaped skin lesion filled with keratin. Parakeratosis and
inflammation near the base of the lesion are common. These tumors may
grow rapidly and may be confused microscopically with squamous cell
carcinoma (hematoxylin and eosin stain). B. Clinical photograph of keratoacanthoma. (Photos courtesy of William
Morris, M.D.)
|
MELANOCYTIC TUMORS Melanoma Cutaneous malignant melanoma is the most rapidly increasing form of cancer
in the United States. Cutaneous melanoma of the periocular skin, however, is
relatively rare and accounts for less than 1% of all
malignant eyelid lesions. Clinically, malignant lesions are usually raised, dark-yet-variably pigmented lesions greater than 10 mm, with
asymmetric and irregular borders. Malignant melanoma, although rare in the periocular region, has the highest
mortality rate of all malignant eyelid tumors. Local, regional, and
distant metastasis appears to be related to both original tumor's
depth of invasion as well as the thickness of the margins of excision. Histologically, melanoma can be classified based on the location in the
skin (epidermal, dermal, subcutaneous), disposition and frequency (pagetoid, lentiginous, nested, single-cell infiltrating, nodular), morphologic features, and cell type (epithelioid, spindle, dendritic, nevuslike, small cell, balloon cell, clear
cell, anaplastic giant cell, rhabdoid, signet ring). The most common
type is intraepidermal pagetoid (also known as superficial spreading) with
epithelioid or polygonal cell types; however, more
than one cell type is often present. Principle histologic features distinguishing
melanoma from dysplastic nevi and Spitz tumors include loss
of rete ridges, uniform cytologic atypia, diminished or absent maturation
of the dermal component of cells, patchy or bandlike mononuclear
cell infiltrates that can be absent in thick melanomas, and increasing
numbers of mitotic figures in the deeper dermis. Often, variable cytoplasm
is present depending on cytologic type, and cells are highly pleomorphic, with
high nuclear-to-cytoplasm ratios, hyperchromatism, prominent
variable nucleoli, and thickened nuclear membranes. APPENDAGEAL (ADNEXAL) TUMORS Sebaceous Carcinoma Sebaceous carcinoma is also rare, comprising less than 1% of all
skin malignancies, and up to 5% of malignant epithelial eyelid
tumors, but is second in mortality only to malignant melanoma. This is
due, in part, to its frequent clinical misdiagnosis, with number one
being chalazion, followed by chronic blepharoconjuncitivitis. Incidence
in Whites is low (1.13–3.2% of malignant eyelid lesions), but
is as high as 32% in Asians. Sebaceous carcinomas
usually originate from the meibomian glands, or less commonly zeisian
glands, and are more commonly found on the upper eyelid. There is
often orbital invasion. Sebaceous carcinomas of the eyelids quite frequently
cause regional metastases, and death may occur. Histologically, irregular lobular masses of cells focally show abundant
foamy cytoplasm, signifying sebaceous differentiation. Many undifferentiated
cells show nuclear atypia with variability in size, shape, and
staining characteristics. The undifferentiated cells show eosinophilic
cytoplasm, but fat stains will demonstrate lipid deposits within these
cells (Fig. 48). Sebaceous carcinoma of the eyelids has a tendency for pagetoid
spread of malignant cells into the conjunctiva or overlying epidermis
of the eyelid, which is a rare finding in extraocular sebaceous carcinomas. 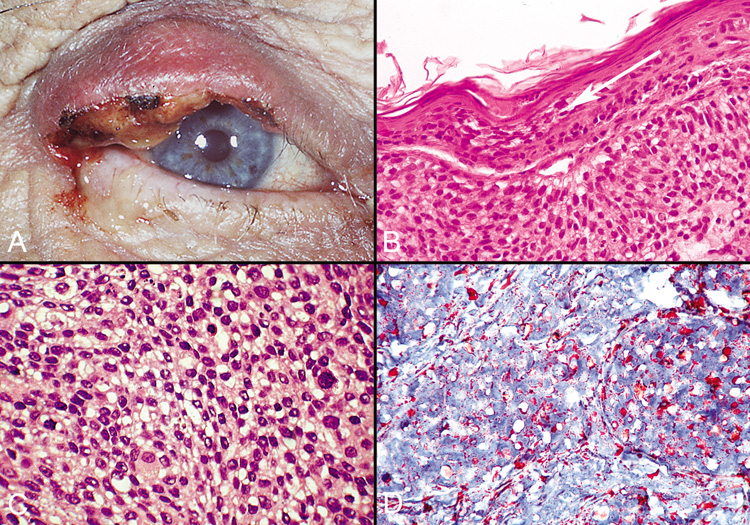 Fig. 48. Sebaceous Carcinoma—A. Clinical photograph of patient with sebaceous carcinoma. B. Photomicrograph showing pagetoid spread of tumor cells within the epithelium (hematoxylin
and eosin stain). C. High-power photomicrograph demonstrating foamy cytoplasm typical
of this tumor (hematoxylin and eosin stain). D. Oil Red O stain identifies the lipid material produced by the tumor cells (Oil
Red O stain). (Photos courtesy of William Morris, M.D.) Fig. 48. Sebaceous Carcinoma—A. Clinical photograph of patient with sebaceous carcinoma. B. Photomicrograph showing pagetoid spread of tumor cells within the epithelium (hematoxylin
and eosin stain). C. High-power photomicrograph demonstrating foamy cytoplasm typical
of this tumor (hematoxylin and eosin stain). D. Oil Red O stain identifies the lipid material produced by the tumor cells (Oil
Red O stain). (Photos courtesy of William Morris, M.D.)
|
FOLLICULAR CARCINOMAS Pilomatrix Carcinoma Pilomatrix carcinoma may develop from malignant transformation of a benign
pilomatricoma or may arise de novo. Clinically, carcinomas cannot
be differentiated from benign lesions. Histologically, the tumor shows proliferations of large anaplastic basophilic
cells with numerous mitoses. Focally, eosinophilic shadow cells
characteristic of matrical differentiation are present. Often, these
tumors show necrosis. Trichilemmal Carcinoma Trichilemmal carcinomas are rare tumors that occur largely on the face
or ears. The tumor is histologically invasive and is composed of atypical
clear cells reminiscent of the outer root sheath of a hair follicle. The
atypical cells are hyperchromatic and pleomorphic and contain abundant
amounts of glycogen in their cytoplasm. Eccrine Carcinoma Malignant sweat gland tumors are rare and, therefore, are difficult to
diagnose clinically and histologically. The rarity of these lesions also
poses a problem with classification. The most common form of eccrine carcinoma is ductal eccrine carcinoma. These
tumors usually occur as nodules in the skin of the head and neck
in middle-aged or elderly patients. Clinically, a diagnosis of
nonmelanoma skin cancer or cyst may be made. The lesions are centered
in the dermis and have a firm consistency. Histologically, the tumor is composed of anastomosing nests and cords of
epithelial cells showing round or oval nuclei and demonstrating variably
developed lumen formation (Fig. 49). Mitotic activity is virtually always present, and the tumor nests
are separated by a fibrous stroma. 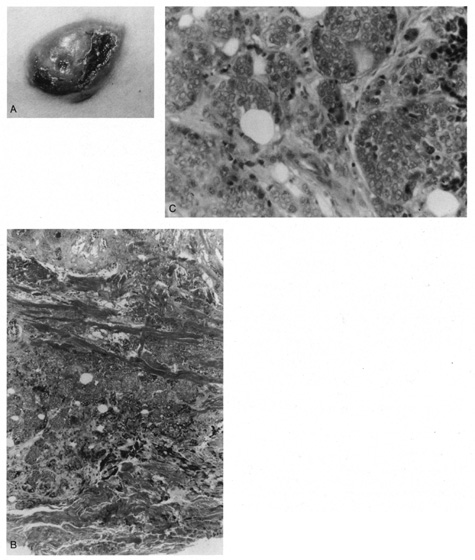 Fig. 49. Eccrine carcinoma. A. Clinical appearance of a large, focal ulcerated nodule. B. Atypical islands with focal duct formation in the deep dermis. C. High magnification shows cellular atypia and duct formation. Fig. 49. Eccrine carcinoma. A. Clinical appearance of a large, focal ulcerated nodule. B. Atypical islands with focal duct formation in the deep dermis. C. High magnification shows cellular atypia and duct formation.
|
Eccrine porocarcinoma is the second most common type of eccrine carcinoma
and tends to occur on the lower extremities of young adults. Histologically, it
is similar to benign eccrine poroma, except that there is
nuclear atypia and numerous mitoses. Other types of eccrine carcinoma are mucinous eccrine carcinoma, adenoid
cystic eccrine carcinoma, and the malignant counterparts of chondroid
syringoma, eccrine spiradenoma, and clear-cell hidradenoma. Apocrine Carcinoma Apocrine carcinomas may occur in one of two clinical forms. The first is
characterized by tumor masses that are located exclusively in the dermis. The
second type occurs as an intraepidermal proliferation (extramammary
Paget's disease) with only rare invasion of the
underlying dermis. Ductopapillary apocrine carcinoma occurs on the eyelids as slowly growing, reddish-pink
firm nodules that are fixed to the underlying
tissue. Histologically, there are solid cellular nests and cords with
focal lumina that infiltrate into the dermis and subcutis. In areas of
glandular differentiation, “decapitation“ secretion is apparent. The
tumor cells have oval vesicular nuclei with prominent nucleoli, and
mitoses are readily apparent. Papillary differentiation characterized
by frondlike projections of tumor cells into cystic spaces may
be found. In most tumors, a mixture of ductal structures and papillary
projections is present. Extramammary Paget's disease may involve the eyelids and typically
presents as an erythematous eczematous plaque with crusting and itching. The
lesion occurs in middle-aged or elderly patients and shows
insidious growth. Histologically, individual or clusters of tumor
cells are seen in a buckshot pattern within the epidermis (Fig. 50). The tumor cells have central oval vesicular nuclei and vacuolated
amphophilic cytoplasm. Mucin stains, such as mucicarmine, will demonstrate
positive staining within the tumor cells. In contrast to Bowen's
disease, the tumor cells do not show intercellular bridges, and
the retention of the basal layer within the epidermis helps differentiate
extramammary Paget's disease from malignant melanoma. 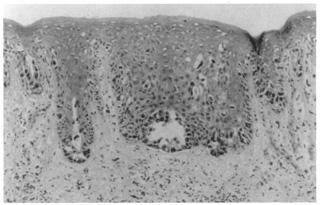 Fig. 50. Extramammary Paget's disease showing a buckshot pattern of atypical
cells in the epidermis with focal duct formation. Fig. 50. Extramammary Paget's disease showing a buckshot pattern of atypical
cells in the epidermis with focal duct formation.
|
OTHER MALIGNANT TUMORS Merkel Cell Carcinoma The merkel cell is a distinctive, nondendritic epithelial clear cell believed
to migrate from the neural crest to the epidermis. Merkel cell
carcinoma typically arises on the head and neck of sun-exposed
skin in elderly patients. The most common clinical appearance is that
of a nonulcerated reddish-purple nodule that may persist for a
few weeks to several years. Histologically, the tumor is purely dermal in location and is composed
of sheets or trabeculae of monotonous small, round tumor cells (Fig. 51). The tumor cells show oval nuclei with inconspicuous nucleoli and
abundant mitotic activity. Differentiation from lymphoma, oat cell carcinoma
of the lung, and other metastatic undifferentiated tumors may
be difficult. Immunohistochemistry and electron microscopy may be needed
for a definitive diagnosis.  Fig. 51. Merkel Cell Tumor—A. Clinical photograph of Merkel cell tumor. B. Photomicrograph of tumor showing monotonous appearance of the tumor cells
with granular nuclear chromatin (hematoxylin and eosin stain). (Photos
courtesy of William Morris, M.D.) Fig. 51. Merkel Cell Tumor—A. Clinical photograph of Merkel cell tumor. B. Photomicrograph of tumor showing monotonous appearance of the tumor cells
with granular nuclear chromatin (hematoxylin and eosin stain). (Photos
courtesy of William Morris, M.D.)
|
Atypical Fibroxanthoma Atypical fibroxanthoma is regarded as a low-grade malignancy and
is considered the superficial counterpart of malignant fibrous histiocytoma, which
it resembles histologically. It has a more favorable prognosis
than malignant fibrous histiocytoma, probably due to its small
size and superficial location. Clinically, the lesions are nodules that
may or may not ulcerate and occur on sun-exposed areas of the
head and neck in elderly patients. Histologically, a dense infiltrate throughout the dermis may extend to
the subcutaneous fat. The infiltrate is composed of cells with pleomorphic
hyperchromatic nuclei in an irregular arrangement. Some cells are
spindle shaped, whereas others appear polygonal with ample foamy vacuolated
cytoplasm. The characteristic feature is the presence of large, bizarre, multinucleated
giant cells showing marked nuclear atypia (Fig. 52). Numerous mitoses are present, many of which are atypical in appearance.  Fig. 52. Atypical fibroxanthoma. Foamy histiocytes and giant cells with atypical
nuclei and mitoses. Fig. 52. Atypical fibroxanthoma. Foamy histiocytes and giant cells with atypical
nuclei and mitoses.
|
Angiosarcoma Cutaneous angiosarcoma most commonly arises on the face and scalp of the
elderly. It may be misdiagnosed as a cellulitis or ecchymosis because
of its asymptomatic benign appearance at presentation. Angiosarcoma
of the face and scalp often invades locally and metastasizes diffusely. Histopathologically, there are atypical pleomorphic endothelial cells forming
irregular vascular channels in the dermis. Mitotic figures are
present, and the overall pattern of the tumor can range from well-differentiated
epithelioid to a poorly differentiated spindle cell
appearance. Immunohistochemistry using factor VIII-related antigen
or Ulex europaeus 1 (UEA-1) will confirm its endothelial
origin. Metastatic Carcinoma Skin metastases are uncommon and usually are seen as a late event in advanced
cancer. Usually, by the time skin metastases are noticed, the tumor
is widely disseminated. Most metastases to the skin occur in patients
between 50 and 70 years of age. In women, the most common skin metastases
are from carcinoma of the breast; in men, cutaneous metastases
from carcinoma of the lung and large intestine are most frequent. In
one large series of 7518 patients, four of 13 patients with ocular malignancy
had skin metastases. The histopathology of cutaneous metastasis simulates the primary tumor, especially
in breast, renal, and colon carcinoma. In some cases, the
tumor can be diagnosed only as a malignant squamous or adenoid proliferation, and
clinical diagnosis must be deferred pending review of the
histology of the primary tumor with or without immunohistochemistry or
electron microscopy. |