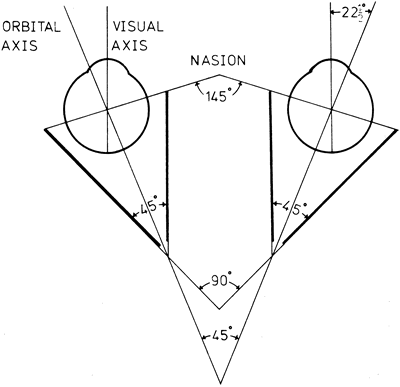
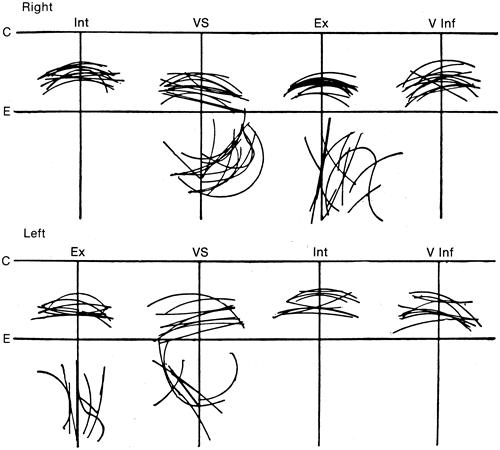
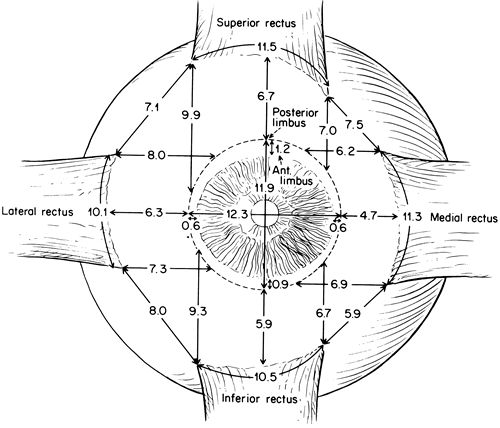
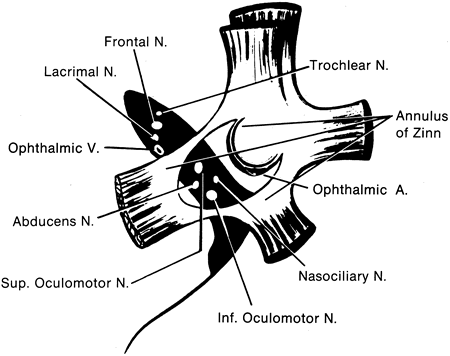
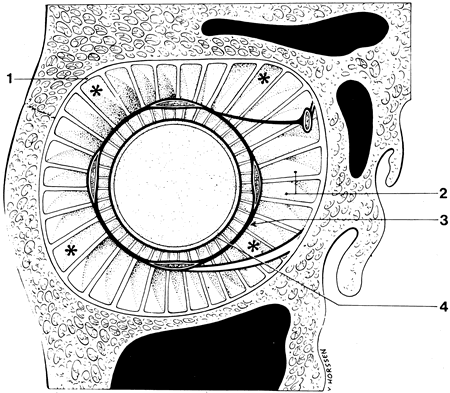
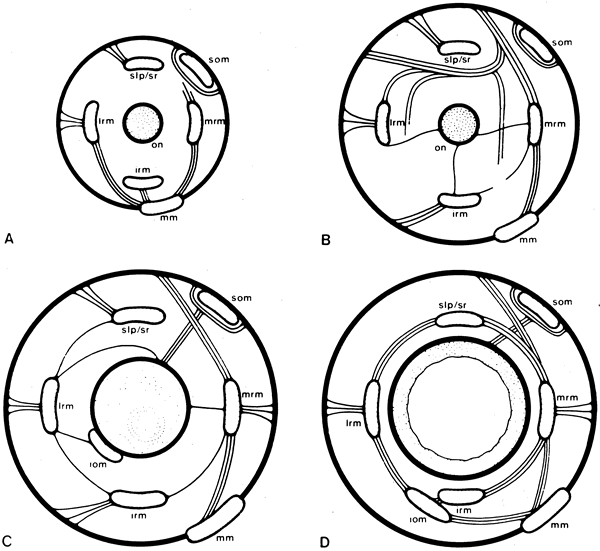
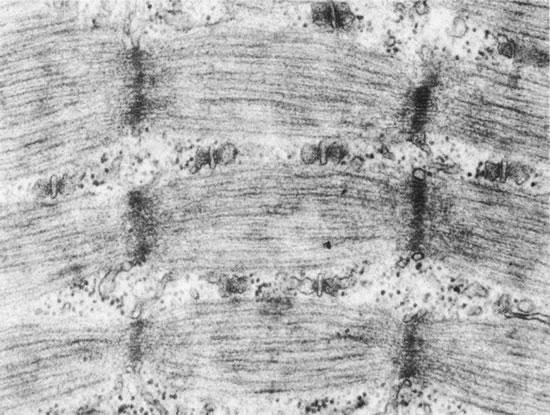
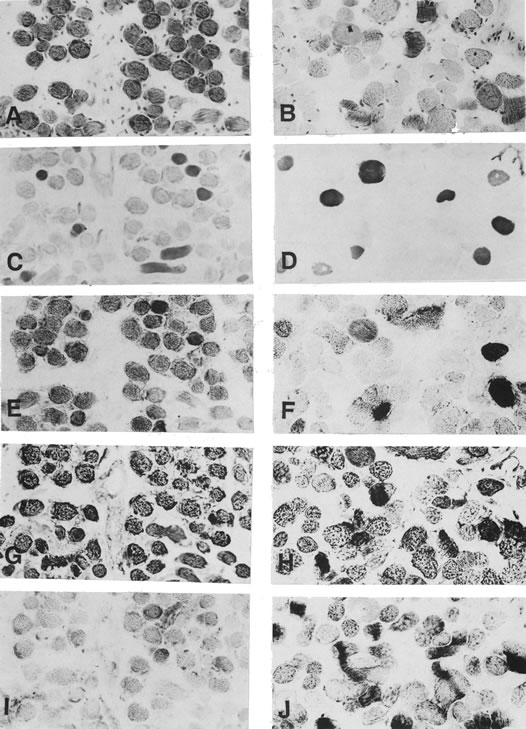
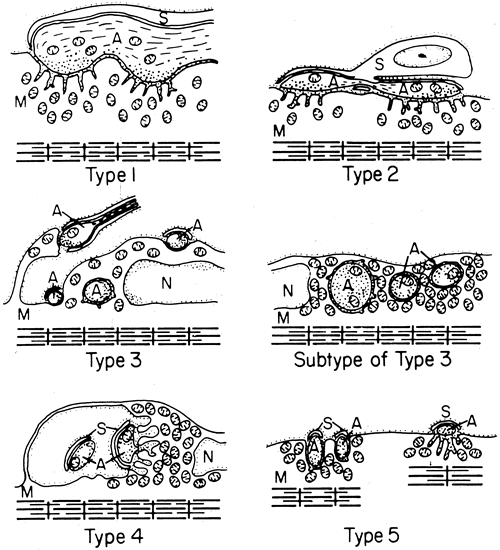
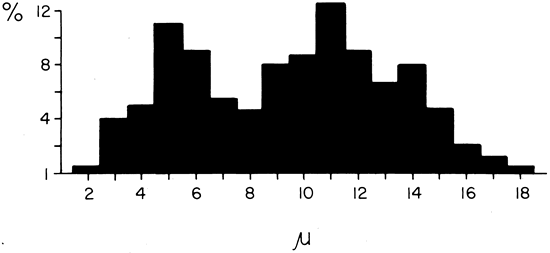
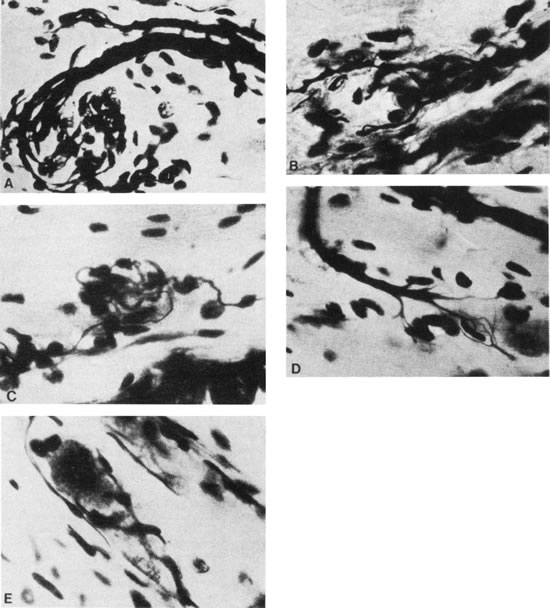
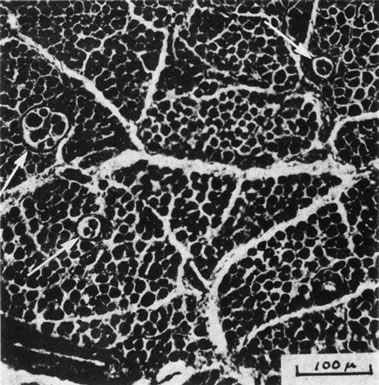
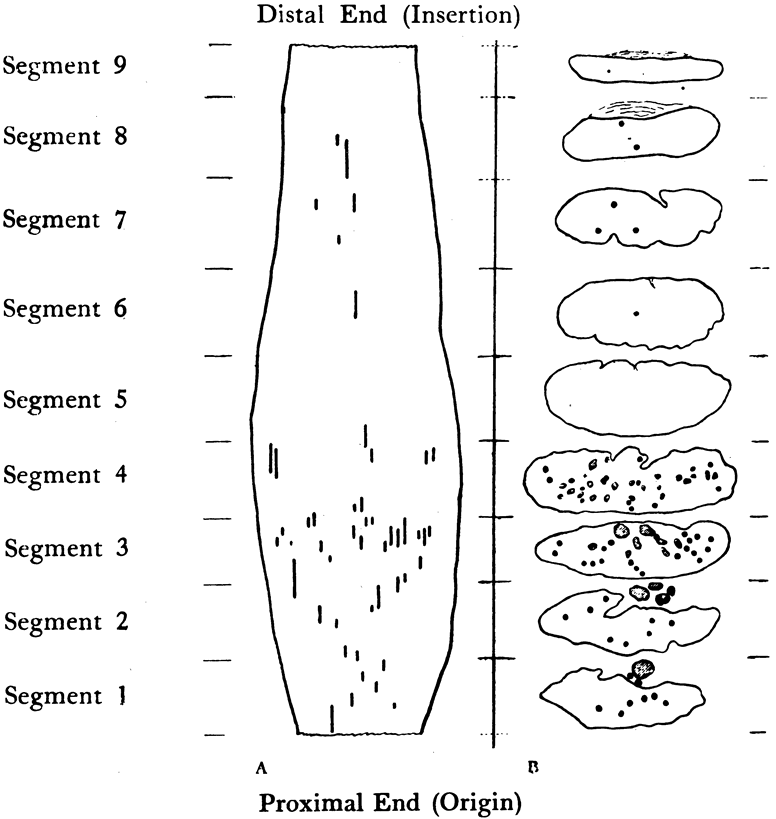
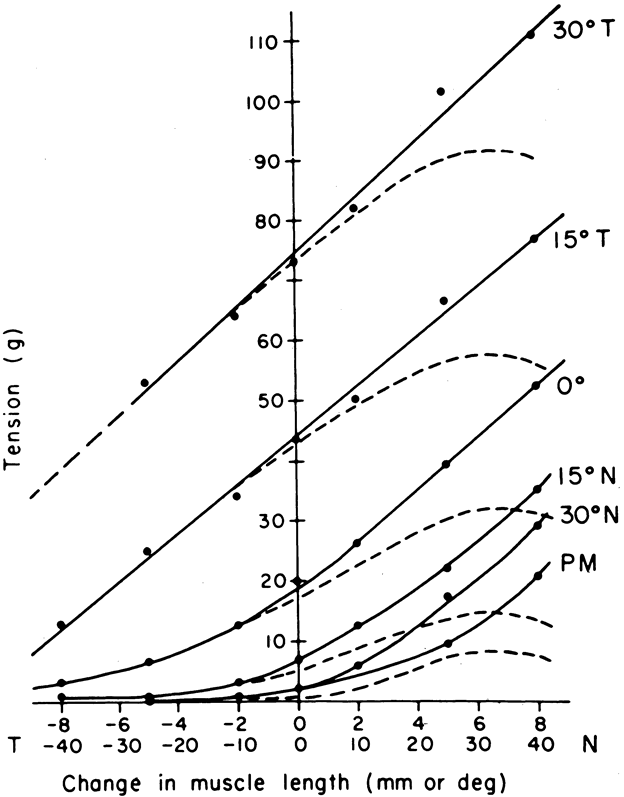
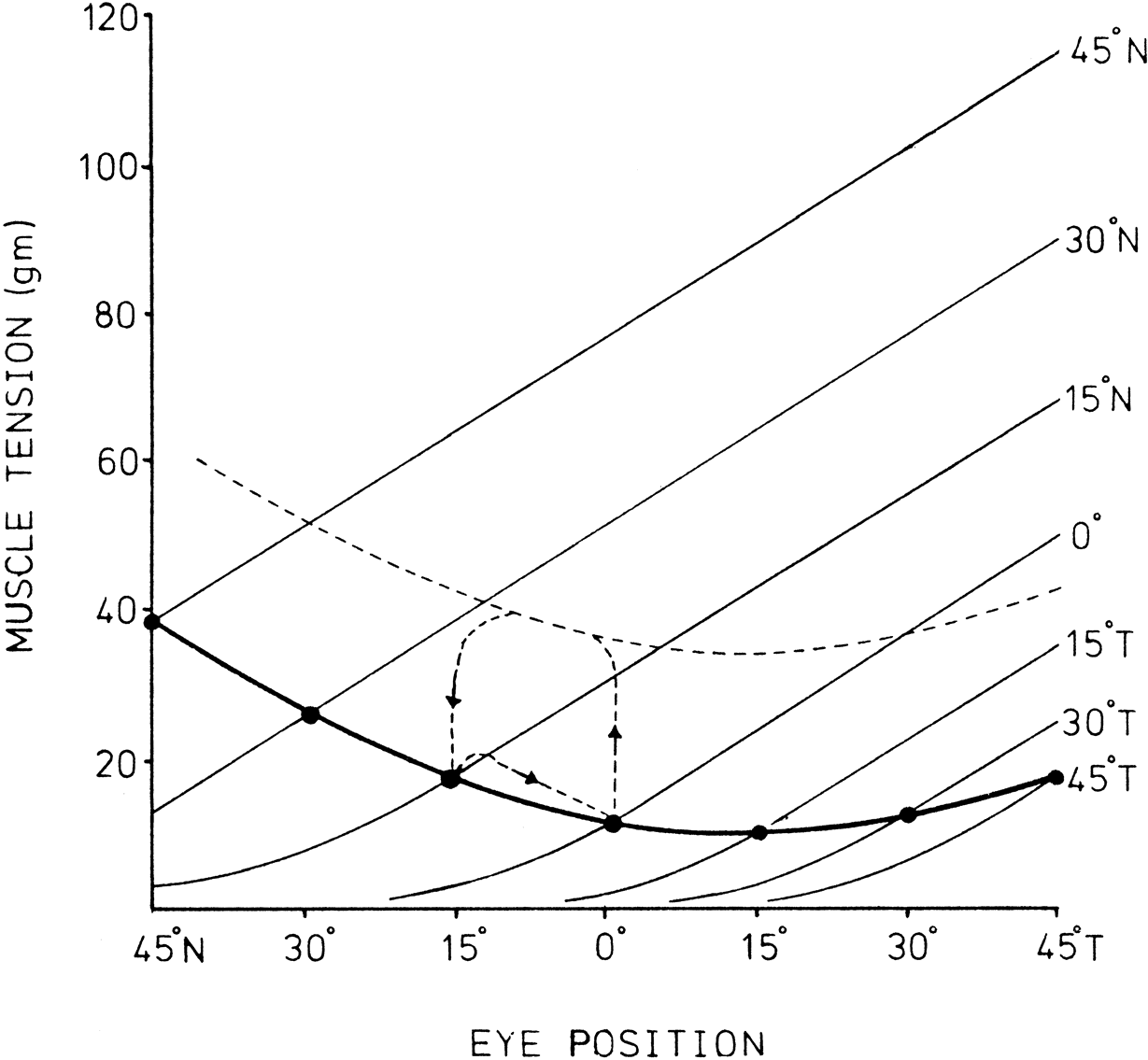
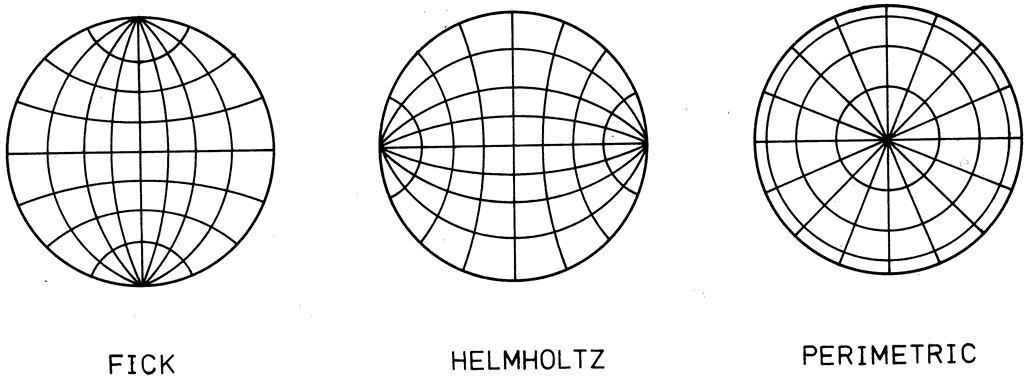
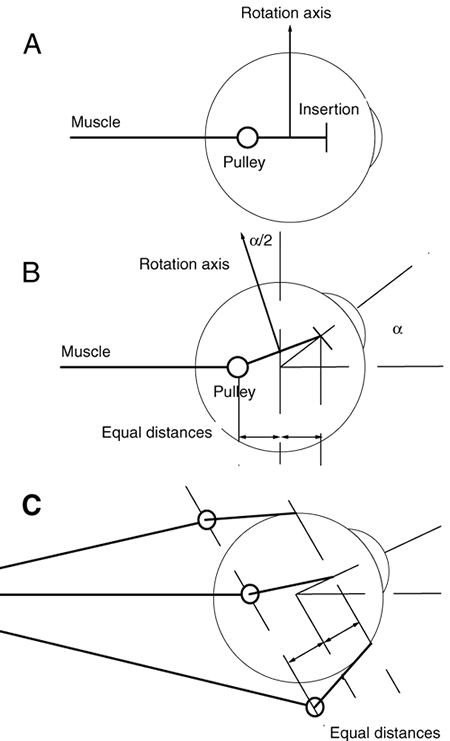
1. Gilbert PW: The origin and development of the human extrinsic ocular muscle. Contrib Embryol Carnegie Inst 36:59, 1957. 2. Mann I: The Development of the Human Eye,2nd ed. New York, Grune & Stratton, 1950. 3. Barber AN: Embryology of the Human Eye. St Louis, Mosby, 1955. 4. Hamilton WJ, Mossman HW: Human Embryology, 4th ed. London, Macmillan, 1976. 5. Iwasaki T: Studies on the initial growth of the extraocular muscles of Japanese. Acta Soc Ophthalmol Jpn 62:2584, 1958. 6. Isomura G: Comparative anatomy of the extrinsic ocular muscles in vertebrates. Anat Anz 150:498, 1981. 7. Neal HV: The history of the eye muscles. J Morphol 30:433, 1918. 8. Cohen B, Henn V: Representation of three-dimensional space in the vestibular, oculomotor, and
visual systems. Ann NY Acad Sci 545:1, 1988. 9. Simpson JI, Graf W: Eye-muscle geometry and compensatory eye movements in lateral-eyed
and frontal-eyed animals. Ann NY Acad Sci 374:20, 1981. 10. Spencer RF, Porter JD: Structural organization of the extraocular muscles. In Büttner-Ennever JA (ed): Neuroanatomy of the oculomotor system, Elsevier, 1988. 11. Meier S: Development of the chick embryo mesoblast: Formation of the embryonic axis
and establishment of the metameric pattern. Dev Biol 73:25, 1979. 12. Noden D: The role of the neural crest in patterning of avian cranial skeletal, connective, and
muscle tissues. Dev Biol 96:144, 1983. 13. Sevel D: The origins and insertions of the extraocular muscles: Development, histologic
features, and clinical significance. Trans Am Ophthalmol Soc 84:488, 1986. 14. Sevel D: A reappraisal of the origin of human extraocular muscles. Ophthalmology 88:1330, 1981. 15. Miller JB, Stockdale FE: Developmental origins of skeletal muscle fibers: Clonal analysis of myogenic
cell lineages based on expression of fast and slow myosin heavy
chains. Proc Natl Acad Sci USA 83:3860, 1986. 16. Miller JB, Stockdale FE: Developmental regulation of the multiple myogenic cell lineages of the
avian embryo. J Cell Biol 103:2197, 1986. 17. Porter JD, Baker RS: Prenatal morphogenesis of primate extraocular muscle: neuromuscular junction
formation and fiber type differentiation. Invest Ophthalmol Vis Sci 33:657, 1992. 18. McMahan UJ, Horton SE, Werle MJ et al: Agrin isoforms and their role in synaptogenesis. Curr Opin Cell Biol 4:869, 1992. 19. Sohal GS, Holt RK: Role of innervation on the embryologic development of skeletal muscle. Cell Tissue Res 210:383, 1980. 20. Altman J, Bayer SA: Development of the brain stem in the rat: V. Thymidine-radiographic study of the time of origin of neurons in
the midbrain tegmentum. J Comp Neurol 198:677, 1981. 21. Shaw MJ, Alley KE: Generation of the ocular motor nuclei and their cell types in the rabbit. J Comp Neurol 200:69, 1981. 22. Sohal GS: Development of the oculomotor nucleus with special reference to the time
of cell origin and cell death. Brain Res 138:217, 1977. 23. Mustafa GY, Gamble HJ: Changes in axonal numbers in developing human trochlear nerve. J Anat (Lond) 128:323, 1979. 24. Sohal GS, Weidman TA: Development of the trochlear nerve: Loss of axons during normal development. Brain Res 142:455, 1978. 25. Hoyt WF, Nachtigaller H: Anomalies of the ocular motor nerves. Am J Ophthalmol 60:443, 1965, 26. Koornneef L: The development of the connective tissue in the human orbit. Acta Morphol Neerl Scand 14:263, 1976. 27. Ruskel GL: The fine structure of innervated myotendinous cylinders in extraocular
muscles of rhesus monkeys. J Neurocytol 7:693, 1978. 28. Harayama K, Amemiya T, Nishimura H: Development of rectus muscles during fetal life: Insertion sites and width. Invest Ophthalmol Vis Sci 19:468, 1980. 29. Kerns JM: Postnatal differentiation of the rat trochlear nerve. J Comp Neurol 189:291, 1980. 30. Nag AC, Cheng M: Differentiation of fiber types in an extraocular muscle of the rat. J Embyrol Exp Morphol 71:171, 1982. 31. Hanson J, Lennerstrand G, Nichols KC: The postnatal development of the inferior oblique muscle of the cat: III. Fiber sizes and histochemical properties. Acta Physiol Scand 108:61, 1980. 32. Schonfelder J, Asmussen G, Schaaf P: Zur postnatalen ontogenese der ausseren Augenmuskeln des Kaninchens. Verh Anat Ges 71:1187, 1977. 33. Porter JD, Baker RS, Spencer RF: Ontogenetic and phylogenetic changes in the extraocular muscle orbital
single innervated fiber type. Invest Ophthalmol Vis Sci 32(Suppl):1242, 1991. 34. Lennerstrand G: Histochemical studies on the inferior oblique muscle of Siamese cats and
domestic cats with unilateral lid suture. Exp Eye Res 30:619, 1980. 35. Lennerstrand G, Hanson J: Contractile properties of extraocular muscle in cats reared with monocular
lid closure and artificial squint. Acta Ophthalmol (Copenh) 57:591, 1979. 36. Kerns JM, Rothblatt LA: The effects of monocular deprivation on the development of the rat trochlear
nerve. Brain Res 230:367, 1981. 37. Andrade FH, Merriam AP, Porter JD: Extraocular muscle gene expression and function after dark rearing. Ann NY Acad Sci 956:391, 2002. 38. McLoon LK, Wirtschafter JD: Continuous myonuclear addition to single extraocular myofibers in uninjured
adult rabbits. Muscle Nerve 25:348, 2002. 39. Duke-Elder S, Wybar KC: System of Ophthalmology. Vol 11. The Anatomy of the Visual System. St. Louis, Mosby, 1961. 40. Bell C: On the motions of the eye in the illustration of the uses of the muscles
and nerves of the orbit. Philos Trans R Soc Lond, part I , p 166, 1823. 41. Motais E: Anatomie de l'Appareil Moteur de l'Oeil de l'Homme et des
Vertebres: Deductions Physiologiques et Chirurgicales (strabisme). Paris, A Delahaye & E Lecrosnier, 1887. 42. Howe L: The Muscles of the Eye. New York, GP Putnam, 1907. 43. Merkel F: Handbuch der topographischen Anatomie, pp 291–293. Vol 1, 2nd ed. Brauschweig, Friedreich Vieweg und Sohn, 1885–1890. 44. Whitnall SE: The Anatomy of the Human Orbit. London, Oxford Medical Publications, 1921. 45. Wolff E: Anatomy of the Eye and Orbit, 5th ed (revised by RJ Last). Philadelphia, WB Saunders, 1961. 46. Adachi B: Die Orbita und die Hauptmass des Schadels der Japaner. Z Morphol Anthropol 7:379, 1904. 47. Goldschmidt M: Beitrag zur Anatomie des Musculus rectus externus und des Musculus rectus
internus bei Hund und Mensch. Ophthalmologica (Basel) 157:381, 1969. 48. Hesser C: Der Bindegewebsapparat und die glatte Muskulatur der Orbita beim Menschen
im normalen Zustande. Anatomische Hefte 49:1, 1913. 49. Fick A: Die Bewegungen des menschlichen Augapfels. Z Rat Med NF 4:101, 1854. 50. Fuchs E: Beitrage zur normalen Anatomie des Augapfels. Graefes Arch Klin Exp Ophthalmol 30(4): 1, 1884. 51. Kato T: Über histologische Untersuchungen der Augenmuskeln von Menschen und
Saugetieren. Okajimas Folia Anat Jpn 16:131, 1938. 52. Motais E, cited in Whitnall SE: The Anatomy of the Human Orbit. London, Oxford Medical Publications, 1921. 53. Nakagawa T: Topographic anatomical studies on the orbit and its contents. Acta Soc Ophthalmol Jpn 69:2155, 1965. 54. Ruete TH: Ein neues Ophthalmotrop. Leipzig, BG Teubner, 1857. 55. Volkmann AW: Zur Mechanik der Augenmuskeln. Ber Verh Konige Sachs Ges Wsch 20:28, 1869. 56. Weiss L: Uber das Verhalten des m. rectus externus und rectus internus bei wachsen
der Divergenz der Orbita. Arch Augenheilkd 29:298, 1894. 57. Zoth O: Augenbewegungen und Gesichtswahrnehmungen. In Nagel W (ed): Handbuch der Physiologic des Menschen, p 209. Vol 3. Braunschweig, Friedreich Vieweg und Sohn, 1905. 58. Howe L: Insertion of the ocular muscles. Trans Amer Ophthalmol Soc 9:668, 1902. 59. Gat L: Ein Bertrag zur Topographie des Ansatzes der veir geraden Augenmuskeln. Ophthalmologica 114:43, 1947. 60. Tillaux P: Traite d'Anatomie Topographique, 6th ed, p 166. Paris, Asselin et Houzeau, 1890. 61. Fink WH: A study of the anatomical variations in the attachments of the oblique
muscles of the eyeball. Trans Am Acad Ophthalmol Otolaryngol 7:500, 1947. 62. Fink WH: Surgery of the Oblique Muscles of the Eye. St Louis, Mosby, 1951. 63. Stager DR, Weakley DR Jr, Stager D: Anterior transposition of the inferior oblique. Arch Ophthalmol 110:360, 1992. 64. Apt L: An anatomical reevaluation of rectus muscle insertions. Tr. Am Ophth Soc 78:365, 1980. 65. Mühlendyck H: Wachstum und Lange der ausseren Augenmuskeln. Ber Dtsch Ophthalmol Ges 75:449, 1978. 66. Schneller : Anatomisch-Physiologische Untersuchungen uber die Augenmuskeln Neugeborener. Albrecht von Graefes Arch Klin Exp Ophthalmol 41:178, 1899. 67. Weiss L: Uber die Washstum des Menschlichen Auges. Anatomische Hefte 8:191, 1897. 68. Miller JM, Robins D: Extraocular muscle sideslip and orbital geometry in monkeys. Vision Res 27:381, 1987. 69. Oh SY, Poukens V, Cohen MS et al JL: Structure-function correlation of laminar vascularity in human rectus
extraocular muscles. Invest Ophthalmol Vis Sci 42:17, 2001. 70. Tenon JR: Memoires et d'Observations sur 1'Anatomie, la Pathologie et la
Chirurgie, et Principalement sur 1'Organe de 1'Oeil, pp 193–203. Paris, Nyon, 1806. 71. Guerin J: Memoire sur la myotomie oculaire par la methode sons-conjonctivale. Gaz Med Paris 2(serie 10):81, 1842. 72. Jones LT: A new concept of the orbital fascia and rectus muscle sheaths and its surgical
implications. Trans Am Acad Ophthalmol Otolaryngol 72:755, 1968. 73. Koornneef L: The first results of a new anatomical method of approach to the human orbit
following a clini cal enquiry. Acta Morphol Neerl Scand 12:259, 1974. 74. Koornneef L: Details of the orbital connective tissue system in the adult. Acta Morphol Neerl Scand 15:1, 1977. 75. Koornneef L: The architecture of the musuclo-fibrous apparatus in the human orbit. Acta Morphol Neerl Scand 15:35, 1977. 76. Koornneef L: New insights in the human orbital connective tissue. Arch Ophthalmol 95:1269, 1976. 77. Porter JD, Poukens V, Baker RS et al: Structure-function correlations in the human medial rectus extraocular
muscle pulleys. Invest Ophthalmol Vis Sci 37:468, 1996. 78. Kono R, Poukens V, Demer DL: Quantitative analysis of the structure of the human extraocular muscle
pulley system. Invest Ophthalmol Vis Sci 43:2923, 2002. 79. Demer JL, Miller JM, Poukens V et al: Evidence for fibromuscular pulleys of the recti extraocular muscles. Invest Ophthalmol Vis Sci 36:1125, 1995. 80. Demer JL, Miller JM: Orbital imaging in strabismsus surgery. In Rosenbaum AL and Santiago P (eds): Clinical Strabismus Management: Principles and Techniques, p 84–98. St. Louis, Mosby, 1999. 81. Demer JL, Poukens V, Miller JM et al: Innervation of extraocular pulley smooth muscle in monkeys and humans. Invest Ophthalmol Vis Sci 38:1774, 1997. 82. Clark RA, Miller JM, Demer JL: Location and stability of rectus muscle pulleys. Invest Ophthalmol Vis Sci 38:227, 1997. 83. Oh SY, Clark RA, Velez F, Rosenbaum AL et al: Incomitant strabismus associated with instability of rectus pulleys. Invest Ophthalmol Vis Sci 43:2169, 2002. 84. Demer JL, Miller JM: Magnetic resonance imaging of the functional anatomy of the superior oblique
muscle. Invest Ophthalmol Vis Sci 36:906, 1995. 85. Mwasi LM, Raviola G: Morphology and permeability properties of blood capillaries in extraocular
muscle of macaque monkey. Graefes Arch Clin Exp Ophthalmol 223:9, 1985. 86. Schiefferdecker P: Eine Eigentumlichkeit im Baue der Augenmuskeln. Dtsch Med Wochnschr 30:725, 1904. 87. Valu L: Über die normale Struktur und Alternenderungen der Bindegewebsfasern
der aussern Augenmuskeln. Albrecht von Graefes Arch Klin Exp Ophthalmol 169:272, 1966. 88. Burke RE: Motor units: Anatomy, physiology, and functional organization. In Brooks VB (ed): Handbook of Physiology, Section 1: The Nervous System. Volume II. Motor Control, Part 1, pp 345-422. Bethesda, MD, American Physiological Society, 1981. 89. Brooke MH, Kaiser KK: Muscle fiber types: How many and what kind?Arch Neurol 23:369, 1970. 90. Peter JB, Bernard RJ, Edgerton VR, et al: Metabolic profiles of three types of skeletal muscle in guinea pigs and
rabbits. Biochemistry 11:2627, 1972. 91. Moore GE, Schachat FH: Molecular heterogeneity of histochemical fiber types: A comparison of fast
muscles. J Muscle Res Cell Motil 6:513, 1985. 92. Pette D, Vrbova G: Neural control of phenotypic expression in mammalian muscle fibers. Muscle Nerve 8:676, 1985. 93. Gauthier GF, Lowey S: Distribution of myosin isoenzymes among skeletal muscle fiber types. J Cell Biol 81:10, 1979. 94. Pierobon-Bormioli SP, Sartore S, Vitadello M et al: “Slow” myosins in vertebrate skeletal muscle: An im-munofluorescence
study. J Cell Biol 85:672, 1980. 95. Pierobon-Bormioli SP, Sartore S, Dalla Libera L, et al: “Fast” isomyosins and fiber types in mammalian skeletal muscle. J Histochem Cytochem 29:1179, 1981. 96. Schiaffino S, Hanzlikova V, Pierobon S: Relations between structure and function in rat skeletal muscle fibers. J Cell Biol 47:107, 1970. 97. Eisenberg BR, Kuda AM: Discrimination between fiber populations in mammalian skeletal muscle by
using ul-trastructural parameters. J Ultrastruct Res 54:76, 1976. 98. Schmalbruch H: The membrane systems in different fiber types of the triceps surae muscle
of cat. Cell Tissue Res 204:187, 1979. 99. Wooten GF, Reis DJ: Blood flow in extraocular muscle of cat. Arch Neurol 26:350, 1972. 100. Wohlfart G: Untersuchungen uber die Gruppierring von Muskelfasern verschiedener Grosse
und Struktur innerhalb der primaren Muskelfaserbundel in der Skeletmuskulatur, sowie
Beobachtungen uber die Innervation diesen Bundel. Z Mikr Anat Forsch 37:621, 1935. 101. Ringel SP, Wilson WB, Barden MT, Kaiser KK: Histochemistry of human extraocular muscle. Arch Ophthalmol 96:1067, 1978. 102. Martinez AJ, Hay S, McNeer KW: Extraocular muscles, light microscopy and ultrastructural features. Acta Neuropathol (Berl) 34:237, 1976. 103. Cilimbaris PA: Histologische Untersuchungen uber die Muskelspindeln der Augenmuskeln. Arch Mikrosk Anat 75:692, 1910. 104. Thulin I: Histologie des muscles ocularies chez l'homme et les singes. Compt Rend Soc Biol 76:490, 1914. 105. Demer JL, Oh SY, Poukens V: Evidence for active control of rectus extraocular muscle pulleys. Invest Ophthalmol Vis Sci 41:1280, 2000. 106. Oh SY, Poukens V, Demer JL: Quantitative analysis of rectus extraocular muscle layers in monkey and
humans. Invest Ophthalmol Vis Sci 42:10, 2001. 107. Siebeck R, Kruger P: Die histologische Struktur der ausseren Augenmuskeln als Ausdruck ihrer
Funktion. Albrecht von Graefes Arch Klin Exp Ophthalmol 156:637, 1955. 108. Lockhart RD, Brandt W: Length of striated muscle fibers. J Anat 72:470, 1938. 109. Hines M: Studies on innervation of skeletal muscle: III. Innervation of extrinsic eye muscles of rabbit. Am J Anat 47:1, 1931. 110. Alvarado JA, van Horn C: Muscle cell types of the cat inferior oblique. In Lennerstrand G, Bach-y-Rita P (eds): Basic Mechanisms of Ocular Motility, pp 15–45. Oxford, Pergamon Press, 1975 111. Gould RP: The microanatomy of muscle. In Bourne GH (ed): The Structure and Function of Muscle. New York, Academic Press, 1973. 112. Voss H: Beitraege zur mikroskopischen Anatomie der Augenmuskeln des Menschen; Faserdicke, Muskelspindeln
Ringbinden. Anat Anz 104:345, 1957. 113. Pachter BR, Davidowitz J, Breinin GM: Light and electron microscopic serial analysis of mouse extraocular muscle: Morphology, innervation and topographical organization of component
fiber populations. Tissue Cell 8:547, 1976. 114. Davidowitz J, Philips G, Breinin GM: Organization of the orbital surface layer in rabbit superior rectus. Invest Ophthalmol 16:711, 1977. 115. Cooper S, Daniel PM: Muscle spindles in human extrinsic eye muscles. Brain 72:1, 1949.. 116. Mayr R: Structure and distribution of fiber types in the external eye muscles of
the rat. Tissue Cell 3:433, 1971. 117. Harker DW: The structure and innervation of sheep superior rectus and levator palpebrae
extraocular muscles: I. Extrafusal muscle fibers. Invest Ophthalmol 11:956, 969, 1972. 118. Floyd K: Junctions between muscle fibers in cat extraocular muscles. Nature 227:185, 1970. 119. Mayr R, Gottschall J, Gruber H, Neuhuber W: Internal structure of cat extraocular muscle. Anat Embryol (Berl) 148:25, 1975. 120. Mayr R, Zenker W, Gruber H: Zwischensehnenfreie Skeletmuskelfaser Verbindungen. Z Zellforsch 79:319, 1967. 121. Teravainen H: Localization of acetyl cholinesterase activity in myotendinous and myomyous
junctions of the striated skeletal muscles of the rat. Experientia 25:524, 1969. 122. Sommerkamp H: Das Substrat der Dauerverkurzung am Froschmuskel. Arch Exp Pathol Pharmakol 128:99, 1928. 123. Kruger P: Uber einen moglichen Zusammenhang zwischen Struktur, Funktion und Chemischen
Beschaffenheit der Muskeln. Biol Zentralbl 49:616, 1929. 124. Spencer RF, McNeer KW: Botulinum toxin paralysis of adult monkey extraocular muscle. Arch Ophthalmol 105:1703, 1987. 125. Carry MR, O'Keefe K, Ringel SP: Histochemistry of mouse extraocular muscle. Anat Embryol 164:403, 1982. 126. Hess A: The structure of slow and fast extrafusal muscle fibers in the extraocular
muscles and their nerve endings in guinea pigs. J Cell Comp Physiol 58:63, 1961. 127. Hess A, Pilar G: Slow fibers in the extraocular muscles of the cat. J Physiol (Lond) 169:780, 1963. 128. Pilar G: Further study of the electrical and mechanical responses of slow fibers
in cat extraocular muscles. J Gen Physiol 50:2289, 1963 129. Pilar G, Hess A: Differences in internal structure and nerve terminals of the slow and twitch
muscle fibers in the cat. Anat Rec 154:243, 1966. 130. Cheng-Minoda K, Davidowitz J, Liebowitz A, Breinin GM: Fine structure of extraocular muscle in rabbit. J Cell Biol 39:193, 1968. 131. Matyushkin DP: Phasic and tonic neuromotor units in the oculomotor apparatus of the rabbit. Sechenov Physiol J USSR 47:65, 1961. 132. Cheng K, Breinin GM: A comparison of the fine structure of extraocular and interosseous muscles
in the monkey. Invest Ophthalmol 5:535, 1966. 133. Mayr R, Stockinger L, Zenker W: Electronenmikroskopische Untersuchungen an unterschiedlich innervierten
Muskelfasern der ausseren Augenmuskulatur des Rhesusaffen. Z Zellforsch 75:434, 1966. 134. Brandt DE, Leeson CR: Structural differences of fast and slow fibers in human extraocular muscle. Am J Oph thalmol 62:478, 1966. 135. Dietert SE: The demonstration of different types of muscle fibers in human extraocular
muscle by electron microscopy and cholinesterase staining. Invest Ophthalmol 4:51, 1965 136. Mukuno K: Fine structure of the human extraocular muscles: II. Two distinct types of muscle fibers. Acta Soc Ophthalmol Jpn 71:907, 1967. 137. Miller JE: Cellular organization of rhesus extraocular muscle. Invest Ophthalmol 6:18, 1967. 138. Pachter BR: Rat extraocular muscle: I. Three-dimensional cytoarchitecture, component fiber populations
and innervation. J Anat (Lond) 137:161, 1983. 139. Chiarandini DJ, Davidowitz J: Structure and function of extraocular muscle fibers. In Zadunaisky JA, Davson H (eds): Current Topics in Eye Research, pp 91–142. Vol 1. New York, Academic Press, 1979. 140. Peachey LD, Takeichi M, Nag AC: Muscle fiber types and innervation in adult cat extraocular muscles. In Milhorat AT (ed): Exploratory Concepts in Muscular Dystrophy, pp 246–254. Vol 1. Amsterdam, Excerpta Medica Foundation, 1974. 141. Pachter BR: Fiber composition of the superior rectus extraocular muscle of the rhesus
monkey. J Morphol 174:237, 1982. 142. Spencer RF, Porter JD: Innervation and structure of extraocular muscles in the monkey in comparison
to those of the cat. J Comp Neurol 198:649, 1981. 143. Spencer RF, Porter JD: Structural organization of the ex traocular muscles. In Büttner-Ennever (ed): Neuroanatomy of the Oculomotor System, pp 33–79. Amsterdam, Elsevier, 1988. 144. Peachey LD: The structure of the extraocular muscle fibers of mammals. In Bach-y-Rita P, Collins CC, Hyde JE (eds): The Control of Eye Movements, pp 47–66. New York, Academic Press, 1971. 145. Porter KR, Palade GE: Studies on the endoplasmic reticulum: III. Its form and distribution in striated muscle cells. J Biophys Biochem Cytol 3:269, 1957. 146. Page S: Structure of the sarcoplasmic reticulum in vertebrate muscle. Br Med Bull 24:170, 1968. 147. Prineas J, Ng RCY: Ultrastructural features of intracellular lipid in normal human muscle. Neurology (Minneap) 17:1092, 1967. 148. Galavazi G, te Lintel Hekkert TJ: Identification of ribosomes and their polysomal configurations in sections
of skeletal muscle of the adult rat. In Bocciarelli DR (ed): Electronmicroscopy 1968, pp 301–302. Abstracts of the 4th European Regional Conference on Electron Microscopy. Rome, Tipographia Poliglotta Vaticana, 1968. 149. Mukuno K: The fine structure of the human extraocular muscle: II. The classifications of muscle fibers. Jpn J Ophthalmol 12:111, 1968. 150. Shafiq SA, Gorycki M, Goldstone L et al: Fine structure of fiber types in normal human muscle. Anat Rec 156:283, 1966. 151. Ogata T, Murata F: Cytological features of three fiber types in human striated muscle. Tohoku J Exp Med 99:225, 1969. 152. Page SG: A comparison of the fine structure of frog slow and twitch muscle fibers. J Cell Biol 26:477, 1965. 153. Hess A: The structure of vertebrate slow and twitch muscle fibers. Invest Ophthalmol 6:217, 1967. 154. Moore GE, Schachat FH: Molecular heterogeneity of histochemical fiber types: A comparison of fast
fibers. J Muscle Res Cell Motil 6:513, 1985. 155. Reichmann H, Srihari T: Enzyme activities, histochemistry and myosin light chain pattern in extraocular
muscles of rabbit. Histochemistry 78:111, 1983. 156. Sartore S, Mascarello F, Rowlerson A et al: Fibre types in extraocular muscles: A new myosin isoform in the fast fibers. J Muscle Res Cell Motil 8:161, 1987. 157. Pierobon-Bormioli S, Torresan P, Sartore S et al: Immunohistochemical identification of slow-tonic fibers in human
extrinsic eye muscles. Invest Ophthalmol Vis Sci 18:303, 1979. 158. Wieczorek DF, Periasamy M, Butler-Browne GS et al: Co-expression of multiple myosin heavy chain genes, in addition
to a tissue-specific one, in extraocular musculature. J Cell Biol 101:618, 1985. 159. McLoon LK, RiosL , Wirtschafter J: Complex three-dimensional patterns of myosin isoform expression: differences
between and within specific extraocular muscles. J Muscle Res Cell Motil. 20:771, 1999. 160. Butler-Browne GS, Herlicoviez D, Whalen RG: Effects of hypothyroidism on myosin isozyme transitions in de veloping
cat muscle. FEBS Lett 66:71, 1984. 161. Gambke B, Lyons GE, Haselgrove J et al: Thyroidal and neural control of myosin transitions during development of
rat fast and slow muscles. FEBS Lett 156:335, 1983. 162. Vita GF, Mastaglia FL: The effects of thyroidectomy on the extraocular muscle of the rat: A histochemical
study. Neuropathol Appl Neurobiol 6:449, 1980. 163. Jolesz F, Sreter FA: Development, innervation and activity-induced changes in skeletal
muscle. Ann Rev Physiol 43:531, 1981. 164. Medford RM, Nguyen HT, Nadal-Ginard B: Transcriptional and cell-cyclemediated regulation of myosin heavy
chain gene expression during muscle cell differentiation. J Biol Chem 258:11063, 1983. 165. Porter JD, Khanna HJ, Kaminski HJ et al: Extraocular muscle is defined by a fundamentally distinct gene expression
profile. Proc Nat Acad Sci USA 98:12062, 2001. 166. Porter JD, Cheng G: Transcriptional profile of rat extraocular muscle by serial analysis of
gene expression. Invest Ophthalmol Vis Sci 43:1048, 2002. 167. Cheng G, Porter JD: Transcriptional profile of rat extraocuular muscle by serial analysis of
gene expression. Invest Ophthalmol Vis Sci:43:1048, 2002. 168. Barnard J, Edgerton VR, Furukawa T et al.: Histochemical, biochemical and contractile properties of red, white and
intermediate fibers. Am J Physiol 220:410, 1971. 169. Brooke MH, Kaiser KK: Muscle fiber types: How many and what kind?Arch Neurol 23:369, 1970. 170. Burke RE, Levine DN, Zajac FE et al: Mammalian motor units: Physiological-histochemical correlations
in three types in cat gastrocnemius. Science 174:709, 1971. 171. Dubowitz V, Pearse AGE: Reciprocal relationship of phosphorylase and oxidative enzymes in skeletal
muscle. Nature 185:701, 1960. 172. Dubowitz V, Pearse AGE: A comparative histochemical study of oxidative enzyme and phosphorylase
activity in skeletal muscle. Histochemie 2:105, 1960. 173. Engel WK: The essentiality of histo- and cytochemical studies in the investigation
of neuromuscular disease. Neurology (Minneap) 12:778, 1962. 174. Engel WK, Brooke MH: Muscle biopsy as a clinical diagnostic aid. In Fields WS (ed): Neurological Diagnostic Techniques, pp 90–146. Springfield, IL, Charles C Thomas, 1966. 175. Henneman E, Olson CB: Relations between structure and function in the design of skeletal muscles. J Neurophysiol 28:581, 1965. 176. Peter JB, Barnard VR, Edgerton VR et al.: Metabolic profiles of the three fibers of skeletal muscles in guinea pigs
and rabbits. Biochemistry 11:2627, 1972. 177. Romanul FCA: Enzymes in muscle: I. Histochemical studies of enzymes in individual muscle fibers. Arch Neurol 11:355, 1964. 178. Stein JM, Padykula HA: Histochemical classification of individual skeletal muscle fibers of the
rat. Am J Anat 110:103, 1962. 179. Padykula HA, Herman E: Factors affecting the activity of adenosine triphosphatase and other phosphatases
as measured by histochemical techniques. J Histochem Cytochem 3 :161, 1955. 180. Padykula HA, Herman E: The specificity of the histochemical method for adenosine triphosphatase. J Histo chem Cytochem 3:170, 1955. 181. Padykula HA, Gauthier GF: Morphological and cytochemical characteristics of fiber types in normal
mammalian skeletal muscle. In Milhorat AT (ed): Exploratory Concepts in Muscular Dystrophy, pp 117–128. New York, Excerpta Medica Foundation, 1967. 182. Yellin H: Unique intrafusal and extraocular muscle fibers exhibiting dual actomysin
ATPase activity. Exp Neurol 25:153, 1969. 183. Yellin H, Guth L: The histochemical classification of muscle fibers. Exp Neurol 26:424, 1970. 184. Gauthier GF: On the relationship of ultrastructural and cytochemical features to color
in mammalian skeletal muscle. Z Zellforsch Mikrosk Anat 95:462, 1969. 185. Hanson J, Lennerstrand G: Contractile and histochemical properties of the inferior oblique muscle
in the rat and in the cat. Acta Ophthalmol (Copenh) 55:88, 1977. 186. Vita GF, Mastaglia FL, Johnson MA: A histochemical study of fiber types in rat extraocular muscles. Neuro pathol Appl Neurobiol 6:449, 1980. 187. Pachter B, Colbjornsen C: Rat extraocular muscle: II. Histochemical fiber types. J Anat (Lond) 137:161, 1983. 188. Pachter B: Rat extraocular muscle: III. Histochemical variability along the length of multiply-innervated
fibers of the orbital surface layer. Histochemistry 80:535, 1984. 189. Asmussen G, Kiessling A, Wohlrab F: Histochemische charakterisierung der verschiedenen muskelfasertypen in
den ausseren Augenmuskeln von Saugetieren. Acta Anat 79:526, 1971. 190. Barmack NH: Laminar organization of the extraocular muscle of the rabbit. Exp Neurol 59:304, 1978. 191. Durston JHJ: Histochemistry of primate extraocular muscles and the changes of denervation. Br J Ophthalmol 58:193, 1974 192. Ringel SP, Engel WK, Bender AN et al: Histochemistry and acetylcholine receptor distribution in normal and de-nervated
monkey extraocular muscles. Neurology (Minn) 28:55, 1978. 193. Hoogengraad TU, Jennekens FGI, Tan KEWP: Histochemical fiber type in human extraocular muscles, an in vestigation
of the inferior oblique muscle. Acta Neuropathol (Berl) 45:73, 1979. 194. Harriman DG: Histochemical fiber types in human extraocular muscle. Bristol Med Chir J 90:27, 1975. 195. Fenn WO: The relation between the work performed and the energy liberated in muscular
contraction. J Physiol (Lond) 58:373, 1924. 196. Hill AV: The heat of shortening and the dynamic constants of muscle. Proc R Soc Med [B] 126:136, 1938. 197. Collins CC, Scott AB, O'Meara D: Muscle tension during unrestrained human eye movements. J Physiol (Lond) 245:351, 1975. 198. Collins CC: The human oculomotor control system. In Lennerstrand G, Bach-y-Rita P (eds): Basic Mechanisms of Ocular Motility and Their Clinical Implications. Oxford, Pergamon Press, 1975. 199. Frohse F: Über die Verzweigung der Nerven zu und in den menschlichen Muskeln. Anat Anz 14:321, 1898. 200. Saito A, Zacks SI: Ultrastructure of Schwann and perineural sheaths at the mouse neuromuscular
junction. Anat Rec 164:379, 1969. 201. Ruskel GL: Sheathing of muscle fibres at neuromuscular junctions and at extrajunctional
loci in human extraocular muscles. J Anat (Lond) 138:33, 1984. 202. Cheng K: Cholinesterase activity in human extraocular muscles. Jpn J Ophthalmol 7:174, 1963. 203. Namba T, Nakamura T, Grob D: Motor nerve endings in human extraocular muscle. Neurology (Minneap) 18:403, 1968. 204. Zenker W, Anzenbacher H: On the different forms of myoneural junction in two types of muscle fiber
from the external ocular muscles of the rhesus monkey. J Cell Comp Physiol 63:273, 1964. 205. Hess A: Further morphological observations of “en plaque” and “en
grappe” nerve endings on mammalian extrafusal muscle fibers
with the cholinesterase technique. Rev Can Biol 21:241, 1962. 206. Teravainen H: Electron microscopic and histochemical observations on different types
of nerve endings on the extraocular muscles of the rat. Z Zellforsch 90:372, 1968. 207. Tschiriew S: Sur les terminaisons nerveuses dans les muscles stries. Arch Physiol Normal Pathol 6:89, 295, 1879. 208. Retzuis G: Zur Kenntniss der motorischen. Nervenendigungen. Biologishe Untersuchungen NeueFolge3:41, 1892. 209. Woollard H: The innervation of the ocular muscles. J Anat 65:215, 1931. 210. Boeke J: Die Beziehungen der Nervenfasen zu den Bindegewebselementen und Tastzellen. Z Mikrosk Anat Forsch 4:448, 1926. 211. Hirano N: Histologische Untersuchungen uber die nervose Innervation der menschlichen
ausseren Augenmuskeln. Albrecht von Graefes Arch Klin Exp Ophthalmol 142:560, 1941. 212. Coers C: Structure and organization of myoneural junction. Int Rev Cytol 22:239, 1967. 213. Corbin KB, Oliver RK: The origin of fibers to the grape-like endings in the insertional
third of the extraocular muscles. J Comp Neurol 77:171, 1942. 214. Teravainen H, Huikuri K: Effect of oculomotor and trigeminal nerve section on the ultrastructure
of different myoneural junctions in the rat extraocular muscles. Z Zellforsch 102:466, 1969. 215. Kupfer C: Motor innervation of extraocular muscle. J Physiol (Lond) 153:522, 1960. 216. Anzenbacher H, Zenker W: Über die Grossenbeziehungen der muskelfasern zu ihren motorischen
Endplatten und Nerven. Z Zellforsch 60:860, 1963. 217. Mukuno K: Fine structure of the human extraocular muscles: III. Neuromuscular junctions in the normal human extraocular muscles. Acta Soc Ophthalmol Jpn 72:104, 1968. 218. Padykula HA, Gauthier GF: The ultrastructure of the neuromuscular junctions of mammalian red, white, and
intermediate skeletal muscle fibers. J Cell Biol 46:27, 1970. 219. Bach-y-Rita P, Lennerstrand G: Absence of polyneuronal innervation in cat extraocular muscles. J Physiol (Lond) 244:613, 1975. 220. Bach-y-Rita P, Ito F: In vivo studies on fast and slow muscle fibers in cat extraocular muscles. J Gen Physiol 49:1177, 1966. 221. Lennerstrand G: Electrical activity and isometric tension in motor units of the cat's
inferior oblique muscles. Acta Physiol Scand 91:458, 1974. 222. Daniel P: Spiral nerve endings in the extrinsic eye muscles of man. J Anat 80:189, 1946. 223. Sas J, Appeltauer C: Atypical muscle spindles in the extrinsic eye muscles of man. Acta Anat 55:311, 1963. 224. Cooper S, Fillenz M: Afferent discharges in response to stretch from the extraocular muscles
of the cat and monkey and the innervation of these muscles. J Physiol (Lond) 127:400, 1955 225. Ruskel GL, Wilson J: Spiral nerve endings and dapple motor end plates in monkey extraocular
muscles. J Anat (Lond) 136:85, 1983. 226. Ruskel GL: Spiral nerve endings in human extraocular muscles terminate in motor end
plates. J Anat (Lond) 139:33, 1984. 227. Fukuda M: Studies on the nerve endings in the extrinsic eye muscles of the rabbit. Jpn J Ophthalmol 2:93, 1958 228. Rosenthal H: De numero atque mensura microscopica fibrillarum elementarium systematis
cerebrospinalis symbolae. Dissertation, Bratislava, 1845, cited in Vierordt H: Anatomische, physiologische
und physikalische Daten und Tabellen. 3rd ed. Jena, G. Fischer, 1906. 229. Tergast P: Ueber das Verhaltniss von Nerve und Muskel. Arch Mikrosk Anat 9:36, 1873. 230. Bors E: Über das zahlenverhaltnis zwischen Nerven und Muskelfasern. Anat Anz 60:415, 1926. 231. Bjorkman A, Wohlfart G: Faseranalyse der Nn oculomotorius, trochlearis und abducens des Menschen
und des N abducens verschiedener Tiere. Z Mikrosk Anat Forsch 39:631, 1936. 232. Torre M: Nombre et dimensions des unités motrices dans les muscles extrinséques
de 1'oeil et en général dans les muscles
squélettiques reliés à des organes de sens. Schweiz Arch Neurol Psychiatry 72:362, 1953. 233. Mühlendyck H: Die Grosse der motorischen Einheiten der unterschiedlich innervierten Augenmuskelfasern. In Kommerell G (ed): Augenbewegungsstürungen. Neurophysiologie und Klinik. Munich, Bergmann Verlag, 1978. 234. Ruskel GL: Fibre analysis of the nerve to the inferior oblique muscle in monkeys. J Anat (Lond) 137:445, 1983. 235. O'Leary J, Heinbecker P, Bishop GH: Analysis of function of nerve to muscle. Am J Physiol 110:636, 1934. 236. Kuffler SW, Hunt CC, Quilliam JP: Function of medullated small-nerve fibers in mammalian ventral roots: Efferent
muscle spindle innervation. J Neurophysiol 14:29, 1951. 237. Wolter J: Morphology of the sensory nerve apparatus in striated muscle of the human
eye. Arch Ophthalmol 53:201, 1955. 238. Manni E, Bortolami R, Derin PL: Presence of cell bodies of the afferents from the eye muscles in the semilunar
ganglion. Arch Ital Biol 108:106, 1970. 239. Batini C, Buisseret P: Sensory peripheral pathway from extrinsic eye muscles. Arch Ital Biol 112:18, 1974. 240. Bortolami R, Lucchi ML, Pettorossi VE et al: Localization and somatotopy of sensory cells innervating the extraocular
muscles of lamb, pig, and cat: Histochemical and electrophysiological
investigation. Arch Ital Biol 125:1, 1987. 241. Porter JD, Spencer RF: Localization and morphology of cat extraocular muscle afferent neurones
identified by retrograde transport of horseradish peroxidase. J Comp Neurol 204:56, 1982. 242. Daunicht WJ: Proprioception in extraocular muscles of the rat. Brain Res 278:291, 1983. 243. Daunicht WJ, Jaworski E, Eckmiller R: Afferent innervation of extraocular muscles in the rat studied by retrograde
and anterograde horseradish peroxidase transport. Neurosci Lett 56:143, 1985. 244. Manni E, Palmieri G, Marini R: Central pathway of the extraocular muscle proprioception. Exp Neurol 42:181, 1974. 245. Alvarado-Mallart MR, Batini C, Buisseret-Delmas C et al: Trigeminal representations of the masticatory and extraocular proprioceptors
as revealed by horseradish peroxidase retrograde transport. Exp Brain Res 23:167, 1975. 246. Tarkhan AA: The innervation of the extrinsic ocular muscles. J Anat 68:293, 1934. 247. Cooper S, Daniel PM, Whitteridge D: Muscle spindles and other sensory endings in the extrinsic eye muscles: The
physiology and anatomy of these receptors and of their connections
with the brain stem. Brain 78:564, 1955. 248. Tozer FM, Sherrington CS: Receptors and afferents of the third, fourth, and sixth cranial nerves. Proc R Soc Lond [B] 82:450, 1910. 249. Warwick R: Oculomotor organization. In Bender MB (ed): The Oculomotor System, pp 173-204. New York, Harper & Row, 1964. 250. Taren JA: An anatomic demonstration of afferent fibers in the IV, V, and VI cranial
nerves of the Macaca mulatta. Am J Ophthalmol 58:408, 1964. 251. Porter JD: Brainstem terminations of extraocular muscle primary afferent neurons in
the monkey. J Comp Neurol 247:133, 1986. 252. Porter JD, Guthrie BL, Sparks DL: Innervation of monkey extraocular muscles: Localization of sensory and
motor neurons by retrograde transport of horseradish peroxidase. J Comp Neurol 218:208, 1983. 253. Edney DP, Porter JD: Neck muscle afferent projections to the brainstem of the monkey: Implications
for the neural control of gaze. J Comp Neurol 250:389, 1986. 254. Hosokawa H: Proprioceptive innervation of striated muscles in the territory of cranial
nerves. Texas Rep Biol Med 19:406, 1961. 255. Sinclair JG: A developmental study of the fourth cranial nerve. Texas Rep Biol Med 16:253, 1958. 256. Maier A, DeSantis M, Eldred E: The occurrence of muscle spindles in extraocular muscles of various vertebrates. J Morphol 143:397, 1974. 257. Siemerling E: Ein Fall von Gummoser Erkrankung der Hirnbasis mit Betheiligung des chiasma
Nervorum Opticorum. Arch Psychiatr Nervenkr 19:401, 1888. 258. Crevatin F: Über muskelspindeln von Saugetieren. Anat Anz 19:173, 1901. 259. Buzzard F: A note on the occurrence of muscle-spindles in ocular muscles. Proc R Soc Med 1/11 (Neurol Sect):83, 1908. 260. Sunderland S: A preliminary note on the presence of neuromuscular spindles in extrinsic
ocular muscles in man. AnatRec 103:561, 1949. 261. Merrillees NCR, Sunderland S, Haylow W: Neuromuscular spindles in the extraocular muscles in man. Anat Rec 108:23, 1950. 262. Lukas JR, Aigner M, Blumer R, Heinzl H, Mayr R: Number and distribution of neuromuscular spindles in human extraocular
muscles. Invest Ophthalmol Vis Sci 35:4317, 1994. 263. Ruskell GL: Extraocular muscle proprioceptors and proprioception. Prog Retinal Eye Res 18:269, 1999. 264. Bruenech JR, Ruskell GL: Muscle spindles in extraocular muscles of human infants. Cells Tissues Organs 169:388, 2001. 265. Inoue T: A study of the muscle spindle in the human ocular muscles. Igaku Kenkyu 28:4759, 1958. 266. Matthews PBC: Muscle spindles and their motor control. Physiol Rev 44:219, 1964. 267. Matthews PCB: Mammalian Muscle Receptors and Their Central Actions. London, Arnold, 1972. 268. Mukuno K, Nomura T: Fine structure of the muscle spindles in the human extraocular muscles. Acta Soc Oph thalmol Jpn 73:2119, 1969. 269. Adal MN: The fine structure of the sensory region of cat muscle spindles. J Ultrastruct Res 26:332, 1969. 270. Corvaja N, Marinozzi V, Pompeiano O: Muscle spindles in the lumbrical muscle of the adult cat: Electron microscopic
observations and functional considerations. Arch Ital Biol 107:365, 1969. 271. Merrillees NCR: The fine structure of muscle spindles in the lumbrical muscle of the rat. J Biophys Biochem Cytol 7:725, 1960. 272. Gruner JE: La structure fine du fuseau neuromusculaire humain. Rev Neurol 104:490, 1961. 273. Spiro AJ, Beilin RL: Human muscle spindle histochemistry. Arch Neurol 20:271, 1969. 274. Dogiel AS: Die Endigungen der sensiblen Nerven in den Augenmuskeln and deren Sehen
beim Menschen and den Säugetieren. Arch Mikroscop Anat 68:501, 1906. 275. Ruskel GL: The fine structure of innervated myotendinous cylinders in extraocular
muscles of rhesus monkeys. J Neurocytol 7:693, 1978. 276. Richmond FJR, Johnston WSW, Baker RS et al.: Palisade endings in human extraocular muscles. Invest Ophthalmol Vis Sci 25:471, 1984. 277. Sodi A, Corsi M, Faussone Pellegrini MS, Salvi G: Fine structure of the receptors at the myotendinour junction of human extraocular
muscles. Histol Histopath 3:103, 1988. 278. Alvarado-Mallart RM, Pinĉon-Raymond M: The palisade endings of the cat extraocular muscles, a light and electron
microscopic study. Tissue Cell 11:567, 1979. 279. Golgi C: Sui nervi dei tendini, e di un nuovo organo nervaso terminale muscula tendinea. Mem Accad Sci Torin 32:359, 1880. 280. Marchi V: Ueber die Terminolorgan der Nerven (Golgi's Nervenkorperchen) in
den Schnen der Augenmuskeln. Albrecht von Graefes Arch Klin Exp Ophthalmol 28:203, 1882. 281. Ciaccio GV: Sur les plaques nerveuses finales dans des vertèbres. Arch Ital Biol 14:31, 1891. 282. Ruskel GL: The incidence and variety of Golgi tendon organs in extraocular muscles
of the rhesus monkey. J Neurocytol 8:639, 1979. 283. Huber C, DeWitt LMA: A contribution on the nerve terminations in neurotendinous end organs. J Comp Neurol 10:159, 1900. 284. Merrillees NCR: Some observations on the fine structure of a Golgi tendon organ of a rat. In Barker D (ed): Symposium on Muscle Receptors, pp 199–205. Hong Kong, Hong Kong University Press, 1962. 285. Cooper S, Eccles JC: The isometric responses of mammalian muscles. J Physiol (Lond) 69:377, 1930. 286. Duke-Elder WS, Duke-Elder PM: The contraction of the extrinsic muscles of the eye by choline and nicotine. Proc R Soc [B] 107:332, 1930. 287. Hofmann H, Lembeck F: The response of neuromuscular blocking agents on extraocular muscle and
intraocular pressure. Proc R Soc Med 62:1217, 1969. 288. Eakins KE, Katz R: The pharmacology of extraocular muscle. In Bach-y-Rita P, Collins CC (eds): The Control of Eye Movements, pp 237–258. New York, Academic Press, 1971. 289. Hill AV: Series elastic component of muscle. Proc R Soc Lond 137:273, 1950. 290. Sakmann B, Neher E (eds): Single-Channel Recording. New York, Plenum Press, 1983. 291. Neher E, Sakmann B: Single-channel currents recorded from membrane of denervated frog
muscle fibers. Nature 260:799, 1976. 292. Liebovitch LS, Tóth TI: Using fractals to understand the opening and closing of ion channels. Ann Biomed Eng 18:177, 1990. 293. Liebovitch LS: Analysis of fractal ion channel gating kinetics: Kinetic rates, energy
levels, and activation energies. Math Biosci 93:97, 1989. 294. Nachsen DA, Blaustein MP: Some properties of potassium-stimulated calcium influx in presynaptic
nerve end ings. J Gen Physiol 76:709, 1980 295. Rahamimoff R, DeRiemer SA, Ginsburg S, Kaiserman I, Sakmann B, Shapira R, Stadler H, Yakir N: Ionic channels in synaptic vesicles: Are they involved in transmitter release?Q J Exp Physiol 74:1019, 1989 296. Scott AB: Botulinum toxin injection into extraocular muscles as an alternative to
strabismus surgery. Ophthalmology 87:1044, 1980 297. Eggers HM: Oculinum for strabismus. In Spaeth G, Katz L (eds): Current Therapy in Ophthalmic Surgery, pp 288–291. Philadelphia, BC Decker, 1989 298. Burgen ASV, Dickens F, Zalman LJ: Action of botulinum toxin. J Physiol (Lend) 109:10, 1949. 299. Black JD, Dolly JO: Interaction of I25I-labelled botulinum neurotoxins with nerve terminals: II. Autoradiographic evidence for its uptake into motor nerves and acceptor-mediated
endocytosis. J Cell Biol 103:535, 1983. 300. Donovan JJ, Middlebrook JL: Ion-conducting channels produced by botulinum toxin in planar lipid
membranes. Biochem 25:2872, 1986. 301. Koniarek J, Welter P, Liebovitch LS, Eggers HM: Botulinum toxin induced ion channels in extraocular muscle fiber membranes. Invest Ophthalmol Suppl 33:1099, 1992. 302. Thesleff S, Molgó J: A new type of transmitter release at the neuromuscular junction. Neuroscience 9:1, 1983. 303. Kim YI, Lφmo T, Lupa MT, Thesleff S: Miniature end-plate potentials in rat skeletal muscle poisoned with
botulinum toxin. J Physiol (Lond) 356:587, 1984. 304. Katz B: The Release of Neural Transmitter Substances. Liverpool, Liverpool University Press, 1969. 305. Fertuck HC, Salpeter MM: Localization of acetylcholine receptor by 125I labelled α-bungarotoxin binding at mouse motor end plates. Proc Natl Acad Sci USA 71:1376, 1974. 306. Takeuchi A, Takeuchi N: On the permeability of end-plate membrane during the action of transmitter. J Physiol (Lond) 154:52, 1960. 307. Burton F, Dörstelmann U, Hutter OF: Single-channel activity in sarcolemmal vesicles from human and other
mammalian muscles. Muscle Nerve 11:1029, 1988. 308. Koniarek J, Welter P, Liebovitch LS et al: Ion channels in extraocular muscle fibers and cultured myoblasts. Invest Ophthalmol Suppl 32:1242, 1991. 309. Jacoby J: Single channel currents recorded from singly-and multiply-innervated
fibers of extraocular muscle. Invest Ophthalmol Suppl 32:1242, 1991. 310. Kuffler SW, Vaughan Williams EM: Properties of the “slow” skeletal muscle fibres of the frog. J Physiol (Lond) 121:318, 1953. 311. Lannergren J: The structure and function of twitch and slow fibers in amphibian skeletal
muscle. In Lennerstrand G, Bach-y-Rita P (eds): Basic Mechanisms of Ocular Motility and Their Clinical Implications, pp 63–84. Oxford, Pergamon Press, 1975. 312. Pilar G: Further study of the electrical and mechanical responses of slow fibers
in cat extraocular muscles. J Gen Physiol 50:2289, 1967. 313. Stefani E, Steinbach AB: Resting potential and electrical properties of frog slow muscle fibres: Effect
of different external solutions. J Physiol (Lond) 203:383, 1969. 314. Kuffler SW, Vaughan Williams EM: Small-nerve junction potentials: The distribution of small motor
nerves to frog skeletal muscle, and the membrane characteristics of the
fibers they innervate. J Physiol (Lond) 121:289, 1953. 315. Kuffler SW, Gerard RW: The small-nerve motor system to skeletal muscle. J Neurophysiol 10:383, 1974. 316. Ozawa T, Cheng-Minoda K, Davidowitz J, Breinin GM: Correlation of potential and fiber type in extraocular muscle. Doc Ophthalmol 26:192, 1969. 317. Ridgeway EB, Gordon AM: Muscle activation: Effects of small length changes on calcium release in
single fibers. Science 189:881, 1975. 318. Hellam DC, Podolsky RJ: Force measurements in skinned fibres. J Physiol (Lond) 200:807, 1969. 319. Chiarandini DJ: Activation of two types of fibers in rat extraocular muscles. J Physiol (Lond) 259:199, 1976. 320. Huxley HE: The structural basis of muscle contraction. Proc R Soc Lond 178:131, 1971. 321. Huxley AF: Muscular contraction. J Physiol (Lond) 243:1, 1974. 322. Huxley AF, Simmons RM: Mechanical transients and the origin of muscular force. Cold Spring Harbor Symp Quant Biol 37:669, 1973 323. Barany M: ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol 50(Suppl):197, 1967 324. Gordon AM, Huxley AF, Julian FJ: The variation in isometric tension with sarcomere length in vertebrate
muscle fibers. J Physiol (Lond) 184:170, 1966 325. Close RJ, Luff AR: Dynamic properties of the inferior rectus muscle of the rat. J Physiol (Lond) 236:259, 1973 326. Browne JS: The contractile properties of slow muscle fibers in sheep extraocular muscle. J Physiol (Lond) 254:535, 1976. 327. Goldberg SJ, Lennerstrand G, Hall CD: Motor unit responses in the lateral rectus muscle of the cat: Intracellular
current injection of abducens nucleus neurons. Acta Physiol Scand 96:58, 1976. 328. Nelson JS, Goldberg SJ, McClung JR: Motoneuron electrophysiological and muscle contractile properties of superior
oblique motor units in the cat. J Neurophysiol 55:715, 1986. 329. Lennerstrand G: Motor units in eye muscles. In Lennerstrand G, Bach-y-Rita P (eds): Basic Mechanisms of Ocular Motility and Their Clinical Implications, pp 119–143. Oxford, Pergamon Press, 1975. 330. Fuchs AF, Luschei ES: Development of isometric tension in simian extraocular muscle. J Physiol (Lond) 219:155, 1971. 331. Barmack NH, Bell CC, Rence BG: Tension and rate of tension development during isometric responses of ex
traocular muscle. J Neurophysiol 34:1072, 1971 332. Close RI, Luff AR: Dynamic properties of inferior rectus muscle of the rat. J Physiol (Lond) 236:259, 1974. 333. Close RI: Dynamic properties of fast and slow skeletal muscles of the rat after nerve
cross-union. J Physiol (Lond) 204:331, 1969. 334. Robinson DA, O'Meara DM, Scott AB, Collins CC: Mechanical components of human eye movements. J Appl Physiol 26:548, 1969. 335. Miller JM, Robins D: Extraocular muscle forces in alert monkey. Vision Res 32:1099, 1992 336. Buchthal F, Kaiser E, Knappeis GG: Elasticity, viscosity and plasticity in cross striated muscle fiber. Acta Physiol Scand 8:16, 1944. 337. Buchthal F, Rosenfalck P: Elastic properties of striated muscle. In Remington JW (ed): Tissue Elasticity, pp 73–93. Washington, DC, Am Physiol Soc, 1957. 338. Collins CC, Scott AB, O'Meara DM: Elements of the peripheral oculomotor apparatus. Am J Optom 46:510, 1969. 339. Collins CC: Orbital mechanics. In Bach-y-Rita P, Collins CC, Hyde JE (eds): The Control of Eye Movements, pp 283–325. New York, Academic Press, 1971. 340. Hill AV: Energy liberation and “viscosity” in muscle. J Physiol (Lond) 93:4P, 1938. 341. Katz B: The relation between force and speed in muscular contraction. J Physiol (Lond) 96:45, 1939. 342. Robinson DA: The mechanics of human saccadic eye movement. J Physiol (Lond) 174:245, 1964. 343. Robinson DA: The mechanics of human smooth pursuit eye movement. J Physiol (Lond) 180:569, 1965. 344. Robinson DA: A quantitative analysis of extraocular muscle cooperation and squint. Invest Ophthalmol 14:801, 1975. 345. Eckmiller R: Hysteresis in static characteristics of eye position coded neurons in the
alert monkey. Pflugers Arch 250:249, 1974. 346. Hilding AC: Normal vitreous, its attachments and dynamics during ocular movement. Arch Ophthalmol 52:497, 1954. 347. Berlin E: Beitrag zur Mechanik der Augenbewegungen. Albrecht von Graefes Arch Klin Exp Ophthalmol 17(2):154, 1871. 348. Fry GA, Hill WW: The mechanics of elevating the eye. Am J Optom 40:707, 1963. 349. Park R, Park G: The center of ocular rotation in the horizontal plane. Am J Physiol 104:545, 1933. 350. Fry GA, Hill WW: The center of rotation of the eye. Am J Optom 39:581, 1962. 351. Enright JT: Saccadic anomalies: Vergence induces large departures from ball-and-socket
behavior. Vision Res 24:301, 1984. 352. Miller JM: Functional anatomy of normal human rectus muscles. Vision Res 29:223, 1989. 353. Simonsz HJ, Harting F, de Waal BJ et al: Sideways displacement and curved path of recti eye muscles. Arch Ophthalmol 103:124, 1985. 354. Enright JT: The aftermath of horizontal saccades: Saccadic retraction and cyclotorsion. Vision Res 26:1807, 1986. 355. Donders FC: Beitrag zur lehre von den Bewegungen des menschlichen Auges. Holländische Beitrage zu den ana-tomischen und physiologischen
Wissenschaften1:105, 379, 1846. 356. Tweed D, Vilis T: Implications of rotational kinematics for the oculomotor system in three
dimensions. J. Neurophysiol 58:832, 1987 357. van den Berg AV: Kinematics of eye movement control. Proc R Soc Lond B Biol Sci 260:190, 1995. 358. Listing JB, cited in Reute CGT: Lehrbuch der Ophthalmologie fur Aerzte und Studirende, pp 37ff. Vol 1, 2nd ed. Braunschweig, Friedreich Vieweg und Sohn, 1855 (Listing did not publish his law). 359. von Helmholtz H: Handbuch der Physiologischen Optik. 3rd ed. Hamburg, Voss, 1910. (English translation by JPC Southall for the Optical Society
of America, 1925.) 360. Howard IP, Templeton WB: Human Spatial Orientation, p 46. New York, John Wiley & Sons, 1966. 361. Landolt E: Table of torsion measurements with convergence in the horizontal plane
and in planes above and below. Cited in Aubert H: Physiologische optik. In Graefe A, Saemisch T (eds): Handbuch der gesammten Augenheilkunde, p 662. Vol 2. Leipzig, Engelmann, 1876. 362. Westheimer G, McKee SP: Failure of Donder's law during smooth pursuit eye movements. Vision Res 13:2145, 1973. 363. Ferman L, Collewijn H, Van der Berg: A direct test of Listing's law:II. Human ocular torsion measured under dynamic conditions. Vision res 27:939, 1987. 364. Haslwanter T, Straumann D, Hepp K et al: Smooth pursuit eye movements obey Listing's law in the monkey. Exp Brain Res 87:470, 1991. 365. Straumann D, Zee D, Solomon D, Kramer PD: Validity of Listing's law during fixations, saccades, smooth pursuit
eye movements and blinks. Exp brain Res 112:135, 1996. 366. Tweed D, Vilis T: Rotation axes of saccades. Ann NY Acad Sci 545:128, 1988. 367. Tscherning M: La loi de Listing. These de Paris, 1887. 368. Quereau JVD: Some aspects of torsion. Arch Ophthalmol 31:783, 1954. 369. Quereau JVD: Rolling of the eye around its visual axis during normal movements. Arch Ophthalmol 53:807, 1955. 370. Hering E: Die Lehre vom binocularen Sehen. Leipzig, Engelmann, 1868. English translation: BridgemanB, StarkL: The Theory of Binocular Vision. New York, Plenum Press, 1977. 371. Tweed D, Cadera W, Vilis T: Computing three-dimensional eye position quaternions and eye velocity
from search coil sugnals. Vision Research 30:97, 1990. 372. Tweed D, Vilis T: Geometric relations of eye position and velocity vectors during saccades. Vision Res. 30:111, 1990. 373. Raphan T: Modeling control of eye orientation in three dimensions. I. Role of muscle pulleys in determining saccadic trajectory. J Neurophysiol 79:2653, 1998. 374. Quaia C, Optican L: Commutative saccadic generator is sufficient to control a 3-D ocular
plant with pulleys. J Neurophysiol 79:3197, 1998. 375. Robinson DA: Oculomotor unit behavior in the monkey. J Neurophysiol 33:393, 1970. 376. Schiller PH: The discharge characteristics of single units in the oculomotor and abducens
nuclei of the unanesthetized monkey. Exp Brain Res 10:347, 1970. 377. Keller EL, Robinson DA: Abducens unit behavior in the monkey during vergence movements. Vision Res 12:369, 1972. 378. Skavenski AA, Robinson DA: Role of abducens neurons in the vestibuloocular reflex. J Neurophysiol 36:724, 1973. 379. Scott AB, Collins CC: Division of labor in the human extraocular muscle. Arch Ophthalmol 90:319, 1973. 380. Henneman E, Somjen G, Carpenter DO: Functional significance of cell size in spinal motoneurons. J Neuro physiol 28:560, 1965. 381. Henneman E, Somjen G, Carpenter DO: Excitability and inhibitability of motoneurons of different sizes. J Neurophysiol 28:599, 1965. 382. Ramon y, Cajal S: Histologie du Systeme de 1'Homme et des Vertebres. Paris, A Maloime, 1909. 383. Sherrington CS: Experimental note on two movements of the eye. J Physiol 17:27, 1894. 384. Breinin G: Electromyography: A tool in ocular and neurologic diagnosis: XI. Muscle palsies. Arch Ophthalmol 57:165, 1957. 385. Strachan IM, Brown BH: Electromyography of extraocular muscles in Duane's syndrome. Br J Ophthalmol 56:594, 1972. 386. Bahill AT, Cuiffreda KJ, Kenyon R, Stark L: Dynamic and static violations of Hering's law of equal innervation. Am J Optom Physiol Opt 53:786, 1976. 387. Bahill AT, Clark MR, Stark L: Glissades-eye movements generated by mismatched componenets of the
saccadic motoneuronal control signal. Math Biosci 26:303, 1975. 387a. Optican LM, Robinson DA: Cerebellar-dependent adaptive control of the primate saccadic system. J Neurophysiol 44:1058, 1980. 388. Westheimer G, McKee SP: Visual acuity in the presence of retinal image motion. J Opt Soc Am 65:847, 1975. 389. Hepp K, Henn V, Villis T, Cohen B: Brainstem regions related to saccade generation. In Wurtz RH, Gold ME (eds): The Neurobiology of Saccadic Eye Movements. Reviews of Oculomotor Research III, p 105. Amsterdam, Elsevier, 1998. 390. Nakayama K: Coordination of extraocular muscles. In Lennerstrand G, Bach-y-Rita P (eds): Basic Mechanisms of Ocular Motility and Their Clinical Implications, pp 193–207. Oxford, Pergamon Press, 1975. 391. Goodwin AW, Fender DH: Recognition of component differences in two-dimensional oculomotor
tracking tasks. Vision Res 13:1905, 1973. 392. Krewson WE: The action of the extraocular muscles. Trans Am Ophthalmol Soc 48:443, 1950 393. Boeder P: The co-operation of extraocular muscles. Am J Ophthalmol 51:469, 1961. 394. Miller JM, Robinson DA: A model of the mechanics of binocular alignment. Comput Biomed Res 17:436, 1984. 395. Miller JM, Pavlovski , Shaemeva, I: Orbit 1.8 Gaze Mechanics Simulation. Eidactics, SanFrancisco, CA, 1999. 396. Miller JM, Demer JL: Biomechanical Modeling in Strabismus Surgery. In Rosenbaum AL, Santiago P (eds): Clinical Strabismus Management: Principles and Techniques, pp 99–113. St. Louis, Mosby, 1999. 397. Robinson DA: The functional behavior of the peripheral ocular motor apparatus: A review. In Kommerell G (ed): Disorders of Ocular Motility: Neurophysiological and Clinical Aspects, pp 43–61. Presented before the Symposium of Deutschen Ophthalmologischen Gesellschaft, Freiburg, April 1977. Munich, Bergman, 1978. 398. Bahill AT, Adler D, Stark L: Most naturally occurring human saccades have magnitudes of 15 degrees or
less. Invest Ophthalmol 14:468, 1975. 399. Matthews BHC: The responses of a muscle spindle during active contraction of a muscle. J Physiol (Lond) 72:153, 1931. 400. Harvey RJ, Matthews PB: The response of de-efferented muscle spindle endings in the cat's
soleus to slow extension of the muscle. J Physiol (Lond) 157:370, 1961. 401. Houk J, Henneman E: Responses of Golgi tendon organs to active contractions of the soleus muscle
of the cat. J Neurophysiol 30:466, 1967. 402. Cooper S, Daniel P, Whitteridge D: Afferent discharges from extraocular muscles. J Physiol (Lond) 113:463, 1951. 403. Cooper S, Daniel P, Whitteridge D: Nerve impulses in the brain stem of the goat: Responses with long latencies
obtained by stretching the extrinsic eye muscles. J Physiol (Lond) 120:491, 1953. 404. Whitteridge D: The effect of stimulation of intrafusal muscle fibers on sensitivity to
stretch of extraocular muscle spindles. Q J Exp Physiol 44:385, 1959. 405. Hunt CC, Kuffler SW: Further study of efferent small-nerve fibers to mammalian muscle
spindles. Muscle spindle innervation and activity during contraction. J Physiol (Lond) 113:283, 1951. 406. Bach-y-Rita P, Ito F: Properties of stretch receptors in cat extraocular muscles. J Physiol (Lond) 186:663, 1966. 407. Lennerstrand G: Position and velocity sensitivity of muscle spindles in the cat: I. Primary and secondary endings deprived of fusimotor activation. Acta Physiol Scand 73:281, 1968. 408. Lennerstrand G: Position and velocity sensitivity of muscle spindles in the cat: IV. Interaction between two fusimotor fibers converging on the same spindle
ending. Acta Physiol Scand 74:257, 1968. 409. Baichenko PI, Matyushkin DP, Suvorov UV: Participation of fast and tonic oculomotor systems in stretch re flexes
and labyrinthine reflexes of extraocular muscles. Fiziol Zh SSSR 53:82, 1967. 410. Bach-y-Rita P: Extraocular muscle inhibitory stretch reflex during active contraction. Arch Ital Biol 110:1, 1972. 411. Baker R, Precht W: Electrophysiological properties of trochlear motor neurons as revealed
by IV nerve stimulation. Exp Brain Res 14:124, 1972. 412. Keller EL, Robinson DA: Absence of a stretch reflex in extraocular muscle of the monkey. J Neurophysiol 34:908, 1971. 413. Eldred E, Granit R, Merton PA: Supraspinal control of the muscle spindles and its significance. J Physiol (Lond) 122:498, 1953. 414. Marsden CD, Merton PA, Morton HB: Servo action in human voluntary movement. Nature 238:140, 1972. 415. Whitteridge D: The motor nerve supply to extraocular muscle spindles. Electroencephalogr Clin Neurophysiol 10:353, 1958. 416. Steinbach MJ, Kirschner EL, Arstikaitis MJ: Recession versus marginal myotomy surgery for strabismus: Effects on spatial
localization. Invest Ophthalmol Vis Sci 28:1870, 1987. 417. Irvine SR, Ludvigh EJ: Is ocular proprioceptive sense concerned in vision?Arch Ophthalmol 15:1037, 1936. 418. Brindley GS, Merton PA: The absence of position sense in the human eye. J Physiol (Lond) 153:127, 1960. 419. Skavenski AA: Inflow as a source of extraretinal eye position information. Vision Res 12:221, 1972. 420. Merton PA: The accuracy of directing the eyes and the hand in the dark. J Physiol (Lond) 156:555, 1961. 421. Hansen RM: Spatial localization during pursuit eye movements. Vision Res 19:1213, 1979. 422. Hansen RM, Skavenski AA: Accuracy of eye position information for motor control. Vision Res 17:919, 1977. 423. Carpenter RHS: Movements of the Eyes. 2nd ed. London, Pion, 1988. 424. von Holst E, Mittelstaedt H: Das Reafferenz-pricip. Naturwissenschaften 37:464, 1950. 425. Ludvigh E: Control of ocular movements and visual interpretation of environment. Arch Ophthalmol 48:442, 1952. 426. Fuchs AF, Kornhuber HH: Extraocular muscle afferents to the cerebellum of the cat. J Physiol (Lond) 200:713, 1969. 427. Baker R, Precht W, Llinas R: Mossy and climbing fiber projections of extraocular afferents to the cerebellum. Brain Res 38:440, 1972. 428. Batini C, Buisseret P, Kado RT: Extraocular proprioceptive and trigeminal projections to the Purkinje cells
of the cerebellar cortex. Arch Ital Biol 112:1, 1974. 429. Robinson DA: Adaptive gain control of vestibuloocular reflex by the cerebellum. J Neurophysiol 39:954, 1976. 430. Steinbach MJ: Proprioceptive knowledge of eye position. Vision Res 27:1737, 1987. 431. Crewther DP, Crewther SG, Pettigrew JD: A Role for extraocular afferents in post-critical period reversal
of monocular deprivation. J Physiol 282:181, 1978. 432. Graves AL, Trotter Y, Frégnac Y: Role of extraocular muscle proprioception in the development of depth perception
in cats. J Neurophysiol 58:816, 1987. 433. Trotter Y, Frégnac Y, Buisseret P: The period of susceptibility of visual cortical binocularity to unilateral
proprioceptive deafferentation of extraocular muscles. J Neurophysiol 58:795, 1987. |