The reason that cortical opacities are consistently conical in shape, grow slowly over decades, evolve in specific quadrants rather than in random locations, and finally affect only anterior or posterior segments of fibers is poorly understood. Nonprimate animal studies have not been useful in elucidating the aforementioned specific characteristics of human cortical cataracts because the cortical opacities of these models are not consistently conical in shape, do not necessarily arise in a quadrantic order, and commonly involve immature (elongating) as well as mature fibers. However, a comparison of the morphology of normal primate (monkey and baboon) and human lenses at different ages with that of human cortical cataracts removed surgically (intra- and extracapsular techniques) strongly suggests that cortical cataracts are a function of improper sutural formation during specific periods of lens development, growth, and aging.
NORMAL HUMAN LENS SUTURE DEVELOPMENT AS A FUNCTION OF AGE
To facilitate an understanding of abnormal human suture development in cortical cataracts, we briefly review normal human suture development as a function of age.
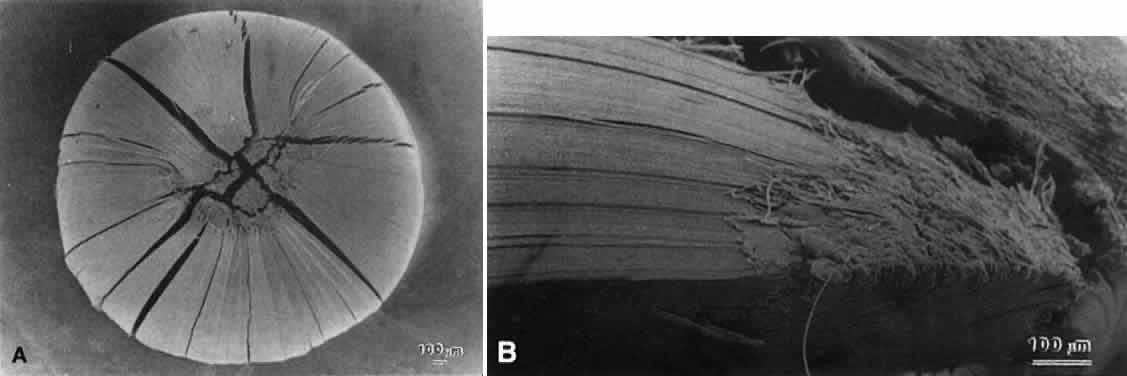
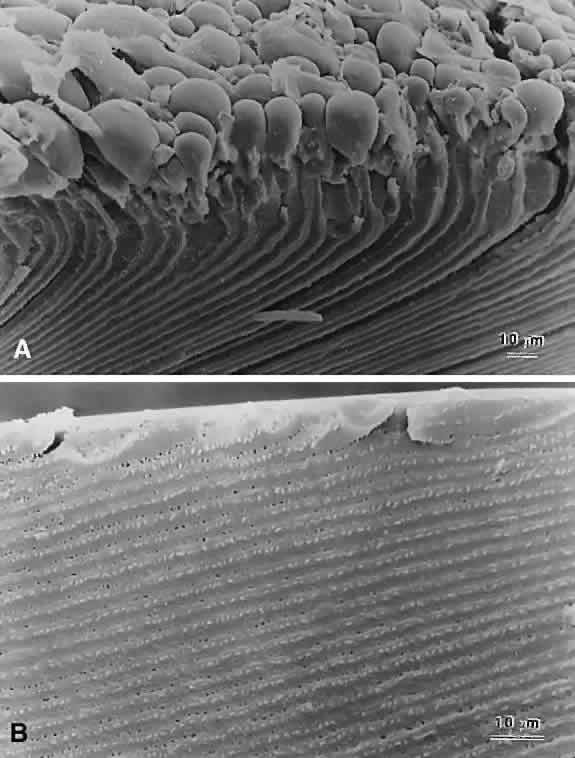
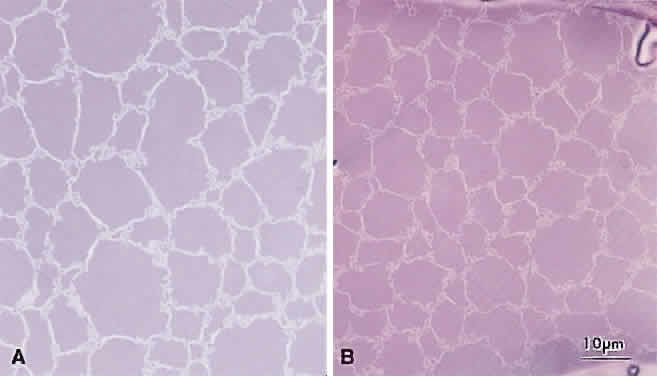
Lens sutures are formed by the overlap of fiber ends anteriorly and posteriorly in each shell.1 Although fibers generally are uniform in shape along their length, their ends are not uniform in shape or size. Thus, sutures are naturally occurring regions of disorder in the otherwise ordered lens. Indeed, although individual fibers cannot be resolved at low magnification, the sutures are recognized easily (Figs. 1, 2, 3). The higher visibility of sutures is related to the increased degree of scatter as light passes through these naturally occurring disordered regions. In contrast, the inability to resolve individual fibers at the same magnification is related to the minimal amount of scatter as light is transmitted through their uniform shape and ordered arrangement. Thus, an understanding of lens sutural anatomy is integral to comprehending lens function or dysfunction.
|
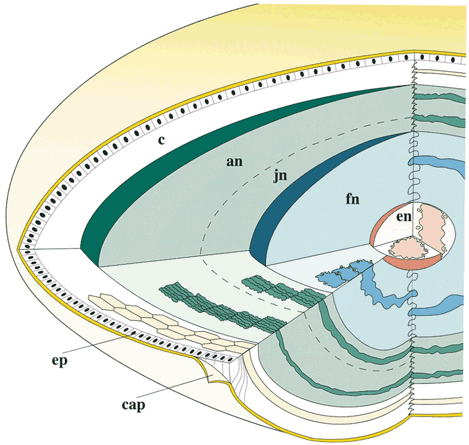
Although fibers are arranged end to end around a polar axis, they are not “meridians.” That is, fibers neither extend from pole to pole nor are they fusiform or tapered at their ends. Rather, there are two distinct types of mature secondary fibers: straight fibers and S-shaped fibers (Fig. 4). A straight fiber is crescent-shaped, with its entire length lying within a plane passed through the visual axis defined by its equatorial location. However, only one end of a straight fiber extends to a pole. An S fiber has a crescent shape, but in addition, its ends exhibit precise curvature away from the poles in diametrically opposite directions. This means that neither of the ends extend to a pole. Thus, the entire length of these fibers does not lie within a plane passed through the visual axis defined by their equatorial location.
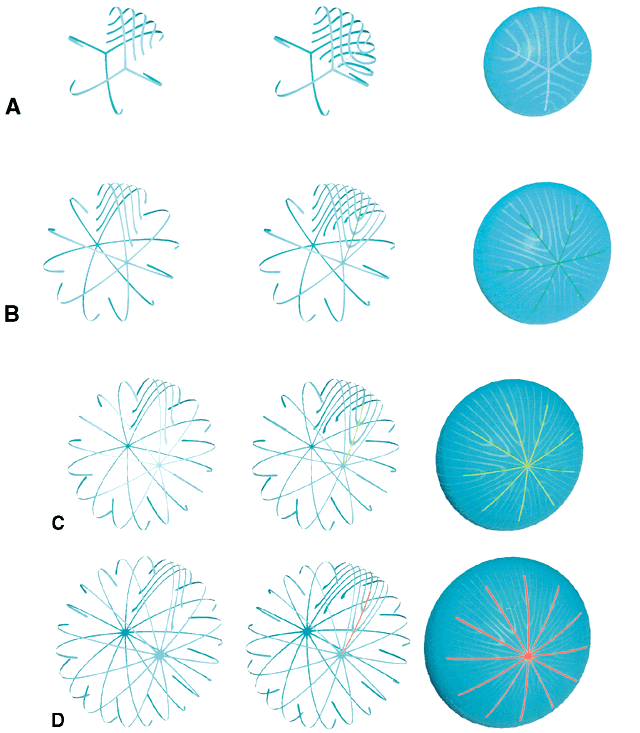
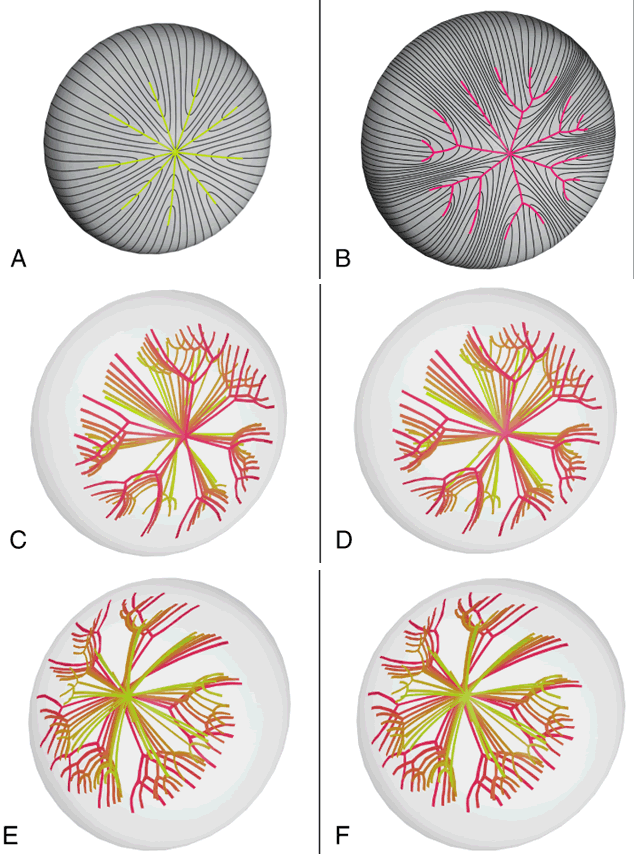
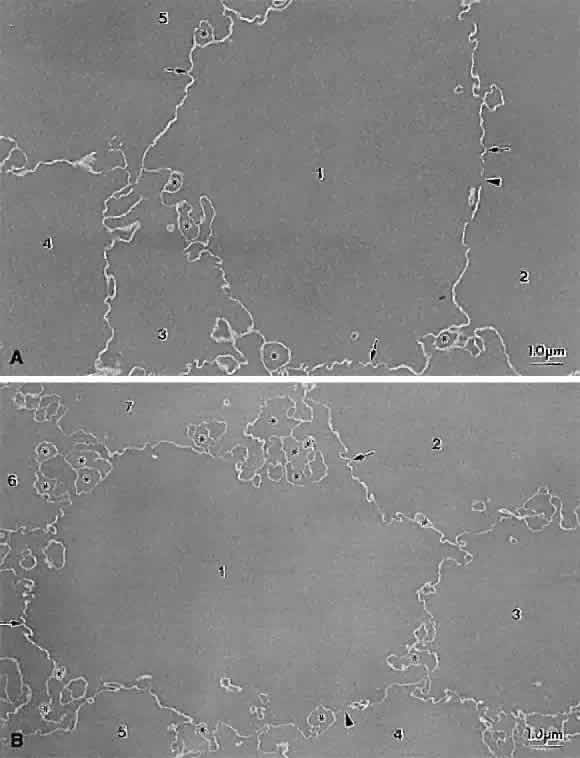
As a result of the variations in secondary fiber shape (failure of ends to extend to the poles and opposite end curvature), the ends of secondary fibers in all growth shells become aligned as specific longitudinal arc lengths. The overlapped ends of fibers in growth shells produce “suture branches” along these defined longitudinal arc lengths, and the origin of suture branches are defined by the ends of straight fibers. All the suture branches extend to confluence at the poles and combine to form discrete anterior and posterior suture “patterns.”
Throughout life, variably straight or S-shaped secondary fibers are arranged specifically to form suture branches and patterns in each growth shell. During the fetal period, as successive growth shells are formed, six straight fibers evolve in specific positions around the equator to subdivide shells into six equal segments. All other fibers evolve into S fibers arranged as six distinct groups, positioned between the straight fibers. Because of opposite-end curvature, the anterior and posterior ends of these fibers become aligned as offset anterior and posterior longitudinal arc lengths. The ends of two proximal groups of S fibers overlap to produce suture branches. The location and boundaries of suture branches are defined by the ends of straight fibers. Also, as a result of opposite-end curvature, while the anterior ends of fibers in proximal groups overlap to form anterior suture branches, their posterior ends overlap with different groups to form posterior branches.
In the anatomic position, the three anterior and three posterior suture branches are oriented at 120° angles to one another to produce respectively, upright Y and inverted Y suture patterns, that in turn are offset by 60°. In successive shells, suture branches formed during fetal development are positioned in identical locations so that continuous triangular suture planes are formed extending from the embryonic nucleus (primary fiber mass) to the lens periphery at birth. From an optical standpoint, the construction of suture planes has a significant negative effect on lens optics2–4 (i.e., increased spherical aberration or focal length variability [FLV] and sharpness of focus). The results of correlative laser scan, scanning electron microscopy (SEM), and three-dimensional computer-assisted drawing (3D-CAD) analysis of nonprimate lenses with Y sutures comparable to human lenses at birth, or of nonprimate lenses with “line” sutures, the simplest form of a branched suture pattern, have shown that FLV is minimal, and therefore, sharpness of focus is greatest, when a low-powered helium-neon laser passes through ordered radial cell columns. These studies also reveal that FLV is maximal, and therefore, sharpness of focus is least, when the beam passes through disordered suture planes.2–3
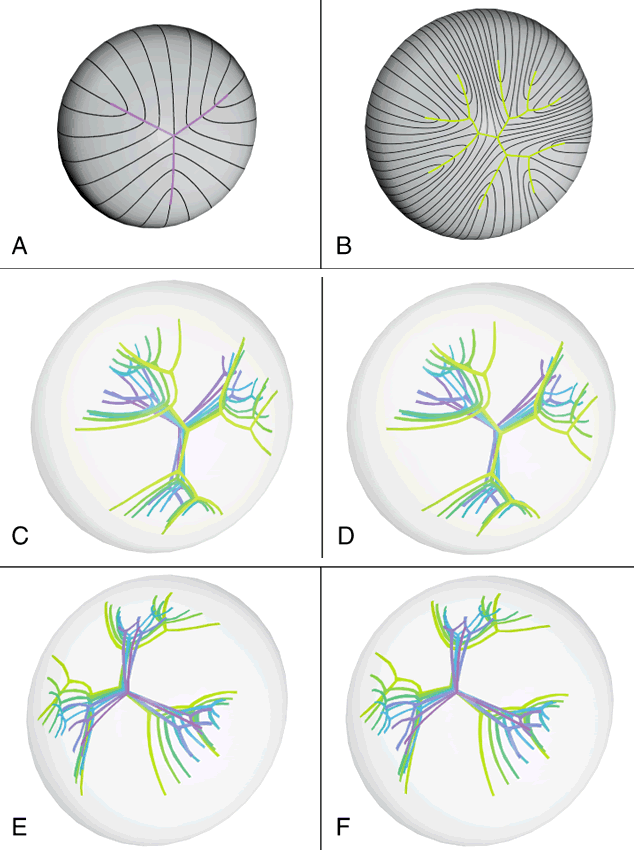
However, in comparable studies of primate lenses, it can be shown that an increased number of suture branches as a function of age results in superior optical quality. The reason for this paradox is that after birth, primates employ a similar but fundamentally different growth scheme than nonprimate lenses. After birth, humans produce progressively more complex sutures throughout distinct periods of growth. During the evolution of these sutures, the original six identical and symmetrically positioned Y suture branches formed throughout the fetal period serve as templates for the eventual formation of 12, 18, and 24 suture branches.
The second generation of sutures in humans, the “simple star” suture, is formed from birth through early childhood. Unlike the first generation Y suture, however, the fibers of successive shells are not identical in shape or position. As a result, these suture branches are out of register and thus, “discontinuous” suture planes are formed extending from the fetal nucleus to the lens periphery. Throughout adolescence and adulthood, “star” and “complex star” sutures—the third and fourth generation of sutures, respectively, are formed in a similar manner. In star and complex star sutures, respectively, 18 and 24 suture branches eventually are formed in successive shells. Thus, adult lenses have only small triangular suture planes within the fetal nucleus, overlain by progressively more complex suture patterns in successive infantile, juvenile, and adult nuclear and cortical shells. Because the branches of the progressively more complex suture patterns in successive shells are neither identical in size nor aligned in register, they form discontinuous suture planes extending from the fetal nucleus to the lens periphery. Thus, in human lenses, the negative influence exerted by suture planes on FLV is minimized effectively. In this manner, human lenses are optically superior to nonprimate lenses with line and Y suture lenses, at least in part, because of their more complex sutures.
Also, the evolution of suture branches does not occur simultaneously within a growth shell. Suture formation normally commences in the inferonasal quadrant and then proceeds in turn in the superotemporal, inferotemporal, and finally, the superonasal quadrants of the lens. The specific starting point is not without developmental or pathologic precedent. Colobomas of the eye (e.g., retina, ciliary body, iris, choroid, lens, and zonules), the failure or arrest of normal embryonic fissure closure during embryogenesis, typically occur in the inferonasal quadrant.5 In fact, this embryologic defect is considered atypical if it occurs in another quadrant.
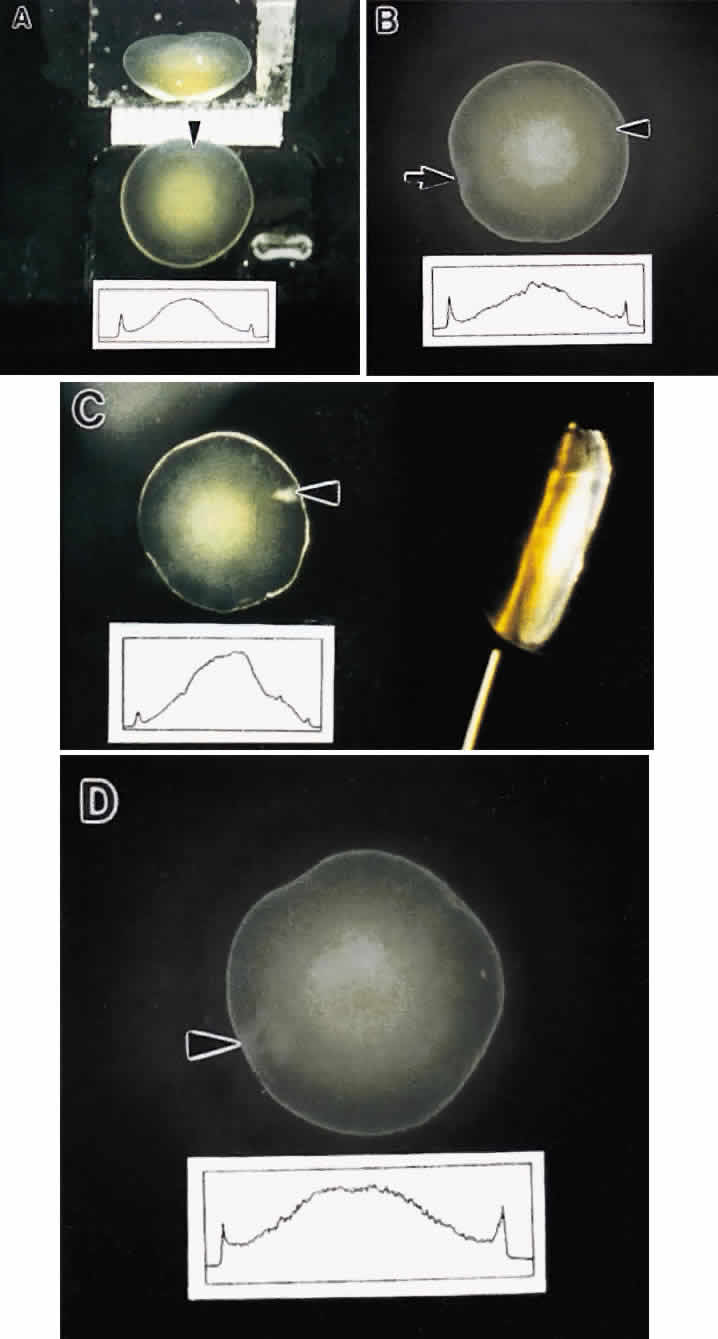
Slit-lamp biomicroscopy reveals four distinct and reproducible zones of discontinuity in aged emmetropic human lenses.6 These zones are formed by 4, 9, 19, and 46 years of age. The temporal development of the zones of discontinuity essentially is identical to the progressive iteration of the four generations of human lens sutures. Furthermore, the anatomic location and measure of the normal zones of discontinuity are coincident with the four distinct generations of sutures. This leads to the compelling argument that the zones of discontinuity and the distinct generations of sutures are one and the same. Indeed, the sharply demarcated zones of discontinuity are not coincident with alterations in human fiber membrane surface structure7–11 or variations in concentration and density of fiber crystalline and cytoskeletal proteins12,13 as a function of development, growth, and age. Furthermore, because the aforementioned changes in lens morphology as a function of age are common to both primate and nonprimate lenses, all lenses—not only primate lenses—should develop zones of discontinuity throughout life. Thus, the abnormal slit-lamp profiles of some cataractous lenses (particularly diabetic and cortical) are likely to be recordings of abnormal suture development. Correlative SEM and 3D-CAD analysis of human cortical cataract lenses surgically removed by the extracapsular technique confirm this premise.
ABNORMAL HUMAN SUTURE DEVELOPMENT AS A FUNCTION OF AGE
As a result of its inverted developmental and growth scheme, the lens retains every fiber formed throughout life within the concentric growth shells. By noting the axial dimensions of the lens at different ages, intact suture patterns formed at different ages can be retrieved for comparative studies between normal and human cortical cataracts.
The first generation of sutures in cortical cataracts has normal Y patterns. However, examination of cortical cataracts removed from patients in the fourth through fifth decades reveal that the second through fourth generations of sutures failed to form normally (Fig. 5). In concentric growth shells, radially enlarging subbranches are formed in identical locations. As a result, continuous suture planes, in the shape of cones extending from the periphery to the pole, are formed over a period of 3 decades, beginning shortly after birth.
Examination of cortical cataracts removed from patients in the seventh through eighth decades reveal that although the first through third generations of sutures are normal, the fourth generation is abnormal (Fig. 6). In concentric growth shells formed after the third generation of sutures (the star suture), radially enlarging subbranches are formed in identical locations. As described previously, continuous suture planes, in the shape of cones extending from the periphery to the pole, are formed over three decades, but in this case the onset of abnormal suture formation does not occur until middle age. The production of continuous pyramid-like planes in concentric growth shells likely would cause a significant increase in both FLV and scatter.2–4
In consideration of the aforementioned facts, the following should be noted:
- The evolution of identical but radially enlarged abnormal suture subbranches
in concentric growth shells results in the formation of abnormal
suture planes in cortical cataracts that essentially are identical in
shape to the conical opacities observed clinically by slit-lamp biomicroscopy.
- The initial abnormal suture planes are seen most frequently in the inferonasal
quadrant, as are the first conical opacities of cortical cataracts.
- The temporal formation of cortical opacities and abnormal suture planes
are synchronous.
- Abnormal suture planes only involve the anterior or alternatively the posterior
segments of fibers, and cortical opacities only occur in the
anterior or posterior cortex.
Thus, a compelling argument can be made that abnormal suture planes and cortical cataract opacities are one and the same.
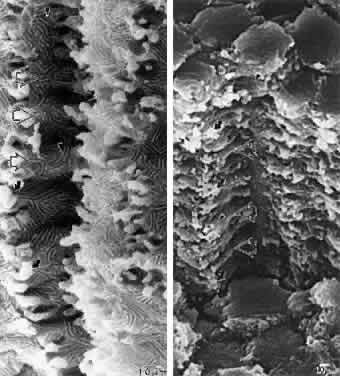
At surgery, the cortex of cortical cataract lenses is softer than the nucleus. Histologically, the cortex of these lenses feature swollen and liquefied fibers. It has been proposed that an increased uptake of water forces the cataractous cortical fibers apart between radial cell columns and concentric growth shells (lamellar separation). This proposition leads to speculation that the lens may be attempting to alter the structure of a localized site of increased scatter. Cortical opacities, seen to greatest advantage by retroillumination slit-lamp biomicroscopy, correspond to areas of lamellar separation that in turn are believed to correlate with “fiber folds” seen by SEM.14
Ultrastructurally, the opacities are characterized by variable amounts of degenerated cortical fiber fragments and ceroid bodies (lipofuscin), presumably the result of enzymatic digestion. Morgagnian cataracts are considered a special form of mixed cortical and nuclear cataract typified by complete liquefaction of the entire cortex. Again, it is tempting to speculate that the zones of liquefaction are an attempt by the lens to eliminate areas of scatter. This premise is strengthened by the fact that the nucleus in aged cortical cataract lenses has normal sutures and thus, would not need to undergo any internal alterations to improve light transmission. It also should be noted that to date, aged nuclear cataracts also seem to have normal sutural anatomy.