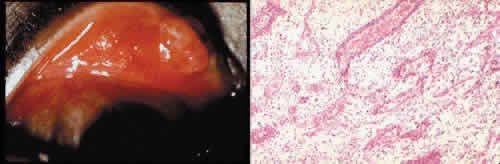
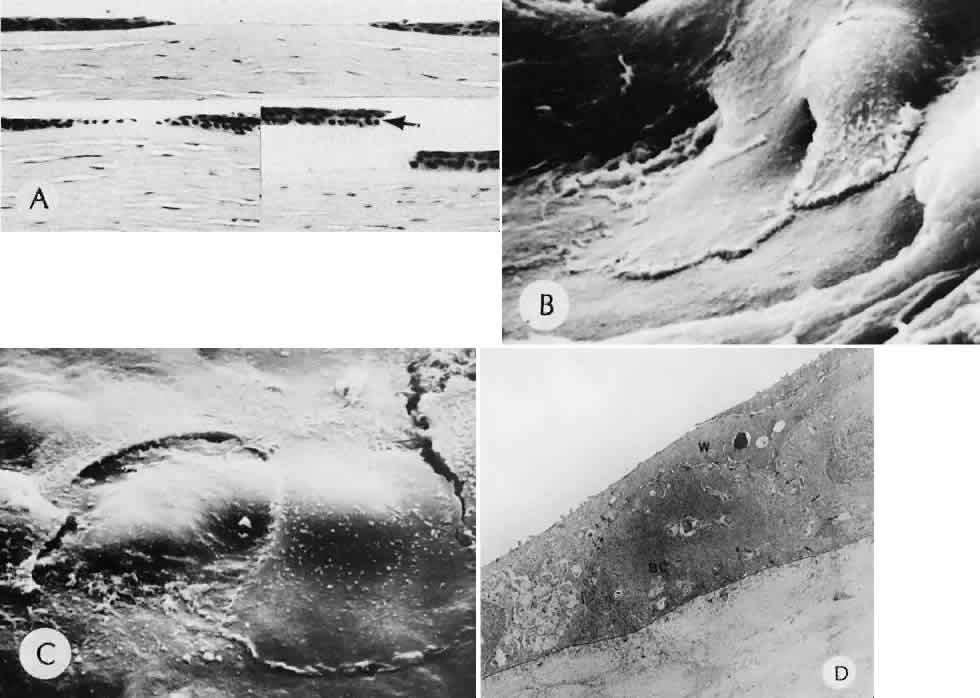
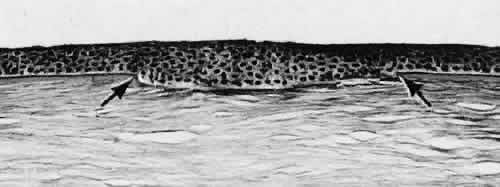
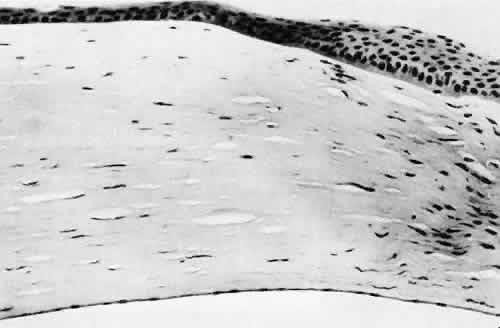
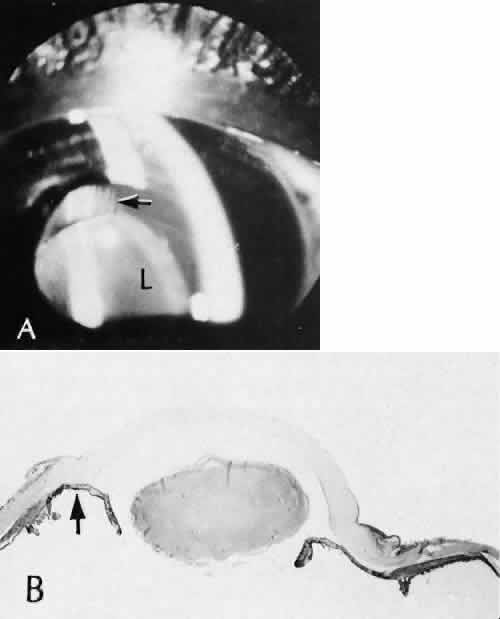
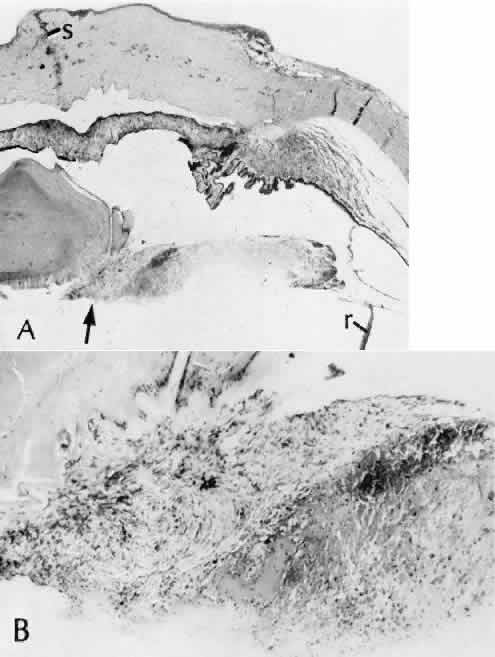
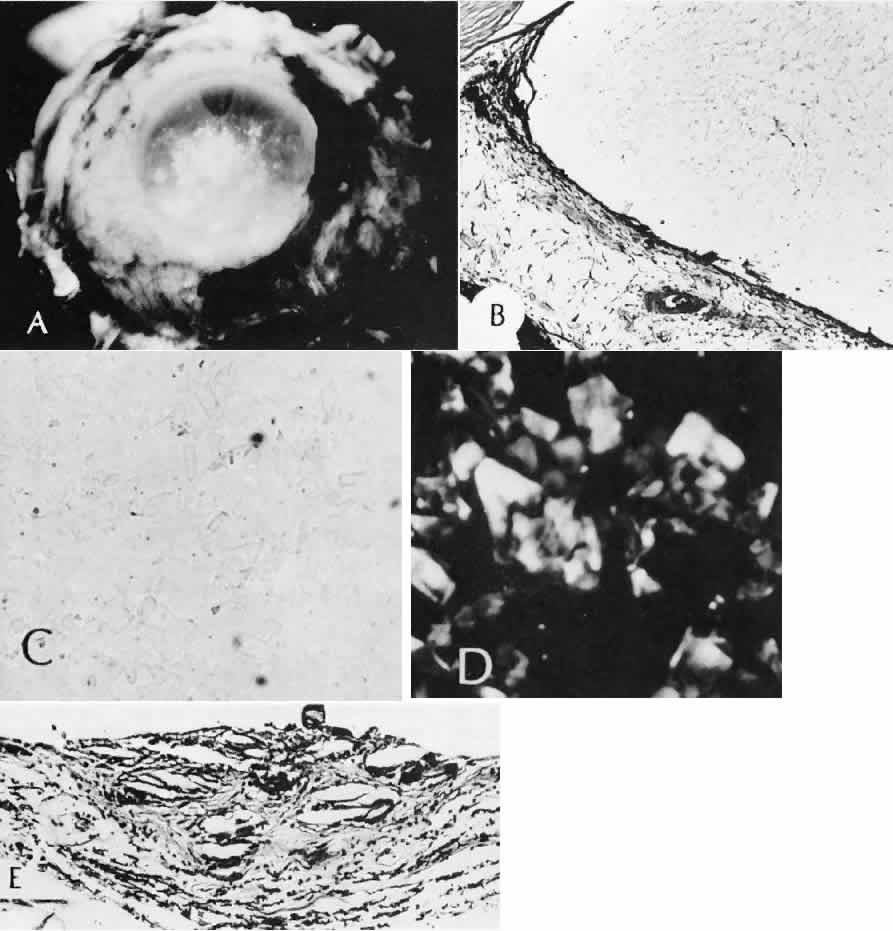
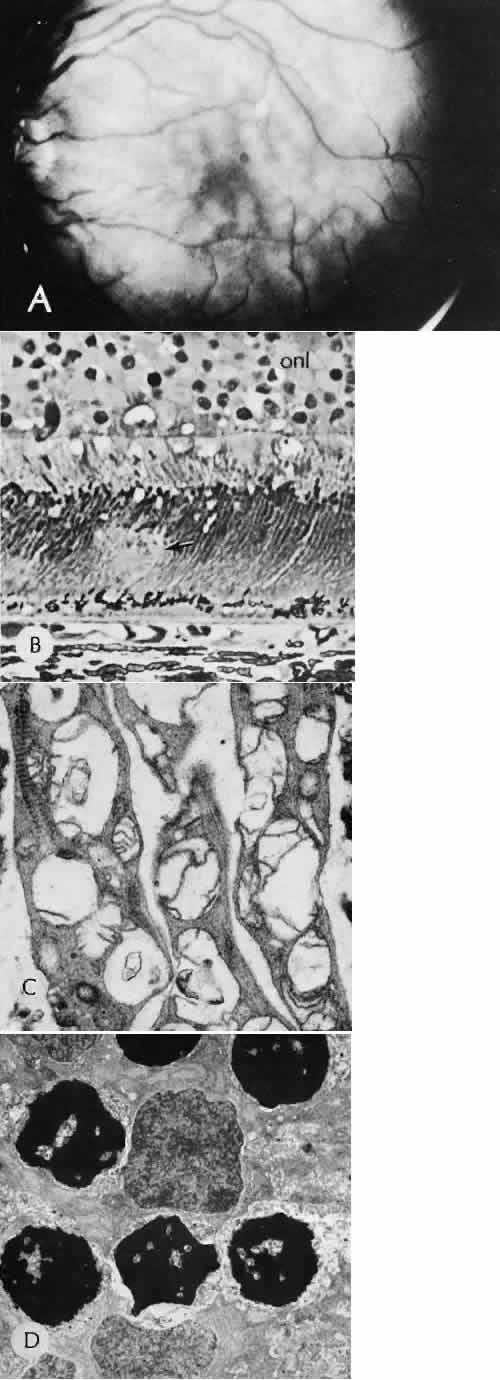
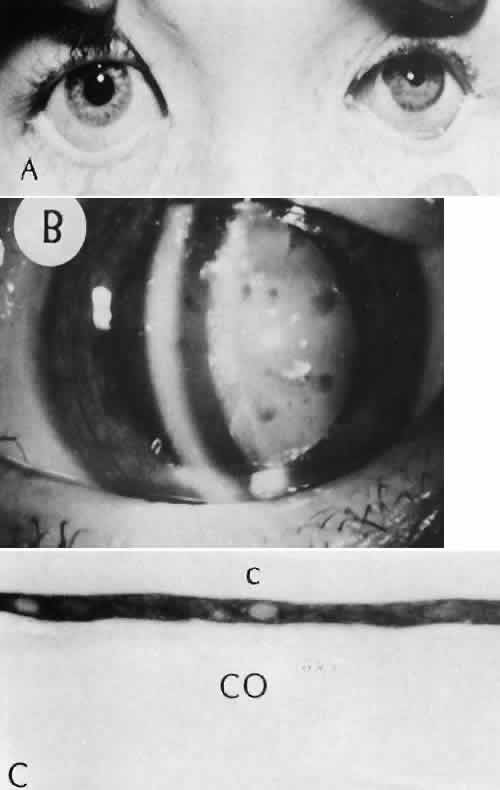
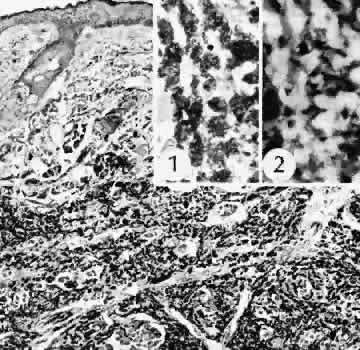
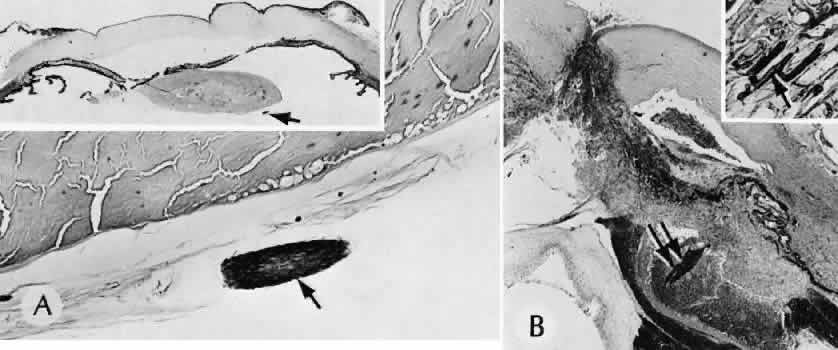
1. Martin P: Wound healing—aiming for perfect skin regeneration. [Review] [92 refs]. Science 276(5309):75–81, 1997. 2. Stocum DL: New tissues from old [editorial]. Science 276(5309):15, 1997. 3. Hicks CR et al: Keratoprostheses: advancing toward a true artificial cornea. [Review] [137 refs]. Survey of Ophthalmology 42(2):175-89, 1997 4. Germain L et al: Can we produce a human corneal equivalent by tissue engineering? Progress in Retinal & Eye Research 19(5):497–527, 2000. 5. Eckes B et al: Interactions of fibroblasts with the extracellular matrix: implications
for the understanding of fibrosis. [Review] [108 refs]. Springer Seminars in Immunopathology 21(4):415-29, 1999 6. Gerwins PE, Skoldenberg L, Claesson-Welsh: Function of fibroblast growth factors and vascular endothelial growth factors
and their receptors in angiogenesis. [Review] [94 refs]. Critical Reviews in Oncology-Hematology 34(3):185-94, 2000. 7. Gabbiani G, E.a.c.i.o.t.m.c.: [Review], Evolution and clinical implications of the myofibroblast
concept. Cardiovascular Research 38 39:545–548, 1998. 8. Thoft RA, Wiley LA, Sundarraj N: The multipotential cells of the limbus. Eye 3(Pt 2):109-13, 1989. 9. Dua HS, Azuara-Blanco A: Limbal stem cells of the corneal epithelium. Survey of Ophthalmology 44(5):415–425, 2000. 10. Wirtschafter JD et al: Mucocutaneous junction as the major source of replacement palpebral conjunctival
epithelial cells. Investigative Ophthalmology & Visual Science 40(13):3138–3146, 1999. 11. Greenhalgh D: The role of apoptosis in wound healing. International Journal of Biochemistry & Cell Biology 30:1019–1030, 1998. 12. Wilson: Stimulus-specific and cell type-specific cascades: emerging principles
relating to control of apoptosis in the eye. [Review] [99 refs]. Experimental Eye Research 69:255–266, 1999. 13. Helena MC et al: Keratocyte apoptosis afer corneal surgery. Investigative Ophthalmology & Visual Science 39(2):276–283, 1998. 14. Wilson: Role of aptosis in wound healing in the cornea. Cornea 19(Suppl):7–12, 2000. 15. Messadi D et al: Expression of apoptosis-associated genes by human dermal scar fibroblasts. Wound Repair Regeneration 7(6):511–517, 1999. 16. Kenney M, Brown D, Rajeev B: Everett Kinsey lecture. The elusive causes of keratoconus: a working hypothesis. CLAO 26:10–13, 2000. 17. Hiscott P et al: Repair in avascular tissues: fibrosis in the transparent structures of
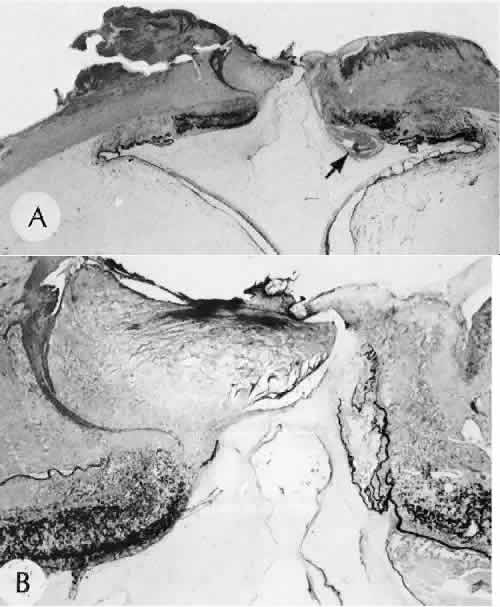
the eye and thrombospondin 1. [Review]. Histology & Histopathology 13:1309–1320, 1999. 18. Kuwabara T, Perkins DG, Cogan DG: Sliding of the epithelium in experimental corneal wounds. Investigative Ophthalmology, 15(1):4–14, 1976. 19. Buck P: Cell migration in repair of mouse corneal epithelium. Investigative Ophthalmology and Visual Science 19:767, 1979. 20. Khodadoust A et al: Adhesion of regenerating corneal epithelium. American Journal of Ophthalmology 65:339, 1968. 21. Cameron J, Flaxman B, Yanoff M: Invitro studies of corneal wound healing: Epithelial-endothelial interactions. Investigative Ophthalmology Visual Science 13:575, 1974. 22. Waring GOD et al: The corneal endothelium. Normal and pathologic structure and function. Ophthalmology 89(6):531–590, 1982. 23. Waring GOD: Posterior collagenous layer of the cornea. Ultrastructural classification
of abnormal collagenous tissue posterior to Descemet's membrane
in 30 cases. Archives of Ophthalmology 100(1):122–134, 1982. 24. Olson RJ, Levenson JE: Migration of donor endothelium in keratoplasty. American Journal of Ophthalmology 84(5):711–714, 1977. 25. Edelhauser HF: The resiliency of the corneal endothelium to refractive and intraocular
surgery. [Review] [62 refs]. Cornea 19(3):263–273, 2000. 26. Geggel HS, Friend J, Thoft RA: Conjunctival epithelial wound healing. Investigative
Ophthalmology & Visual Scence 25(7)860–863, 1984. 27. Wirtschafter JD et al: Palpebral conjunctival transient amplifying cells
originate at the mucocutaneous junction and their progeny migrate toward
the fornix. Transactions of the American Ophthalmological Society 95:417–429; discussion 429–432, 1997. 28. Huang A, Tseng J, Kenyon KR: Morphogenesis of rat conjunctival goblet cells. Investigative Ophthalmology & Visual Science 29(6):969–975, 1988. 29. Pellegrini G et al: Location and clonal analysis of stem cells and their differentiated progeny
in the human ocular surface. Journal of Cell Biology 145(4):769–782, 1999. 30. Iliev ME et al: Transconjunctival application of mitomycin C in combination with laser
sclerostomy ab interno: a long-term morphological study of the postoperative
healing process. Experimental Eye Research 64(6):1013–1026, 1997. 31. Oshima Y et al: Comparative study of intraocular lens implantation through 3.0 mm temporal
clear corneal and superior scleral tunnel self-sealing incisions. Journal of Cataract & Refractive Surgery 23(3):347–353, 1997. 32. Anders N et al: Postoperative astigmatism and relative strength of tunnel incisions: a
prospective clinical trial. Journal of Cataract & Refractive Surgery 23(3):332–336, 1997. 33. Oshika T et al: Three year prospective, randomized evaluation of intraocular lens implantation
through 3.2 and 5.5 mm incisions. Journal of Cataract & Refractive Surgery 24(4):509–514, 1998. 34. Flaxel JT, Swan KC: Limbal wound healing after cataract extraction. A histologic study. Archives of Ophthalmology 81(5):653–659, 1969. 35. Flaxel JT: Histology of cataract extractions. Archives of Ophthalmology 83(4):436–444, 1970. 36. Luntz MH, Kaufmann JC, Spiller M: Sutures and iris wound-healing in the baboon. Advances in Ophthalmology 30:171–184, 1975. 37. Tetsumoto K, Kuchle M, Naumann GO: Late histopathological findings of neodymium:YAG laser iridotomies in humans. Archives of Ophthalmoogy 110(8):1119–1123, 1992. 38. Font RL, Brownstein S: A light and electron microscopic study of anterior subcapsular cataracts. American Journal of Ophthalmology 78(6):972–984, 1974. 39. Pau H, Novotny GE, Arnold G: Ultrastructural investigation of extracellular structures in subcapsular
white corrugated cataract (anterior capsular cataract). Graefes Archive for Clinical & Experimental Ophthalmology 223(2):96–100, 1985. 40. Azuma N, Hara T: Extracellular matrix of opacified anterior capsule after endocapsular cataract
surgery. Graefes Archive for Clinical & Experimental Ophthalmology 236(7):531–536, 1998. 41. Saika S et al: Immunolocalization of prolyl 4-hydroxylase subunits, alpha-smooth muscle
actin, and extracellular matrix compoents in human lens capsules with
lens implants. Experimental Eye Research 66:283–294, 1998. 42. Kato K, Kurosaka D, Nagamoto T: Apoptotic cell death in rabbit lens after lens extraction. Investigative Ophthalmology and Visual Science 38:2322–2330, 1997. 43. Wallow IH, Tso MO: Repair after xenon arc photocoagulation. 2. A clinical and light microscopic
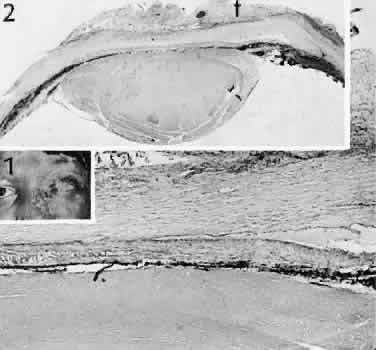
study of the evolution of retinal lesions in the rhesus monkey. American Journal of Ophthalmology 75(4):610–626, 1973. 44. Tso MO, Wallow IH, Elgin S: Experimental photocoagulation of the human retina. Archives of Ophthalmology 95(6):1035–1040, 1977. 45. Wallow IH, Tso MO, Elgin S: Experimental photocoagulation of the human retina. II. Electron microscopic
study. Archives of Ophthalmology 95(6):1041–1050, 1977. 46. Yoon YH, Marmor MF: Rapid enhancement of retinal adhesion by laser photocoagulation. Ophthalmology 95(10):1385–1388, 1988. 47. Miller B, et al: Effect of the vitreous on retinal wound-healing. Graefes Archive for Clinical & Experimental Ophthalmology 224(6):576–579, 1986. 48. Yamana T et al: The process of closure of experimental retinal holes in rabbit eyes. Gaefes Archive for Clinical & Experimental Ophthalmology 238(1):81–87, 2000. 49. Ozaki S et al: Influence of the sensory retina on healing of the rabbit retinal pigment
epithelium. Current Eye Research 16(4):349–358, 1997. 50. Perry DD, Reddick RL, Risco JM: Choroidal microvascular repair after argon laser photocoagulation. Ultrastructural
observations. Investigative Ophthalmology & Visual Science 25(9):1019–1026, 1984. 51. Perry DD, Risco JM: Choroidal microvascular repair after argon laser photocoagulation. American Journal of Ophthalmology 93(6):787–793, 1982. 52. Hayashi A et al: Surgically induced degeneration and regeneration of the choriocapillaris
in rabbit. Graefes Archive for Clinical & Experimental Ophthalmology 237(8):668–677, 1999. 53. Pollack A, Korte GE: Restoration of the outer blood-retinal barrier after krypton laser photocoagulation. Ophthalmic Research 25(4):201–209, 1993. 54. Miller H et al: Pathogenesis of laser-induced choroidal subretinal neovascularization. Investigative Ophthalmology & Visual Science 31(5):899–908, 1990. 55. Jacobs B, Gaynes B, Dutsch T: Refractive astigmatism after oblique clear corneal phacoemulsification
cataract incision. Journal of Cataract and Refractive Surgery 25:949–962, 1999. 56. Buzard K, Febbara J: Transconjunctival corneoscleral tunnel "blue line cataract incision. Journal of Cataract and Refractive Surgery 26:242–249, 2000. 57. Lindstrom RL, Hardten DR, Chu YR: Laser In Situ keratomileusis (LASIK) for
the treatment of low moderate, and high myopia. Transactions of the
American Ophthalmological Society 95:285–296; discussion 296–306, 1997. 58. McDonnell PJ: Emergence of refractive surgery. Archives of Ophthalmology 118(8):1119–1120, 2000. 59. Sachs HG, Lohmann CP, Op de Laak JP: Intraocular pressure in sections with 2 microkeratomes in vitro. Ophthalmologe 94(10):707–709, 1997. 60. Seiler T, Quurke AW: Iatrogenic keratectasia after LASIK i a case of forme fruste keratoconus. Journal of Cataract & Refractive Surgery 24(7):1007–1009, 1998. 61. Seiler TK, Koufala, Richter G: Iatrogenic keratectasia after laser in situ keratomileusis. Journal of Refractive Surgery 14(3):312–317, 1998. 62. Probst LE, Machat JJ: Mathematics of laser in situ kera-tomileusis for high myopia. Journal of Cataract & Re-fractive Surgery 24(2):190–195, 1998. 63. Doughty MJ, Zaman ML: Human corneal thickness and its impact on intraocular pressure measures: a
review and meta-analysis approach. [Review] [517 refs]. Survey ofOphthalmology 44(5):367–408, 2000. 64. Price FW Jr, Koller DL, Price MO: Central corneal pachymetry in patients undergoing laser in situ keratomileusis. Ophthalmology 106(11):2216–2220, 1999. 65. Maldonado MJ et al: Optical coherence tomography evaluation of the corneal
cap and stromal bed features after laser in situ keratomileusis for
high myopia and astigmatism. Ophthalmology 107(1):81–87; discussion 88, 2000. 66. Binder PS et al: Comparison of two microkeratome systems. Journal of Refractive Surgery 13(2):142–153, 1997. 67. Behrens A et al: Experimental evaluation of two current-generation automated microkeratomes: the
Hansatome and the Supratome. American Journal of Ophthalmology 129(1):59–67, 2000. 68. Behrens A et al: Evaluation of corneal flap dimensions and cut quality using the Automated
Corneal Shaper microkeratome. Journal of Refractive Surgery, 16(1):83–89, 2000. 69. Ye HQ, Azar DT: Expression of gelatinases A and B, and TIMPs 1 and 2 during corneal wound
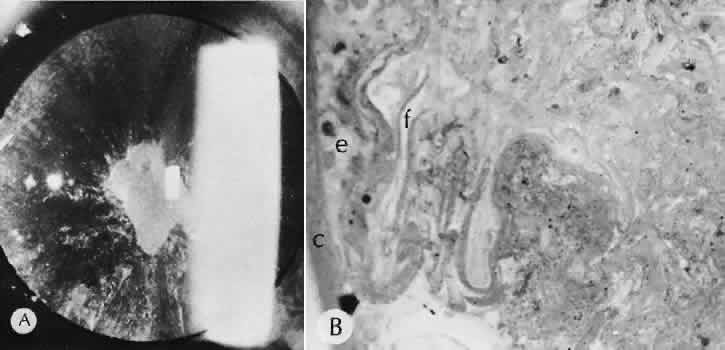
healing. Investigative Ophthalmology & Visual Science 39(6):913–921, 1998. 70. Kato T et al: Corneal wound healing following laser in situ keratomileusis (LASIK): a
histopathological study in rabbits. British Journal of Ophthalmology, 83(11):1302–1305, 1999. 71. Goggin M et al: Regression after photorefractive keratectomy for myopia. Journal
of Cataract & Refractive Surgery 22(2):194–196 1996. 72. Ramirez-Florez S, Maurice DM: Inflammatory cells, refractive regression, and haze after excimer laser
PRK [published erratum appears in J Refract Surg 1996 Sep-Oct;12(6):676] [see
comments]. Journal of Refractive Surgery 12(3):370–381, 1996. 73. Wachtlin J et al: Immunohistology of corneal wound healing after photorefractive keratectomy
and laser in situ keratomileusis. wachtlin;atsymbol;ukbf.fu-berlin.de. Journal of Refractive Surgery 15(4):451–458, 1999. 74. Polunin GS et al: The corneal barrier function in myopic eyes after laser in situ keratomileusis
and after photorefractive keratectomy in eyes with haze formation. Journal of Refractive Surgery 15(Suppl 2):S221–S224, 1999. 75. Vesaluoma M et al: Corneal stromal changes induced by myopic LASIK. Investigative Ophthalmology & Visual Science 41(2):369–376, 2000. 76. Linna TU et al: Effect of myopic LASIK on corneal sensitivity and morphology of subbasal
nerves. Investigative Ophthalmology & Visual Science 41(2):393–397, 2000. 77. Maurice DM, Monroe F: Cohesive strength of corneal lamellae. Experimental Eye Research 50(1):59–63, 1990. 78. Gimbel HV et al: Incidence and management of intraoperative and early postoperative
complications in 1000 consecutive laser in situ keratomileusis
cases [see comments]. Ophthalmology 105(10):1839–1847; discussion 1847–1848, 1998. 79. Lin RT, Maloney RK: Flap complications associated with lamellar refractive surgery [see
comments]. American Journal of Ophthalmology 127(2):129–136, 1999. 80. Shields MB et al: Clinical and histopathologic observations concerning hypotony after trabeculectomy
with adjunctive mitomycin C. American Journal of Ophthalmology 116(6):673–683, 1993. 81. Pasquale LR et al: Effect of topical mitomycin C on glaucoma filtration surgery in monkeys. Ophthalmology 99(1):14–18, 1992. 82. Jampel HD: Effect of brief exposure to mitomycin C on viability and proliferation
of cultured human Tenon's apsule fibroblasts. Ophthalmology 99(9):1471–1476, 1992. 83. Skuta GL: Antifibrotic agents in glaucoma filtering surgery. International Ophthalmology Clinics 33(4):165–182, 1993. 84. Kroll AJ, Machemer R: Experimental retinal detachment and reattachment: II. Electron microscopy. Bibliotheca Ophthalmologica 79:91–105, 1969. 85. Kroll AJ, Machemer R: Experimental retinal detachment in the owl monkey. V. Electron microscopy
of the reattached retina. American Journal of Ophthalmology 67(1):117–130, 1969. 86. Bettman JW Jr: Pathology of complications of intraocular surgery. American Journal of Ophthalmology 68(6):1037–1050, 1969. 87. Arango JL, Margo CE: Wound complications following cataract surgery. A case-control study. Archives of Ophthalmology 116(8):1021–1024, 1998. 88. Linebarger EJ et al: Phacoemulsification and modern cataract surgery. [Review] [404 refs]. Survey of Ophthalmology 44(2):123–147, 1999. 89. Pieramici D, Green WR, Stark WJ: Stripping of Descemet's membrane: a clinicopathologic correlation. [Review] [21 refs]. Ophthalmic Surgery 25(4):226–231, 1994. 90. Amaral CE, Palay DA: Technique for repair of Descemet membrane detachment. American Journal of Ophthalmology 127(1):88–90, 1999. 91. Benson WE et al: Late hyphema due to vascularization of the cataract wound. Annals of Ophthalmology 10(8):1109–1112, 1978. 92. Lumme P, Laatikainen LT: Risk factors for intraoperative and early postoperative complications in
extracapsular cataract surgery. European Journal of Ophthalmology 4(3):151–158, 1994. 93. Winslow RL, Stevenson WD, Yanoff M: Spontaneous expulsive choroidal hemorrhage. Archives of Ophthalmology 92(1):33–36, 1974. 94. Ingraham HJ, Donnenfeld ED, Perry HD: Massive suprachoroidal hemorrhage in penetrating keratoplasty. American Journal of Ophthalmology 108(6):670–675, 1989. 95. Perry HD, Donnenfeld ED: Expulsive choroidal hemorrhage following suture removal after penetratng
keratoplasty. American Journal of Ophthalmology 106(1):99–100, 1988. 96. Carlson AN, Stewart WC, Tso PC: Intraocular lens complications requiring removal or exchange. [Review] [285 refs]. Survey of Ophthalmology 42(5):417–440, 1998. 97. Ruiz RS, Teeters VW: The vitreous wick syndrome. A late complication following cataract extraction. American Journal of Ophthalmology 70(4):483–490, 1970. 98. Lindstrom RL, Doughman DJ: Bacterial endophthalmitis associated with vitreous wick. Annals of Ophthalmology 11(11):1775–1778, 1979. 99. Maguire LJ et al: Bacterial endophthalmitis associated with vitreous wick after penetrating
keratoplasty. American Journal of Ophthalmology 100(6):854–855, 1985. 100. Pulido JS et al: Histoplasma capsulatum endophthalmitis after cataract extraction. Ophthalmology 97(2):217–220, 1990. 101. Kielar RA, Stambaugh JL: Pupillary block glaucoma following intraocular lens implantation. Ophthalmic Surgery 13(8):647–650, 1982. 102. Willis DA, Stewart RH, Kimbrough RL: Pupillary block associated with posterior chamber lenses. Ophthalmic Surgery 16(2):108–109, 1985. 103. Ellingson FT: The uveitis-glaucoma-hyphema syndrome associated with the Mark VIII anterior
chamber lens implant. Journal - American Intra-Ocular Implant Society 4(2):50–53, 1978. 104. Magargal LE et al: Recurrent microhyphema in the pseudophakic eye. Ophthalmology 90(10):1231–1234, 1983. 105. Drews RC, Smith ME, Okun N: Scanning electron microscopy of intraocular lenses. Ophthalmology 85(4):415–424, 1978. 106. Apple DJ, Sims J: Harold Ridley and the invention of the intraocular lens. Survey of Ophthalmology 40(4):279–292, 1996. 107. Samples JR, Van Buskirk EM: Pigmentary glaucoma associated with posterior chamber intraocular lenses. American Journal of Ophthalmology 100(3):385–388, 1985. 108. Insler MS, Zatzkis SM: Pigment dispersion syndrome in pseudophakic corneal transplants. American Journal of Ophthalmology 102(6):762–765, 1986. 109. Mastropasqua L, Lobefalo L, Gallenga PE: Iris chafing in pseudophakia. Documenta Ophthalmologica 87(2):139–144, 1994. 110. Johnson DH, Bourne WM, Campbell RJ: The ultrastructure of Descemet's membrane. II. Aphakic bullous keratopathy. Archives of Ophthalmology 100(12):1948–1951, 1982. 111. Chu MW, Font RL, Koch DD: Visual results and complications following posterior iris-fixated posterior
chamber lenses at penetrating keratoplasty. Ophthalmic Surgery 23(9):608–613, 1992. 112. Buxton JN et al: Donor failure after corneal transplantation surgery. Cornea 7(2):89–95, 1988. 113. Allen HF, Mangiaracine AB: Bacterial endophthalmitis after cataract extraction. II. Incidence in 36,000 consecutive
operations with special reference to preoperative topical
antibiotics. Archives of Ophthalmology 91(1):3–7, 1974. 114. Kloess PM, et al: Bacterial and fungal endophthalmitis after penetrating keratoplasty [published
erratum appears inAm J Ophthalmol 1993 Apr 15;115(4):548]. [Review] [36refs]. American Journal of Ophthalmology 115(3):309–316,1993. 115. Meisler DM et al: Chronic Propionibacterium endophthalmitis after extracapsular cataract
extraction and intraocular lens implantation. American Journal of Ophthalmology 102(6):733–739, 1986. 116. Chien AM et al: Propionibacterium acnes endophthalmitis after intracapsular cataract extraction [see
comments]. Ophthalmology 99(4):487–490, 1992. 117. Clark WL et al: Treatment strategies and visual acuityoutcomes in chronic postoperative
Propionibacteriumacnes endophthalmitis. Ophthalmology 106(9):1665–1670,1999. 118. Kappelhof JP et al: The ring of Soemmerring in the rabbit: A scanning electron microscopic
study. Graefes Archive for Clinical & Experimental Ophthalmology 223(3):111–120, 1985. 119. Apple DJ et al: Posterior capsule opacification. [Review] [430 refs]. Survey of Ophthalmology 37(2):73–116,1992. 120. Tetz MR, Nimsgern C: Posterior capsule opacification. Part 2: Clinical findings. [Review] [103 refs]. Journa of Cataract & Refractive Surgery 25(12):1662–1674, 1999. 121. Coonan P et al: The incidence of retinal detachment following extracapsular cataract extraction. A
ten-year study. Ophthalmology 92(8):1096–1101, 1985. 122. van Oye R, Gelisken O: Pseudophakic glaucoma. International Ophthalmology 8(3):183–186, 1985. 123. Kooner KS et al: Intraocular pressure following ECCE, phacoemulsification, and PC-IOL implantation. Ophthalmic Surgery 19(9):643–646, 1988. 124. Perez-Santonja JJ et al: Laser in situ keratomileusis to correct high myopia. Journal of Cataract & Refractive Surgery 23(3):372–385, 1997. 125. Stulting RD et al: Complications of laser in situ keratomileusis for the correction of myopia. Ophthalmology 106(1):13–20, 1999. 126. Farah SG et al: Laser in situ keratomileusis: literature review of a developing technique. Journal of Cataract & Refractive Surgery 24(7):989–1006, 1998. 127. Wilson SE: LASIK: management of common complications. Laser in situ keratomileusis. Cornea 17(5):459–467, 1998. 128. Reviglio V et al: Mycobacterium chelonae keratitis following laser in situ keratomileusis. Journal of Refractive Surgery 14(3):357–360, 1998. 129. Pirzada WA, Kalaawry H: Laser in situ keratomileusis for myopia of -1 to -3.50 diopters. Journal of Refractive Surgery 13(Suppl 5):S425–S426, 1997. 130. Mulhern MG, Condon PI, O'Keefe M: Endophthalmitis after astigmatic myopic laser in situ keratomileusis. Journal of Cataract & Refractive Surgery, 23(6):948–950, 1997. 131. Perez-Santonja JJ et al: Nocardial keratitis after laser in situ keratomileusis. Journal of Refractive Surgery 13(3):314–317, 1997. 132. Crews KR, Mifflin MD, Olson RJ: Complications of automated lamellar keratectomy [letter] [see
comments]. Archives of Ophthalmology 112(12):1514–1515, 1994. 133. Friedman RF, Chodosh J, Wolf TC: Catastrophic complications of automated lamellar keratoplasty [letter]. Archives of Ophthalmology 115(7):925–926, 1997. 134. Pallikaris IG, Signos DS: Laser in situ keratomileusis to treat myopia: early experience. Journal of Cataract & Refractive Surgery 23(1):39–49, 1997. 135. Sugar A: Outcome of cornea, iris, and lens perforation during automated lamellar
keratectomy [letter] [see comments]. Archives of Ophthalmology 114(9):1144–1145, 1996. 136. Hori Y et al: Medical treatment of operative corneal perforation caused by laser in situ
keratomileusis. Archives of Ophthalmology 117(10):1422–1423, 1999. 137. Joo CK, Kim TG: Corneal perforation during laser in situ keratomileusis. Journal of Cataract & Refractive Surgery 25(8):1165–1167, 1999. 138. Leung AT, Rao SK, Lam DS: Traumatic partial unfolding of laser in situ keratomileusis flap with severe
epithelial ingrowth. Journal of Cataract & Refractive Surgery 26(1):135–139, 2000. 139. Pannu JS: Wrinkled corneal flaps after LASIK [letter; comment]. Journal of Refractive Surgery 13(4):341, 1997. 140. Carpel EF, Carlson KH, Shannon S: Folds and striae in laser in situ keratomileusis flaps. Journal of Refractive Surgery 15(6):687–690, 1999. 141. Helena MC, Meisler D, Wilson SE: Epithelial growth within the lamellar interface after laser in situ keratomileusis (LASIK) [see comments]. Cornea 16(3):300–305, 1997. 142. Castillo A et al: Peripheral melt of flap after laser in situ keratomileusis. Journal of Refractive Surgery 14(1):61–63, 1998. 143. Steinert RF et al: Diffuse interface keratitis after laser in situ keratomileusis (LASIK): A
non-speific syndrome. American Journal of Ophthalmology, 129:380–381, 2000. 144. MacRae S, Macaluso DC, Rich LF: Sterile interface keratitis associated with micropannus hemorrhage after
laser in situ keratomileusis. Journal of Cataract & Refractive Surgery 25(12):1679–1681, 1999. 145. Smith RJ, Maloney RK: Diffuse lamellar keratitis. A new syndrome in lamellar refractive surgery. Ophthalmology 105(9):1721–1726, 1998. 146. Macaluso DC, Rich LF, MacRae S: Sterile interface keratitis after laser in situ keratomieusis: three episodes
in one patient with concomitant contact dermatitis of the eyelids. Journal of Refractive Surgery 15(6):679–682, 1999. 147. Lyle WA, Jin GJ: Interface fluid associated with diffuse lamellar keratitis and epithelial
ingrowth after laser in situ keratomileusis. Journal of Cataract & Refractive Surgery 25(7):1009–1012, 1999. 148. Holland SP et al: Diffuse lamellar keratitis related to endotoxins released from sterilizer
reservoir biofilms. Ophthalmology 107:1227–1234, 2000. 149. Kaufman SC et al: Interface inflammation after laser in situ keratomileusis. Sands of the
Sahara syndrome [see comments]. Journal of Cataract & Refractive Surgery 24(12):1589–1593, 1998. 150. Hirst LW, Vandeleur KW Jr: Laser in situ keratomileusis interface deposits. Journal of Refractive Surgery 14(6):653–654, 1998. 151. Fraenkel GE et al: Central focal interface opacity after laser in situ keratomileusis. Journal of Refractive Surgery 14(5):571–576, 1998. 152. Peacock LW et al: Ocular integrity after refractive procedures [see comments]. Ophthalmology 104(7):1079–1083, 1997. 153. Pearlstein ES et al: Ruptured globe after radial keratotomy. American Journal of Ophthalmology 106(6):755–756, 1988. 154. Zadok D et al: Pneumotonometry versus Goldmann tonometry after laser in situ keratomileusis
for myopia. Journal of Cataract & Refractive Surgery 25(10):1344–1348, 1999. 155. Pastor JC: Proliferative vitreoretinopathy: an overview. [Review] [139 refs]. Survey of Ophthalmology 43(1):3–18, 1998. 156. Chiapella A, Rosenthal A: One year in an eye casualty clinic. British Journal of Ophthalmology 67:685, 1985. 157. Karlson T, Klein B, Klein BE: The incidence of acute hospital-treated eye injuries. Arch Ophthalmol 104:1473, 1986. 158. Parver L: Eye trauma. The neglected disorder. Arch Ophthalmol 104:152, 1986. 159. Pieramici DJ et al: A system for classifying mechanical injuries of the eye (globe). The Ocular
Trauma Classification Group [see comments]. America Journal of Ophthalmology 123(6):820–831, 1997. 160. Sobaci G et al: Deadly weapon-related open-globe injuries: outcome assessment by the ocular
trauma classification system. American Journal of Ophthalmology 129(1):47–53, 2000. 161. Bullock JD et al: Ocular and orbital trauma from water balloon slingshots. A clinical, epidemiologic, and
experimental study. Ophthalmology 104(5):878–887, 1997. 162. Maloney W et al: Specular microscopy of traumatic posterior annular keratopthy. Archives of Ophthalmology 97:1647–1650, 1979. 163. Stulting RM, Rodrigues, Nay R: Ultrastructure of traumatic corneal endothelial rings. American Journal of Ophthalmology 101:156–159, 1986. 164. Cotran P, Bajart A: Congenital corneal opacities. International Ophthalmology Clinics 32:93–105, 1992. 165. Tuft SJ, Gregory WM, Buckley RJ: Acute corneal hydrops in keratoconus. Ophthalmology 101(10):1738–1744, 1994. 166. Shaw EL: Pathophysiology and treatment of corneal hydrops. Ophthalmic Surgery 7(4):33–37, 1976. 167. Feder RS et al: Intrastromal clefts in keratoconus patients with hydrops. American Journal of Ophthalmology, 126(1):9–16, 1998. 168. Margo CE, MW: Mosteller, Corneal pseudocyst following acute hydrops. British Journal of Ophthalmology 71(5):359–360, 1987. 169. O'Grady R, Kirk H, K.H.C.k.A.J.o.O.-Z. RB: Corneal keloids. American Journal of Ophthalmology 73:206–213, 1972. 170. Lahav M et al: Corneal keloids-a shitopathological study. Graefes Arive For Clinical and Experimental Ophthalmology 218:256–261, 1982. 171. Canavan YY, Archer DB: Anterior segment consequences of blunt ocular injury. Br J Ophthalmol 66:549, 1982. 172. Collet B: Traumatic hyphema: a review. Annals of Ophthalmology 14:52–56, 1972. 173. Cassel G, Jeffers J, Jaeger E: Wills Eye Hospital Traumatic Hyphmea Study. Ophthalmic Surgery 16:441–443, 1985. 174. Kennedy R, Brubaker R: Traumatic hyphema in a defined population. American Journal of Ophthalmology 106:123–130, 1988. 175. ÜcRahmani B, Jahadi H, Rajaeefard A: An analysis of risk for secondary hemorrahge in traumatic hyphema. Ophthalmology 106:380–385, 1999. 176. Caprioli J, Sears M: The histopathology of black ball hyphema: a report of two cases. Ophthalmic Surgery 15:491–495, 1984. 177. Tonjum A: Gonioscopy in traumatic hyphema. Acta Ophthalmologica 44:650–664, 1966. 178. Beyer T, Hist L, H.L.C.b.s.a.l.p.A.O. TL: Corneal blood staining at low pressures. Archives of Ophthalmology 103:654, 1985. 179. Messmer EP, Gottsch J, Font RL: Blood staining of the cornea: a histopathologic analysis of 16 cases. Cornea 3(3):205–212, 1984. 180. Gottish JD et al: Corneal blood staining. An animal model. Ophthalmology 93(6):797–802, 1986. 181. Kumar S et al: A quantitative animal model of traumatic iridodialysis. Acta Ophthalmologica 68(5):591–596, 1990. 182. Wolff SM, Zimmerman L: Chronic secondary glaucoma associated with retrodisplacement of the iris
root and deepening of the anterior chamber angle secondary to contusion. American Journal of Ophthalmology 54:547, 1962. 183. Spaeth G: Traumatic hyphema, angle recession. Archives of Ophthalmology 78:714, 1967. 184. Marak GE Jr: Phacoanaphylactic endophthalmitis [published erratum appears in Surv
Ophthalmol 1992 May-Jun;36(6):454]. [Review] [141 refs]. Survey of Ophthalmology 36(5):325–339, 1992. 185. Spraul CW, Grossniklaus HE: Vitreous Hemorrhage. Survey of Ophthalmology 42(1):3–39, 1997. 186. Campbell DG, Essigmann EM: Hemolytic ghost cell glaucoma. Further studies. Archives of Ophthalmology 97(11):2141–2146, 1979. 187. Campbell DG: Ghost cell glaucoma following trauma. Ophthalmology 88(11):1151–1158, 1981. 188. Montenegro MH, Simmons RJ: Ghost cell glaucoma. International Ophthalmology Clinics 35(1):111–115, 1995. 189. Summers CG, Lindstrom RL, Cameron JD: Phase contrast microscopy. Diagnosis of ghost cell glaucoma following cataract
extraction. Survey of Ophthalmology 28(4):342–344, 1984. 190. Mansour AM, Green W, Hogge C: Histopathology of commotio retinae. Retina, 12(1):24–28, 1992. 191. Pulido JS, Blair NP: The blood-retinal barrier in Berlin's edema. Retina 7(4):233–236, 1987. 192. Liem AT, Keunen JE, van Norren D: Reversible cone photoreceptor injury in commotio retinae of the macula. Retina 15(1):58–61, 1995. 193. Tso MO: Pathology of cystoid macular edema. Ophthalmology 89(8):902–915, 1982. 194. Frangieh GT, Green WR, Engel HM: A histopathologic study of macular cysts and holes. Retina 1(4):311–336, 1981. 195. Hochman MA, Seery CM, Zarbin MA: Pathophysiology and management of subretinal hemorrhage. Survey of Ophthalmology 42(3):195–213, 1997. 196. Glatt H, Machemer R: Experimental subretinal hemorrhage in rabbits. American Journal of Ophthalmology 94(6):762–773, 1982. 197. Cox MS, Freeman HM: Retinal detachment due to ocular penetration. I. Clinical characteristics
and surgical results. Archives of Ophthalmology 96(8):1354–1361, 1978. 198. Kempster RC, Green WR, Finkelstein D: Choroidal rupture. Clinicopathologic correlation of an unusual case. Retina 16(1):57–63, 1996. 199. Gross JG et al: Subfoveal neovascular membrane removal in patients with traumatic choroidal
rupture. Ophthalmology 103(4):579–585, 1996. 200. Wagoner MD: Chemical injuries of the eye: current concepts in pathophysiology and therapy. [Review] [428 refs]. Survey of Ophthalmology 41(4):275–313, 1997. |